Organismal Function Enhancement through Biomaterial Intervention
Abstract
:1. Introduction
2. Biointerface Engineering
3. Artificial Organelle
4. Three-Dimensional Multicellular Immune Niche
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cusack, M.; Freer, A. Biomineralization: Elemental and organic influence in carbonate systems. Chem. Rev. 2008, 108, 4433–4454. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Diatoms, biomineralization processes, and genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Hao, H.; Zhang, Y.; Xu, X.; Tang, R. Therapeutic potential of biomineralization-based engineering. Adv. Ther. 2018, 1, 1800079. [Google Scholar] [CrossRef]
- Cao, J.; Zaremba, O.T.; Lei, Q.; Ploetz, E.; Wuttke, S.; Zhu, W. Artificial bioaugmentation of biomacromolecules and living organisms for biomedical applications. ACS Nano 2021, 15, 3900–3926. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Schüler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, R.; Yan, X.; Fan, K. Nanozyme-based artificial organelles: An emerging direction for artificial organelles. Small 2022, 18, 2202294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Wang, Y.; Cui, Y.; Lin, J.; Zhou, Y.; Tang, S.; Zhang, Y.; Hao, H.; Nie, Z.; et al. Material-engineered bioartificial microorganisms enabling efficient scavenging of waterborne viruses. Nat. Commun. 2023, 14, 4658. [Google Scholar] [CrossRef]
- Che, H.; Cao, S.; Van Hest, J.C.M. Feedback-induced temporal control of “breathing” polymersomes to create self-adaptive nanoreactors. J. Am. Chem. Soc. 2018, 140, 5356–5359. [Google Scholar] [CrossRef]
- Zhang, Y.; Habibovic, P. Delivering mechanical stimulation to cells: State of the art in materials and devices design. Adv. Mater. 2022, 34, 2110267. [Google Scholar] [CrossRef]
- He, X.; Zhu, H.; Shang, J.; Li, M.; Zhang, Y.; Zhou, S.; Gong, G.; He, Y.; Blocki, A.; Guo, J. Intratumoral synthesis of transformable metal-phenolic nanoaggregates with enhanced tumor penetration and retention for photothermal immunotherapy. Theranostics 2022, 12, 6258–6272. [Google Scholar] [CrossRef]
- Otrin, L.; Kleineberg, C.; Caire Da Silva, L.; Landfester, K.; Ivanov, I.; Wang, M.; Bednarz, C.; Sundmacher, K.; Vidaković-Koch, T. Artificial organelles for energy regeneration. Adv. Biosyst. 2019, 3, 1800323. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced tumor synergistic therapy by injectable magnetic hydrogel mediated generation of hyperthermia and highly toxic reactive oxygen species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, L.; Van Hest, J.C.M. Compartmentalization approaches in soft matter science: From nanoreactor development to organelle mimics. Adv. Mater. 2016, 28, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Weiden, J.; Tel, J.; Figdor, C.G. Synthetic immune niches for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Hao, H.; Wu, S.; Lin, J.; Zheng, Z.; Zhou, Y.; Zhang, Y.; Guo, Q.; Tian, F.; Zhao, M.; Chen, Y.; et al. Immunization against zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel. Nat. Biomed. Eng. 2023, 7, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Chan, C.T.Y.; Slomovic, S.; Collins, J.J. Next-generation biocontainment systems for engineered organisms. Nat. Chem. Biol. 2018, 14, 530–537. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Mo, L.; Song, Z.; Chen, W.; Deng, Y.; Zhao, H.; Qin, E.; Qin, C.; Tang, R. Eggshell-inspired biomineralization generates vaccines that do not require refrigeration. Angew. Chem. Int. Ed. 2012, 51, 10576–10579. [Google Scholar] [CrossRef]
- Wang, G.; Cao, R.-Y.; Chen, R.; Mo, L.; Han, J.-F.; Wang, X.; Xu, X.; Jiang, T.; Deng, Y.-Q.; Lyu, K.; et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 7619–7624. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.; Li, X.-F.; Li, Y.; Zhu, S.-Y.; Qin, C.-F.; Tang, R. Alumina-encapsulated vaccine formulation with improved thermostability and immunogenicity. Chem. Commun. 2016, 52, 6447–6450. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.-Q.; Yang, D.; Xiao, Y.; Zhao, H.; Nian, Q.-G.; Xu, X.; Li, X.-F.; Tang, R.; Qin, C.-F. Biomimetic inorganic camouflage circumvents antibody-dependent enhancement of infection. Chem. Sci. 2017, 8, 8240–8246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, H.; Lin, J.; Ma, Z.; Li, H.; Nie, Z.; Cui, Y.; Guo, Z.; Zhang, Y.; Wang, X.; et al. Conformation-stabilized amorphous nanocoating for rational design of long-term thermostable viral vaccines. ACS Appl. Mater. Interfaces 2022, 14, 39873–39884. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.; Kawazoe, N.; Chen, G. Encapsulation of individual living cells with enzyme responsive polymer nanoshell. Biomaterials 2019, 197, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, P.; Jiang, W.; Pan, H.; Xu, X.; Tang, R. Yeast cells with an artificial mineral shell: Protection and modification of living cells by biomimetic mineralization. Angew. Chem. Int. Ed. 2008, 47, 3560–3564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Park, J.H.; Kim, M.-H.; Hong, D.; Cho, H.; Yang, S.H.; Choi, I.S. Cytoprotective silica coating of individual mammalian cells through bioinspired silicification. Angew. Chem. Int. Ed. 2014, 53, 8056–8059. [Google Scholar] [CrossRef] [PubMed]
- Youn, W.; Ko, E.H.; Kim, M.-H.; Park, M.; Hong, D.; Seisenbaeva, G.A.; Kessler, V.G.; Choi, I.S. Cytoprotective encapsulation of individual jurkat T cells within durable TiO2 shells for T-cell therapy. Angew. Chem. Int. Ed. 2017, 56, 10702–10706. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, X.; Zhu, G.; Shao, C.; Li, Y.; Ma, W.; Xu, X.; Tang, R. Silicification-induced cell aggregation for the sustainable production of H2 under aerobic conditions. Angew. Chem. Int. Ed. 2015, 54, 11961–11965. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Richardson, J.J.; Doonan, C.J.; Mulet, X.; Ju, Y.; Cui, J.; Caruso, F.; Falcaro, P. An enzyme-coated metal–organic framework shell for synthetically adaptive cell survival. Angew. Chem. Int. Ed. 2017, 56, 8510–8515. [Google Scholar] [CrossRef]
- Pan, J.; Gong, G.; Wang, Q.; Shang, J.; He, Y.; Catania, C.; Birnbaum, D.; Li, Y.; Jia, Z.; Zhang, Y.; et al. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea. Nat. Commun. 2022, 13, 2117. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, L.; Yang, C.; Xu, L.; Wang, G.; Guo, Y.; Cheng, S.; Tian, X.; Wang, C.; Xie, R.; et al. Site-selected in situ polymerization for living cell surface engineering. Nat. Commun. 2023, 14, 7285. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, B.; Yang, X.; Xiao, Y.; Wang, X.; Shao, C.; Tang, R. A drug-free tumor therapy strategy: Cancer-cell-targeting calcification. Angew. Chem. Int. Ed. 2016, 55, 5225–5229. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Gong, G.; Dai, M.; Xiang, Z.; Pan, J.; He, X.; Shang, J.; Blocki, A.M.; Zhao, Z.; Shields, C.W., IV; et al. Systemic tumor suppression via macrophage-driven automated homing of metal-phenolic-gated nanosponges for metastatic melanoma. Adv. Sci. 2023, 10, 2207488. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, M.; Chen, Y.; Liu, Z.; Shao, C.; Jin, B.; Wang, X.; Hui, L.; Wang, S.; Liao, Z.; et al. Surface-anchored framework for generating RhD-epitope stealth red blood cells. Sci. Adv. 2020, 6, eaaw9679. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.P.; De Lima, T.M.; Pithon-Curi, T.C.; Curi, R. The mechanism of indole acetic acid cytotoxicity. Toxicol. Lett. 2004, 148, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, B.; Schattling, P.; Zhang, Y.; Städler, B. Subcompartmentalized nanoreactors as artificial organelle with intracellular activity. Small 2016, 12, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Labay, C.; Jansman, M.M.T.; Ek, P.K.; Hosta-Rigau, L. Intracellular microreactors as artificial organelles to conduct multiple enzymatic reactions simultaneously. Adv. Healthc. Mater. 2017, 6, 1601190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, L.; Qiu, K.; Liao, X.; Rees, T.W.; Zhao, Z.; Ji, L.; Chao, H. An ultrasmall RuO2 nanozyme exhibiting multienzyme-like activity for the prevention of acute kidney injury. ACS Appl. Mater. Interfaces 2020, 12, 31205–31216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, X.; Yang, X.; Jin, B.; Shao, C.; Kang, W.; Tang, R. Nanomaterial-based organelles protect normal cells against chemotherapy-induced cytotoxicity. Adv. Mater. 2018, 30, 1801304. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, J.; Zhang, Q.; Xin, T.; Yu, Y.; Nie, Y.; Zhang, S. Artificial organelles based on cross-linked zwitterionic vesicles. Nano Lett. 2020, 20, 6548–6555. [Google Scholar] [CrossRef]
- Einfalt, T.; Witzigmann, D.; Edlinger, C.; Sieber, S.; Goers, R.; Najer, A.; Spulber, M.; Onaca-Fischer, O.; Huwyler, J.; Palivan, C.G. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat. Commun. 2018, 9, 1127. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.A.; Choi, Y.; Lewin, S.A.; Verbeke, C.S.; Dranoff, G.; Mooney, D.J. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 2015, 33, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Dellacherie, M.O.; Li, A.; Lu, B.Y.; Verbeke, C.S.; Gu, L.; Stafford, A.G.; Doherty, E.J.; Mooney, D.J. Single-shot mesoporous silica rods scaffold for induction of humoral responses against small antigens. Adv. Funct. Mater. 2020, 30, 2002448. [Google Scholar] [CrossRef]
- Shah, N.J.; Najibi, A.J.; Shih, T.-Y.; Mao, A.S.; Sharda, A.; Scadden, D.T.; Mooney, D.J. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 2020, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Najibi, A.J.; Shih, T.-Y.; Mooney, D.J. Cryogel vaccines effectively induce immune responses independent of proximity to the draining lymph nodes. Biomaterials 2022, 281, 121329. [Google Scholar] [CrossRef] [PubMed]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015, 33, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Ma, Q.; Bai, J.; Xu, J.; Fei, Z.; Dong, Z.; Maruyama, A.; Leong, K.W.; Liu, Z.; Wang, C. An implantable blood clot–based immune niche for enhanced cancer vaccination. Sci. Adv. 2020, 6, eabb4639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Fei, Z.; Dai, H.; Fan, Q.; Yang, Q.; Chen, Y.; Wang, B.; Wang, C. 3D printing scaffold vaccine for antitumor immunity. Adv. Mater. 2021, 33, 2106768. [Google Scholar] [CrossRef]
- Dang, W.; Chen, W.-C.; Ju, E.; Xu, Y.; Li, K.; Wang, H.; Wang, K.; Lv, S.; Shao, D.; Tao, Y.; et al. 3D printed hydrogel scaffolds combining glutathione depletion-induced ferroptosis and photothermia-augmented chemodynamic therapy for efficiently inhibiting postoperative tumor recurrence. J. Nanobiotechnol. 2022, 20, 266. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, D.; Lee, J.; Choi, I.S. Cell-in-shell hybrids: Chemical nanoencapsulation of individual cells. Acc. Chem. Res. 2016, 49, 792–800. [Google Scholar] [CrossRef]
- Youn, W.; Kim, J.Y.; Park, J.; Kim, N.; Choi, H.; Cho, H.; Choi, I.S. Single-cell nanoencapsulation: From passive to active shells. Adv. Mater. 2020, 32, 1907001. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, Y.; Wang, X.; Tang, R. Novel nanomaterial–organism hybrids with biomedical potential. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1706. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, B.; Wang, X.; Tang, R. Organism–materials integration: A promising strategy for biomedical applications. Adv. NanoBiomed Res. 2021, 1, 2000044. [Google Scholar] [CrossRef]
- Park, J.; Kim, N.; Han, S.Y.; Rhee, S.Y.; Nguyen, D.T.; Lee, H.; Choi, I.S. A micrometric transformer: Compositional nanoshell transformation of Fe3+-trimesic-acid complex with concomitant payload release in cell-in-catalytic-shell nanobiohybrids. Adv. Sci. 2024, 11, 2306450. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, G.; Tang, R. Nanomodification of living organisms by biomimetic mineralization. Nano Res. 2014, 7, 1404–1428. [Google Scholar] [CrossRef]
- Ma, Z.; Li, B.; Tang, R. Biomineralization: Biomimetic synthesis of materials and biomimetic regulation of organisms. Chin. J. Chem. 2021, 39, 2071–2082. [Google Scholar] [CrossRef]
- Mckenney, P.T.; Driks, A.; Eichenberger, P. The bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Dujardin, E.; Peet, C.; Stubbs, G.; Culver, J.N.; Mann, S. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003, 3, 413–417. [Google Scholar] [CrossRef]
- Laidler, J.R.; Shugart, J.A.; Cady, S.L.; Bahjat, K.S.; Stedman, K.M. Reversible inactivation and desiccation tolerance of silicified viruses. J. Virol. 2013, 87, 13927–13929. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.; Wang, X.; Song, Z.; Tang, R. Mineralized state of the avian influenza virus in the environment. Angew. Chem. Int. Ed. 2017, 56, 12908–12912. [Google Scholar] [CrossRef]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic–organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Fowler, C.E.; Shenton, W.; Stubbs, G.; Mann, S. Tobacco mosaic virus liquid crystals as templates for the interior design of silica mesophases and nanoparticles. Adv. Mater. 2001, 13, 1266–1269. [Google Scholar] [CrossRef]
- Schlehuber, L.D.; Mcfadyen, I.J.; Shu, Y.; Carignan, J.; Duprex, W.P.; Forsyth, W.R.; Ho, J.H.; Kitsos, C.M.; Lee, G.Y.; Levinson, D.A.; et al. Towards ambient temperature-stable vaccines: The identification of thermally stabilizing liquid formulations for measles virus using an innovative high-throughput infectivity assay. Vaccine 2011, 29, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.-J.; Zhou, H.; Nian, Q.-G.; Song, Z.; Deng, Y.-Q.; Wang, X.; Zhu, S.-Y.; Li, X.-F.; Qin, C.-F.; et al. Hydrated silica exterior produced by biomimetic silicification confers viral vaccine heat-resistance. ACS Nano 2015, 9, 799–808. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, H.; Nian, Q.-G.; Yang, Y.; Qin, C.-F.; Tang, R. Robust vaccine formulation produced by assembling a hybrid coating of polyethyleneimine–silica. Chem. Sci. 2016, 7, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, C.; Li, P.; Wu, T.; Zhou, H.; Yang, D.; Liu, Y.; Ma, X.; Song, Z.; Nian, Q.; et al. Vaccine engineering with dual-functional mineral shell: A promising strategy to overcome preexisting immunity. Adv. Mater. 2016, 28, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, Y.; Li, S.; Wang, G.; Qin, E.; Xu, X.; Tang, R.; Qin, C. Biomineralization-based virus shell-engineering: Towards neutralization escape and tropism expansion. Adv. Healthc. Mater. 2012, 1, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, D.; Li, S.; Xu, X.; Qin, C.-F.; Tang, R. Biomineralized vaccine nanohybrid for needle-free intranasal immunization. Biomaterials 2016, 106, 286–294. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O′keeffe, M.; Yaghi, O.M. Reticular chemistry: Occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Ju, E.; Liu, Z.; Chen, Z.; Ren, J.; Qu, X. Metal-organic-framework-based vaccine platforms for enhanced systemic immune and memory response. Adv. Funct. Mater. 2016, 26, 6454–6461. [Google Scholar] [CrossRef]
- Li, S.; Dharmarwardana, M.; Welch, R.P.; Ren, Y.; Thompson, C.M.; Smaldone, R.A.; Gassensmith, J.J. Template-directed synthesis of porous and protective core–shell bionanoparticles. Angew. Chem. Int. Ed. 2016, 55, 10691–10696. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Welch, R.P.; Dharmarwardana, M.; Benjamin, C.E.; Li, S.; Shahrivarkevishahi, A.; Popal, S.; Tuong, L.H.; Creswell, C.T.; Gassensmith, J.J. Enhanced stability and controlled delivery of MOF-encapsulated vaccines and their immunogenic response in vivo. ACS Appl. Mater. Interfaces 2019, 11, 9740–9746. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.L.; More, J.; Rott, J.; Lewin, D. Virus inactivation in albumin by a combination of alkali conditions and high temperature. Biologicals 2011, 39, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.; Sheng, Y.; Zhu, H.; Li, Z.; Su, Z.; Yu, R.; Zhang, S. Synthesis of a crystalline zeolitic imidazole framework-8 nano-coating on single environment-sensitive viral particles for enhanced immune responses. Nanoscale Adv. 2023, 5, 1433–1449. [Google Scholar] [CrossRef]
- Lee, H.; Kim, N.; Rheem, H.B.; Kim, B.J.; Park, J.H.; Choi, I.S. A decade of advances in single-cell nanocoating for mammalian cells. Adv. Healthc. Mater. 2021, 10, 2100347. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Roh, D.-H.; Schmidt, M.; Crotti, L.B.; Varma, A. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 2001, 276, 19679–19682. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Minullina, R.T. Hybrid cellular–inorganic core–shell microparticles: Encapsulation of individual living cells in calcium carbonate microshells. Langmuir 2009, 25, 6617–6621. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Liu, C.; Guan, Y.; Ren, J.; Qu, X. Manganese dioxide nanozymes as responsive cytoprotective shells for individual living cell encapsulation. Angew. Chem. Int. Ed. 2017, 56, 13661–13665. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Goli, P.L.; Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 2007, 7, 877–882. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Lvov, Y.M. “Face-lifting” and “make-up” for microorganisms: Layer-by-layer polyelectrolyte nanocoating. ACS Nano 2012, 6, 4557–4564. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The effect of layer-by-layer assembly coating on the proliferation and differentiation of neural stem cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, H.; Kim, H.-B.; Yang, M.; Heo, J.; Won, Y.; Jang, S.S.; Park, J.K.; Son, Y.; Oh, T.I.; et al. Cytoprotective self-assembled rgd peptide nanofilms for surface modification of viable mesenchymal stem cells. Chem. Mater. 2017, 29, 2055–2065. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Tang, R. Improvement of biological organisms using functional material shells. Adv. Funct. Mater. 2016, 26, 1862–1880. [Google Scholar] [CrossRef]
- Yang, S.H.; Lee, K.-B.; Kong, B.; Kim, J.-H.; Kim, H.-S.; Choi, I.S. Biomimetic encapsulation of individual cells with silica. Angew. Chem. Int. Ed. 2009, 48, 9160–9163. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.M.; Correia, T.R.; Gaspar, V.M.; Rodrigues, J.M.M.; Choi, I.S.; Mano, J.F. Partial coated stem cells with bioinspired silica as new generation of cellular hybrid materials. Adv. Funct. Mater. 2021, 31, 2009619. [Google Scholar] [CrossRef]
- Wang, B.; Liu, P.; Tang, Y.; Pan, H.; Xu, X.; Tang, R. Guarding embryo development of zebrafish by shell engineering: A strategy to shield life from ozone depletion. PLoS ONE 2010, 5, e9963. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Qi, J.; Liu, X.; Wang, L.; Zhang, H.; Xie, H.; Huang, X. Enzyme-modulated anaerobic encapsulation of chlorella cells allows switching from O2 to H2 production. Angew. Chem. Int. Ed. 2019, 58, 3992–3995. [Google Scholar] [CrossRef]
- Riccò, R.; Liang, W.; Li, S.; Gassensmith, J.J.; Caruso, F.; Doonan, C.; Falcaro, P. Metal–organic frameworks for cell and virus biology: A perspective. ACS Nano 2018, 12, 13–23. [Google Scholar] [CrossRef]
- Liang, K.; Richardson, J.J.; Cui, J.; Caruso, F.; Doonan, C.J.; Falcaro, P. Metal–organic framework coatings as cytoprotective exoskeletons for living cells. Adv. Mater. 2016, 28, 7910–7914. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, J.; Amini, S.; Ju, Y.; Agola, J.O.; Zimpel, A.; Shang, J.; Noureddine, A.; Caruso, F.; Wuttke, S.; et al. Supracells: Living mammalian cells protected within functional modular nanoparticle-based exoskeletons. Adv. Mater. 2019, 31, 1900545. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Suástegui, M.; Sakimoto, K.K.; Moody, V.M.; Xiao, G.; Nocera, D.G.; Joshi, N.S. Light-driven fine chemical production in yeast biohybrids. Science 2018, 362, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Pan, D.C.; Qi, Q.M.; Kim, J.; Kapate, N.; Sun, T.; Shields, C.W., IV; Wang, L.L.-W.; Wu, D.; Kwon, C.J.; et al. Engineering of living cells with polyphenol-functionalized biologically active nanocomplexes. Adv. Mater. 2020, 32, 2003492. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, K.; Lee, J.; Choi, J.Y.; Hong, D.; Yang, S.H.; Caruso, F.; Lee, Y.; Choi, I.S. A cytoprotective and degradable metal–polyphenol nanoshell for single-cell encapsulation. Angew. Chem. Int. Ed. 2014, 53, 12420–12425. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.; Choi, J.; Kim, D.; Hong, D.; Park, J.H.; Yang, S.H.; Choi, I.S. Chemical sporulation and germination: Cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid–Feiii complex. Nanoscale 2015, 7, 18918–18922. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; Von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Guo, J.; Tardy, B.L.; Christofferson, A.J.; Dai, Y.; Richardson, J.J.; Zhu, W.; Hu, M.; Ju, Y.; Cui, J.; Dagastine, R.R.; et al. Modular assembly of superstructures from polyphenol-functionalized building blocks. Nat. Nanotechnol. 2016, 11, 1105–1111. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.-B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Dong, H.; Wei, X.; Shi, D.; Li, Y. Ca2+-mediated surface polydopamine engineering to program dendritic cell maturation. ACS Appl. Mater. Interfaces 2020, 12, 4163–4173. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Kim, N.; Youn, W.; Yun, G.; Han, S.Y.; Nguyen, D.T.; Choi, I.S. Cell-in-catalytic-shell nanoarchitectonics: Catalytic empowerment of individual living cells by single-cell nanoencapsulation. Adv. Mater. 2022, 34, 2201247. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, H.; Liu, Z.; Liu, C.; Du, X.; Ren, J.; Qu, X. Hydrogen-bonded organic framework (HOF)-based single-neural stem cell encapsulation and transplantation to remodel impaired neural networks. Angew. Chem. Int. Ed. 2022, 61, e202201485. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Gao, N.; Li, X.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. A biocompatible second near-infrared nanozyme for spatiotemporal and non-invasive attenuation of amyloid deposition through scalp and skull. ACS Nano 2020, 14, 9894–9903. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B. Living cell-mediated in-situ polymerization for biomedical applications. Prog. Polym. Sci. 2022, 129, 101545. [Google Scholar] [CrossRef]
- Niu, J.; Lunn, D.J.; Pusuluri, A.; Yoo, J.I.; O′malley, M.A.; Mitragotri, S.; Soh, H.T.; Hawker, C.J. Engineering live cell surfaces with functional polymers via cytocompatible controlled radical polymerization. Nat. Chem. 2017, 9, 537–545. [Google Scholar] [CrossRef]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Tang, N.; Li, H.; Zhang, L.; Zhang, X.; Chen, Y.; Shou, H.; Feng, S.; Chen, X.; Luo, Y.; Tang, R.; et al. A macromolecular drug for cancer therapy via extracellular calcification. Angew. Chem. Int. Ed. 2021, 60, 6509–6517. [Google Scholar] [CrossRef]
- Ayer, M.; Klok, H.-A. Cell-mediated delivery of synthetic nano- and microparticles. J. Control. Release 2017, 259, 92–104. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Tang, W.; Wen, R.; Zhou, S.; Lee, C.; Wang, H.; Jiang, W.; Delahunty, I.M.; Zhen, Z.; et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv. Mater. 2018, 30, 1805557. [Google Scholar] [CrossRef]
- Brenner, J.S.; Pan, D.C.; Myerson, J.W.; Marcos-Contreras, O.A.; Villa, C.H.; Patel, P.; Hekierski, H.; Chatterjee, S.; Tao, J.-Q.; Parhiz, H.; et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018, 9, 2684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Fehnel, A.; Pan, D.C.; Gao, Y.; Kim, J.; Evans, M.A.; Mandal, A.; Guo, J.; et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat. Biomed. Eng. 2021, 5, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Kim, J.; Suja, V.C.; Kapate, N.; Gao, Y.; Guo, J.; Muzykantov, V.R.; Mitragotri, S. Red blood cell anchoring enables targeted transduction and re-administration of AAV-mediated gene therapy. Adv. Sci. 2022, 9, 2201293. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.G. A strategic approach to the problems of providing rhesus D–negative blood transfusion in geographic areas with low rhd negativity: A nigerian perspective. Transfus. Med. Rev. 2010, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; Zhao, B.; Chen, J.; Zhang, X.; Tang, R. Antigenically shielded universal red blood cells by polydopamine-based cell surface engineering. Chem. Sci. 2014, 5, 3463–3468. [Google Scholar] [CrossRef]
- Oerlemans, R.a.J.F.; Timmermans, S.B.P.E.; Van Hest, J.C.M. Artificial organelles: Towards adding or restoring intracellular activity. ChemBioChem 2021, 22, 2051–2078. [Google Scholar] [CrossRef]
- Chang, F.-P.; Hung, Y.; Chang, J.-H.; Lin, C.-H.; Mou, C.-Y. Enzyme encapsulated hollow silica nanospheres for intracellular biocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 6883–6890. [Google Scholar] [CrossRef]
- Mukerabigwi, J.F.; Ge, Z.; Kataoka, K. Therapeutic nanoreactors as in vivo nanoplatforms for cancer therapy. Chem. A Eur. J. 2018, 24, 15706–15724. [Google Scholar] [CrossRef]
- Guindani, C.; Da Silva, L.C.; Cao, S.; Ivanov, T.; Landfester, K. Synthetic cells: From simple bio-inspired modules to sophisticated integrated systems. Angew. Chem. Int. Ed. 2022, 61, e202110855. [Google Scholar] [CrossRef]
- Destito, P.; Sousa-Castillo, A.; Couceiro, J.R.; López, F.; Correa-Duarte, M.A.; Mascareñas, J.L. Hollow nanoreactors for pd-catalyzed suzuki–miyaura coupling and o-propargyl cleavage reactions in bio-relevant aqueous media. Chem. Sci. 2019, 10, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Castillo, A.; Couceiro, J.R.; Tomás-Gamasa, M.; Mariño-López, A.; López, F.; Baaziz, W.; Ersen, O.; Comesaña-Hermo, M.; Mascareñas, J.L.; Correa-Duarte, M.A. Remote activation of hollow nanoreactors for heterogeneous photocatalysis in biorelevant media. Nano Lett. 2020, 20, 7068–7076. [Google Scholar] [CrossRef] [PubMed]
- Dobrunz, D.; Toma, A.C.; Tanner, P.; Pfohl, T.; Palivan, C.G. Polymer nanoreactors with dual functionality: Simultaneous detoxification of peroxynitrite and oxygen transport. Langmuir 2012, 28, 15889–15899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, J.; Zhou, G.; Liu, T.-M.; Dai, Y.; Nie, G.; Zhao, Q. Remodeling of tumor microenvironment by tumor-targeting nanozymes enhances immune activation of car t cells for combination therapy. Small 2021, 17, 2102624. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Saltzman, W.M.; Piotrowski-Daspit, A.S. Escaping the endosome: Assessing cellular trafficking mechanisms of non-viral vehicles. J. Control. Release 2021, 335, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nymann Westensee, I.; Brodszkij, E.; Städler, B. Cell mimicry as a bottom-up strategy for hierarchical engineering of nature-inspired entities. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1683. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lomora, M.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Belluati, A.; Craciun, I.; Meyer, C.E.; Rigo, S.; Palivan, C.G. Enzymatic reactions in polymeric compartments: Nanotechnology meets nature. Curr. Opin. Biotechnol. 2019, 60, 53–62. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Carlsson, N.; Gustafsson, H.; Thörn, C.; Olsson, L.; Holmberg, K.; Åkerman, B. Enzymes immobilized in mesoporous silica: A physical–chemical perspective. Adv. Colloid Interface Sci. 2014, 205, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Carrillo-Carrión, C.; Destito, P.; Alvarez, A.; Tomás-Gamasa, M.; Pelaz, B.; Lopez, F.; Mascareñas, J.L.; Del Pino, P. Core-shell palladium/MOF platforms as diffusion-controlled nanoreactors in living cells and tissue models. Cell Rep. Phys. Sci. 2020, 1, 100076. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; York-Duran, M.J.; Hosta-Rigau, L. Recent progress in micro/nanoreactors toward the creation of artificial organelles. Adv. Healthc. Mater. 2018, 7, 1700917. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow nano- and microstructures as catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Lu, X.; Kuai, L.; Huang, F.; Jiang, J.; Song, J.; Liu, Y.; Chen, S.; Mao, L.; Peng, W.; Luo, Y.; et al. Single-atom catalysts-based catalytic ros clearance for efficient psoriasis treatment and relapse prevention via restoring esr1. Nat. Commun. 2023, 14, 6767. [Google Scholar] [CrossRef]
- Schmidt, M.; Scherk, C.; Iakovleva, O.; Nolting, H.F.; Meier, B.; Parak, F. The structure of the azide coordinated superoxide dismutase of propionibacterium shermanii investigated by X-ray structure analysis, extended X-ray absorption fine structure, mössbauer and electron paramagnetic resonance spectroscopy. Inorg. Chim. Acta 1998, 275–276, 65–72. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Bian, J.; Zhang, X.; Fu, Y.; Li, Z.; Wei, S.; Xu, Z.; Liu, X.; Liu, Z.; et al. Ag/Pd bimetal nanozyme with enhanced catalytic and photothermal effects for ROS/hyperthermia/chemotherapy triple-modality antitumor therapy. Chem. Eng. J. 2020, 397, 125438. [Google Scholar] [CrossRef]
- Yang, H.; Xu, B.; Li, S.; Wu, Q.; Lu, M.; Han, A.; Liu, H. A photoresponsive nanozyme for synergistic catalytic therapy and dual phototherapy. Small 2021, 17, 2007090. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Gao, F.; Wei, J.-J.; Qian, C.-G.; Sun, M.-J. Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 2019, 19, 4334–4342. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Zhu, T.; Yang, W.; Shi, Y.; Huang, D.; Li, J.; Xiang, S.; Wang, J.; Chen, X.; Zheng, N. Pd@Pt-gox/ha as a novel enzymatic cascade nanoreactor for high-efficiency starving-enhanced chemodynamic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 51249–51262. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, D.; Laurent, A.; Pawlik, T.M.; Lauwers, G.Y.; Vauthey, J.N.; Abdalla, E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. J. Br. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. 2012, 51, 11853–11857. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fei, J.; Li, G.; Yuan, T.; Xu, X.; Li, J. Nanozyme-catalyzed cascade reactions for mitochondria-mimicking oxidative phosphorylation. Angew. Chem. Int. Ed. 2019, 58, 5572–5576. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, K.A.; Devaraj, N.K. Synthesis of lipid membranes for artificial cells. Nat. Rev. Chem. 2021, 5, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Delong, J.P.; Van Etten, J.L.; Al-Ameeli, Z.; Agarkova, I.V.; Dunigan, D.D. The consumption of viruses returns energy to food chains. Proc. Natl. Acad. Sci. USA 2023, 120, e2215000120. [Google Scholar] [CrossRef]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef]
- Wang, X.; Moreno, S.; Boye, S.; Wang, P.; Liu, X.; Lederer, A.; Voit, B.; Appelhans, D. Artificial organelles with orthogonal-responsive membranes for protocell systems: Probing the intrinsic and sequential docking and diffusion of cargo into two coexisting avidin–polymersomes. Adv. Sci. 2021, 8, 2004263. [Google Scholar] [CrossRef]
- Garni, M.; Einfalt, T.; Goers, R.; Palivan, C.G.; Meier, W. Live follow-up of enzymatic reactions inside the cavities of synthetic giant unilamellar vesicles equipped with membrane proteins mimicking cell architecture. ACS Synth. Biol. 2018, 7, 2116–2125. [Google Scholar] [CrossRef]
- Garni, M.; Thamboo, S.; Schoenenberger, C.-A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2017, 1859, 619–638. [Google Scholar] [CrossRef]
- Buddingh’, B.C.; Elzinga, J.; Van Hest, J.C.M. Intercellular communication between artificial cells by allosteric amplification of a molecular signal. Nat. Commun. 2020, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.; Ye, Y.; Bomba, H.N.; Gu, Z. Bioengineering of artificial antigen presenting cells and lymphoid organs. Theranostics 2017, 7, 3504–3516. [Google Scholar] [CrossRef] [PubMed]
- Najibi, A.J.; Mooney, D.J. Cell and tissue engineering in lymph nodes for cancer immunotherapy. Adv. Drug Deliv. Rev. 2020, 161–162, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Ali, O.A.; Huebsch, N.; Cao, L.; Dranoff, G.; Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009, 8, 151–158. [Google Scholar] [CrossRef]
- Fenton, O.S.; Tibbitt, M.W.; Appel, E.A.; Jhunjhunwala, S.; Webber, M.J.; Langer, R. Injectable polymer–nanoparticle hydrogels for local immune cell recruitment. Biomacromolecules 2019, 20, 4430–4436. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Warren Sands, R.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.-Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Benchérif, S.; Mooney, D.J.; Renaud, P. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef]
- Shih, T.-Y.; Blacklow, S.O.; Li, A.W.; Freedman, B.R.; Bencherif, S.; Koshy, S.T.; Darnell, M.C.; Mooney, D.J. Injectable, tough alginate cryogels as cancer vaccines. Adv. Healthc. Mater. 2018, 7, 1701469. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Jia, L.; Pang, M.; Yang, X.; Yang, Y.; Kamel Elyzayati, S.; Liao, Y.; Wang, H.; Zhu, Y.; Wang, Q. Injectable adhesive hydrogel as photothermal-derived antigen reservoir for enhanced anti-tumor immunity. Adv. Funct. Mater. 2021, 31, 2010587. [Google Scholar] [CrossRef]
- Krummel, M.F.; Bartumeus, F.; Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 2016, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, Z.-Y.; Han, Z.; Yuan, Y.; Shao, Y.; Weitz, D. Regulation of cell attachment, spreading, and migration by hydrogel substrates with independently tunable mesh size. Acta Biomater. 2022, 141, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, A.N.; Irvine, D.J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. Part A 2008, 85A, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Dellacherie, M.O.; Seo, B.R.; Mooney, D.J. Macroscale biomaterials strategies for local immunomodulation. Nat. Rev. Mater. 2019, 4, 379–397. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A checkpoint-regulatable immune niche created by injectable hydrogel for tumor therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef] [PubMed]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: Postoperative immunotherapy. Adv. Mater. 2018, 30, 1706719. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, Y.; Teng, X.; Wang, Y.; Huo, S.; Qing, G.; Ni, Q.; Li, X.; Wang, J.; Ye, X.; et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1053–1064. [Google Scholar] [CrossRef]
- Rheem, H.B.; Choi, H.; Yang, S.; Han, S.; Rhee, S.Y.; Jeong, H.; Lee, K.-B.; Lee, Y.; Kim, I.-S.; Lee, H.; et al. Fugetaxis of cell-in-catalytic-coat nanobiohybrids in glucose gradients. Small 2023, 19, 2301431. [Google Scholar] [CrossRef]

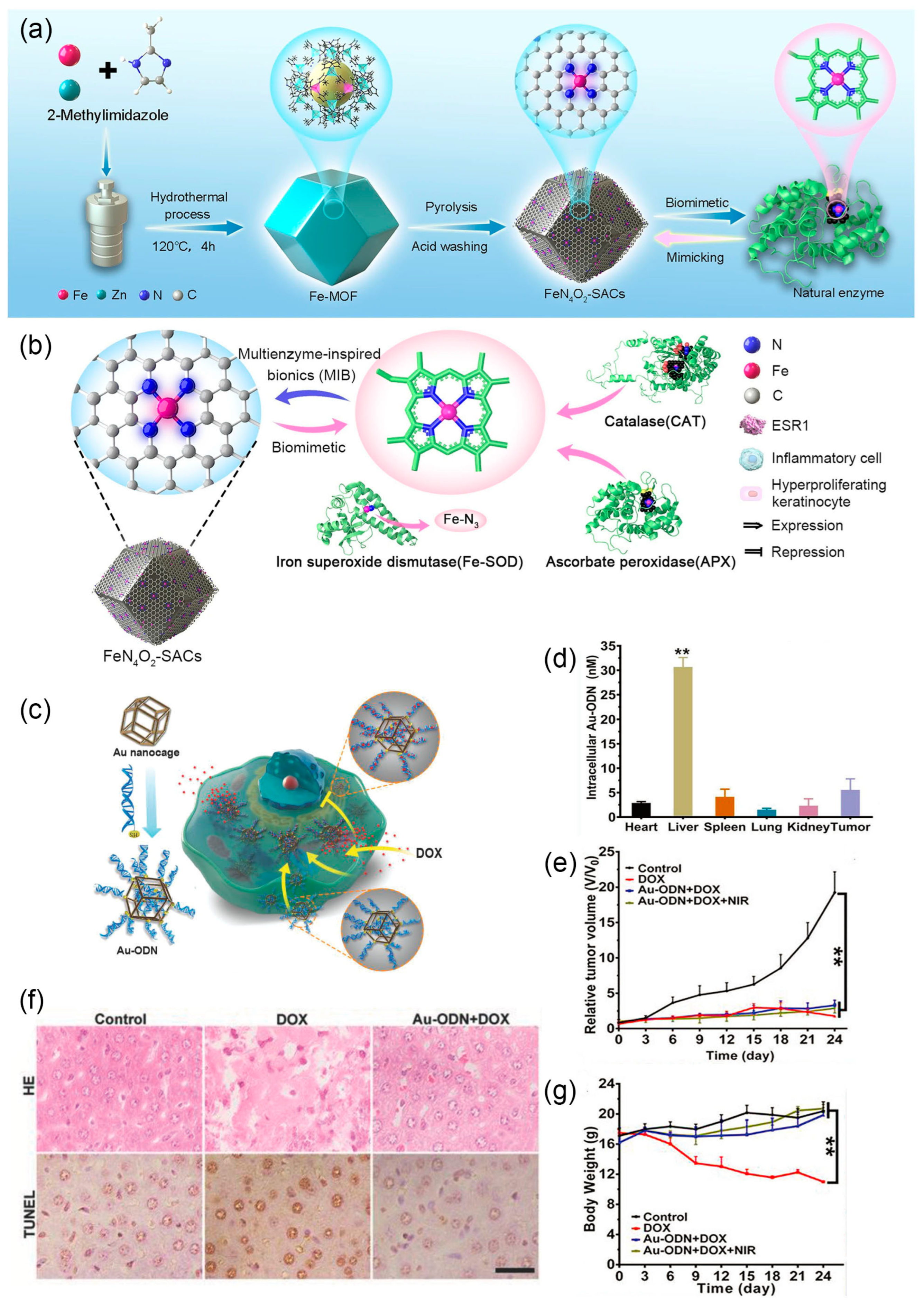

| Strategies | Organisms | Functional Enhancement | Main Biomaterials | References |
|---|---|---|---|---|
| Biointerface engineering | JEV/EV71 | Thermal stability | CaP | [18,19] |
| EV71 | Thermal stability | Aluminum oxide | [20] | |
| DENV | ADE effects elimination | CaP | [21] | |
| Ad5 | Thermal stability | ZIF-8 | [22] | |
| MSCs | Tumor targeting | Gelatin | [23] | |
| Yeast cells | Protection | Polymers, CaP | [24] | |
| Hela | Protection | Silica | [25] | |
| Jurkat T | Protection | TiO2 | [26] | |
| Green algae | Hydrogen production | Silica | [27] | |
| Yeast cells | Catalysis | ZIF-8 | [28] | |
| Probiotics | Protection | Fe3+-TA | [29] | |
| Jurkat T | Functional group | Polymers | [30] | |
| Cancer cells | CCTC | CaP | [31] | |
| Macrophages | Drug delivery | Nanosponges | [32] | |
| RBCs | Blood transfusion | Polymers | [33] | |
| Artificial organelles | Cancer cells | Prodrug therapy | HSN | [34] |
| Macrophages | Catalysis | Polymers | [35,36] | |
| Kidney cells | Antioxidants | RuO2 | [37] | |
| Normal cells | Chemotherapeutic drug capture | Au-ODN | [38] | |
| Normal cells | Inflammation clearance | cZV, CeO2 | [39] | |
| Paramecium caudatum | Waterborne virus clearance | Fe3O4 | [7] | |
| Zebrafish embryos | Stimuli responsive | Liposome | [40] | |
| 3D multicellular immune niches | DCs, T cells | Immune responses | MSRs | [41,42] |
| DCs | Recruitment, activation | Freeze gel | [43,44] | |
| T cells | Migration | PAA, collagen fibers | [45] | |
| Immune cells | Recruitment, activation | Blood clot | [46] | |
| Immune cells | Antiviral immunity | Chitosan | [16] | |
| Immune cells | Immune responses | 3D-printed scaffold | [47,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Zhou, Y.; Ma, Z.; Tang, R.; Wang, X. Organismal Function Enhancement through Biomaterial Intervention. Nanomaterials 2024, 14, 377. https://doi.org/10.3390/nano14040377
Tian F, Zhou Y, Ma Z, Tang R, Wang X. Organismal Function Enhancement through Biomaterial Intervention. Nanomaterials. 2024; 14(4):377. https://doi.org/10.3390/nano14040377
Chicago/Turabian StyleTian, Fengchao, Yuemin Zhou, Zaiqiang Ma, Ruikang Tang, and Xiaoyu Wang. 2024. "Organismal Function Enhancement through Biomaterial Intervention" Nanomaterials 14, no. 4: 377. https://doi.org/10.3390/nano14040377