The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model
Abstract
1. Introduction
2. Materials and Methods
2.1. iPSC Culture
2.2. Cortical Brain Organoid Culture
2.3. STB-MP Characterization and Treatment
2.4. MTT Assay
2.5. RNA Extraction and Reverse Transcription PCR
2.6. Western Blot Analysis
2.7. Histology and Immunofluorescence
2.8. ELISA
2.9. Statistical Analysis
3. Results
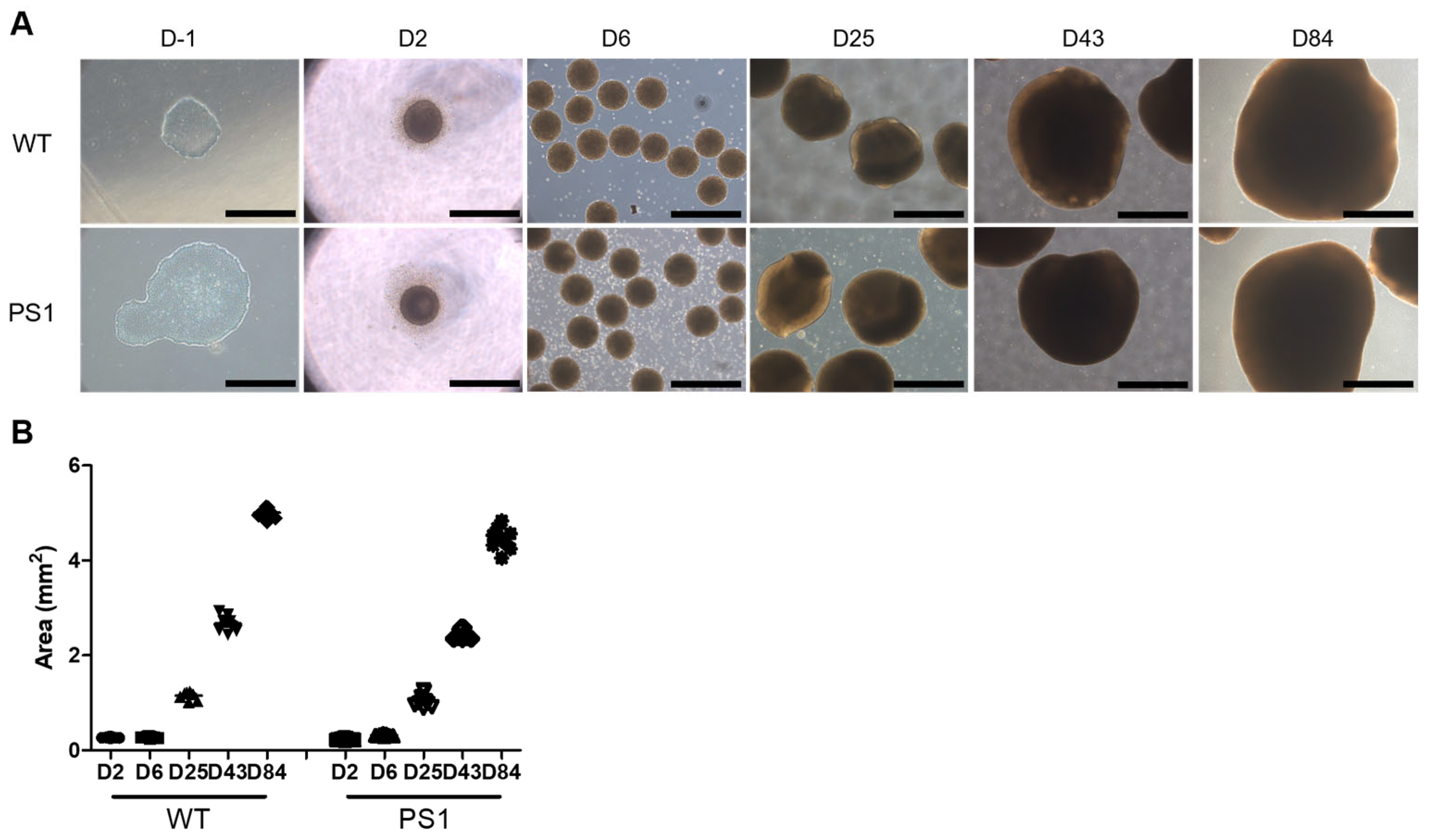
3.1. Generation and Characterization of Cortical Brain Organoids from AD Patient-Derived iPSCs
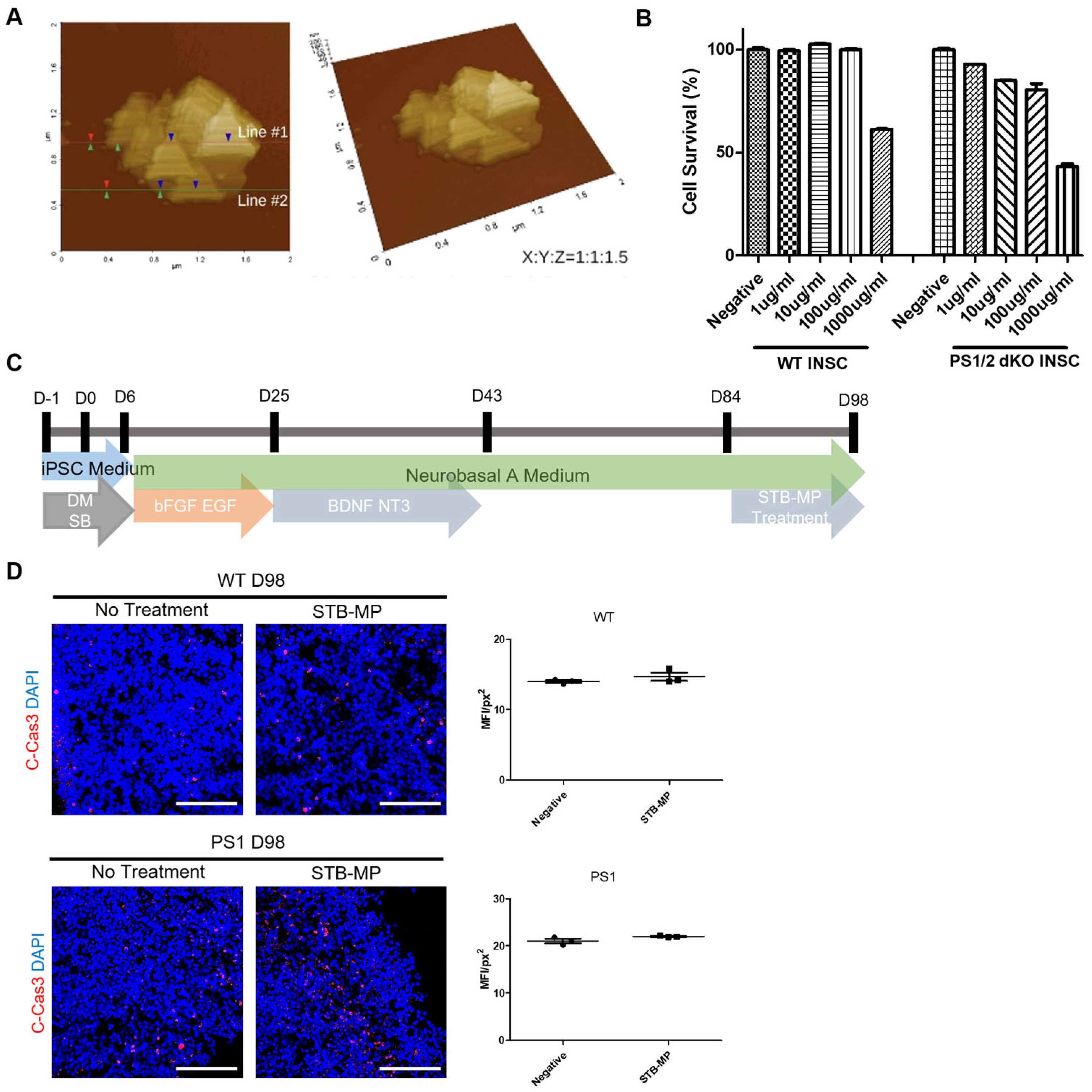
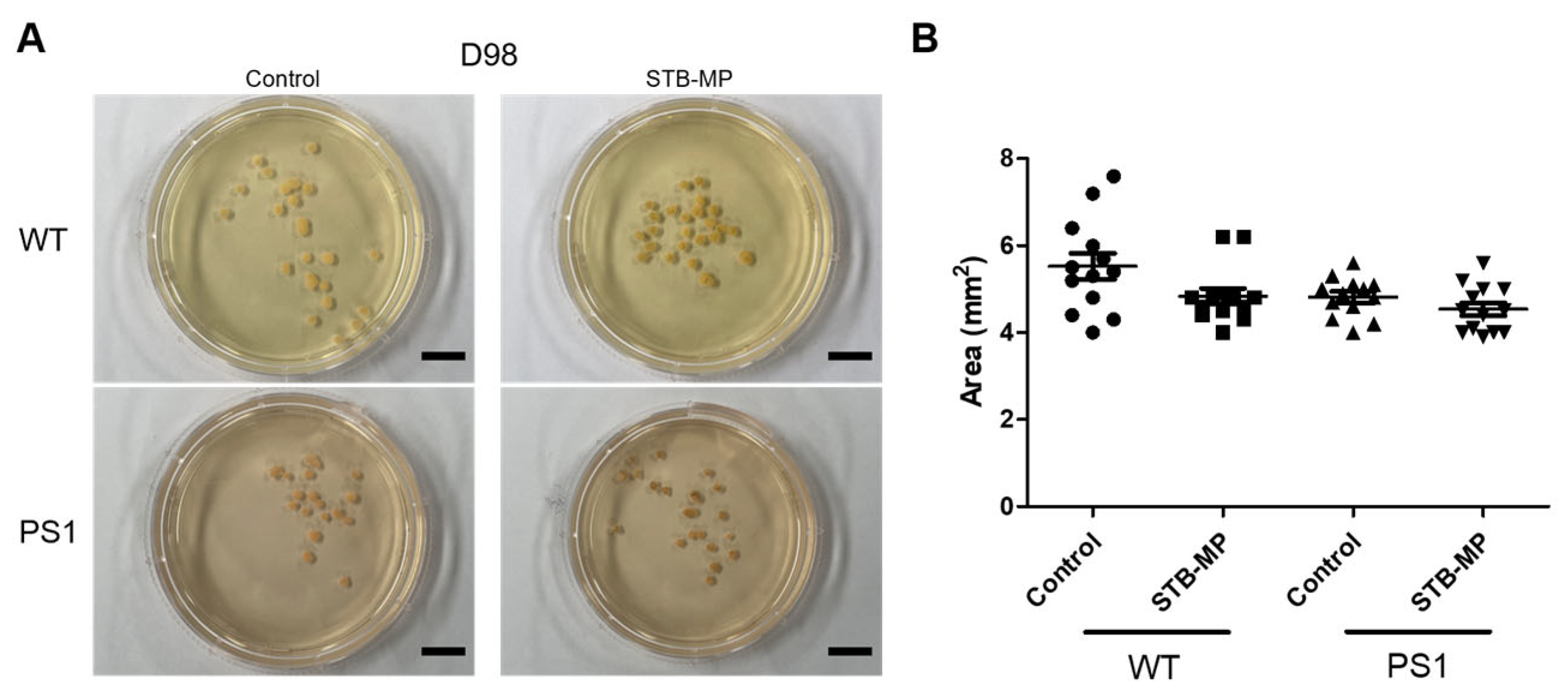
3.2. Characterization of STB-MP Nanoparticle Treatment and Toxicity
3.3. STB-MP Treatment Does Not Affect Neurogenesis in Cortical Brain Organoids
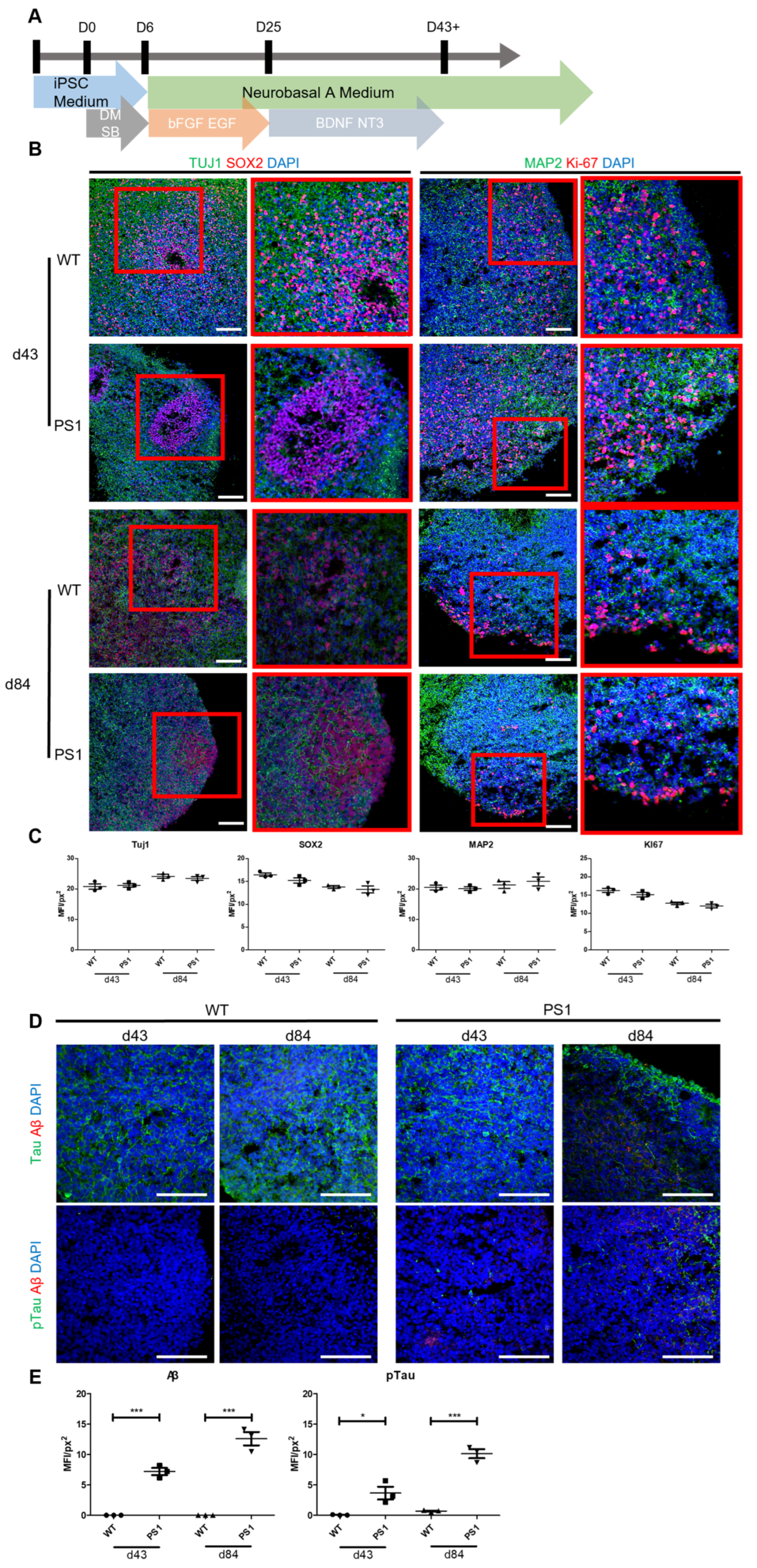
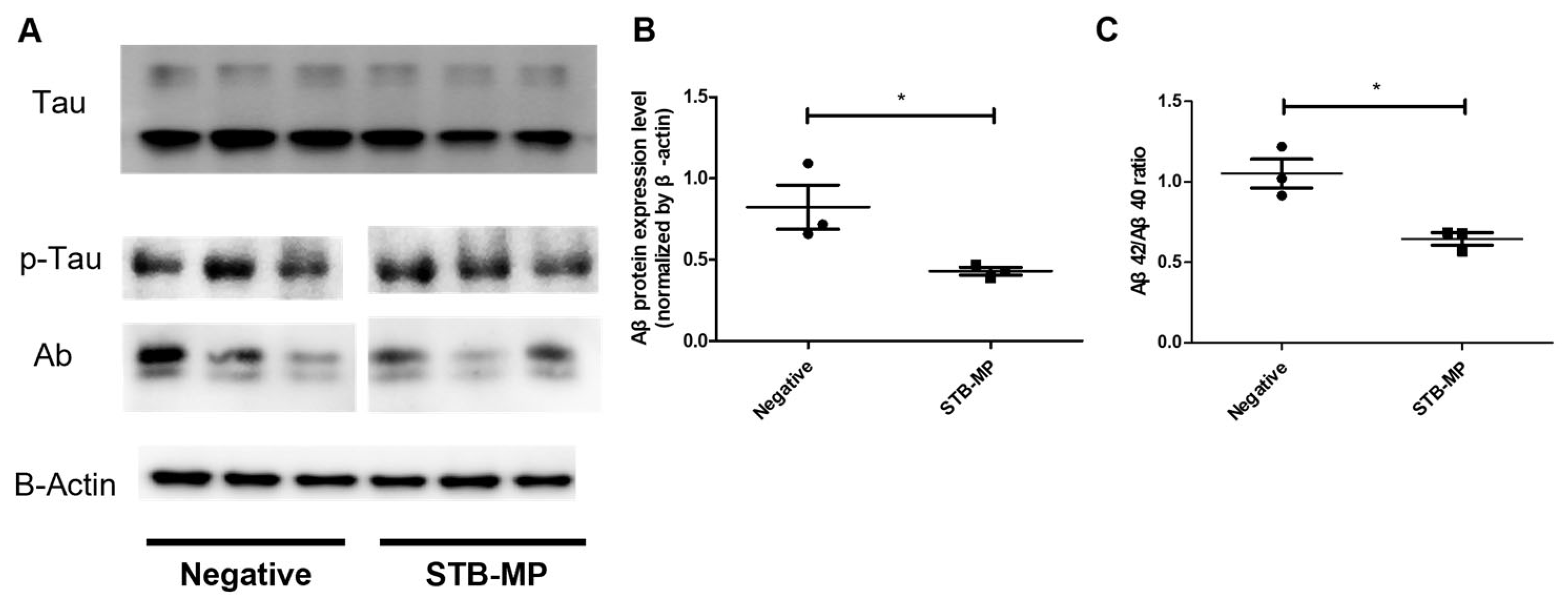
3.4. STB-MP Treatment Reduces Aβ Expression
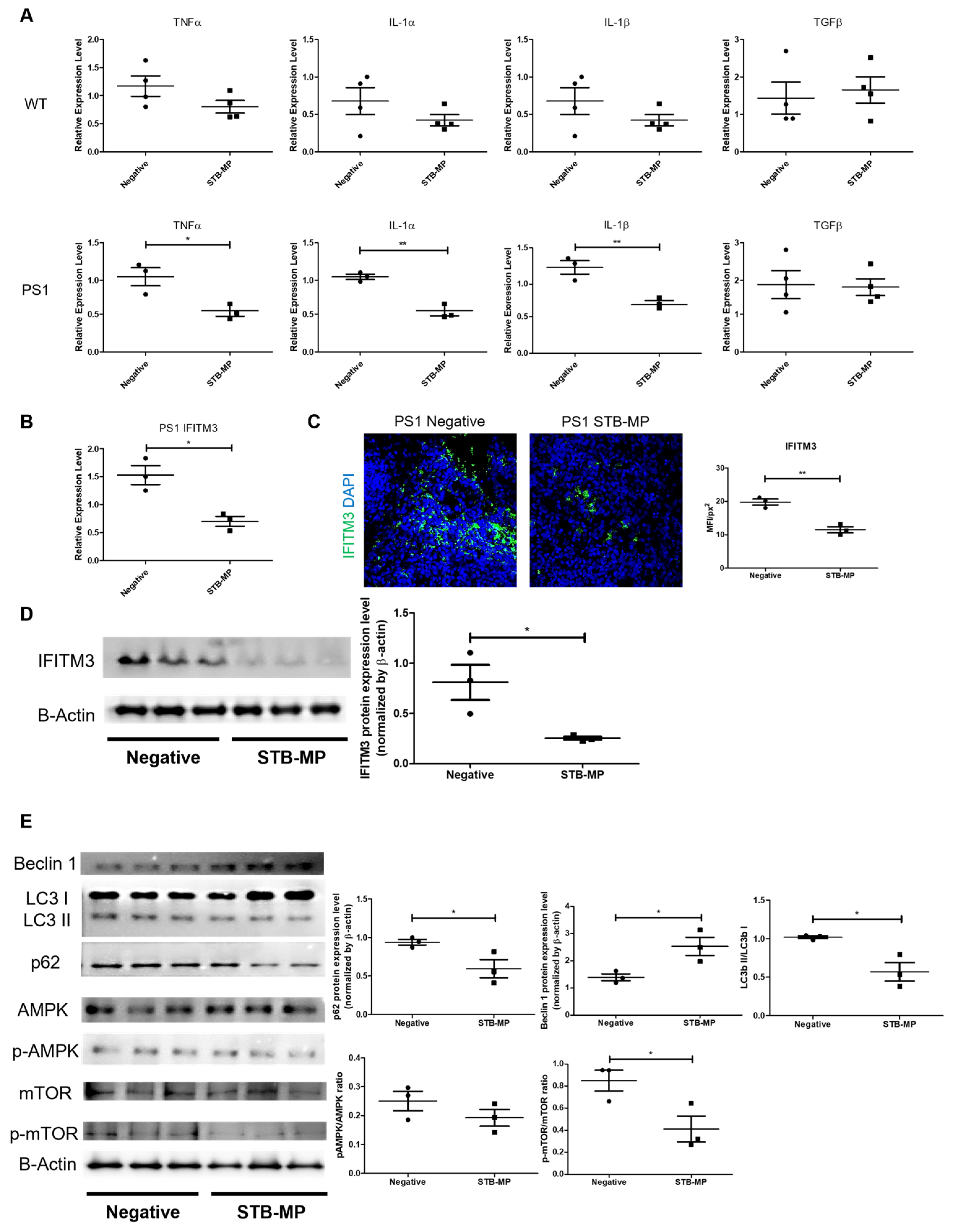
3.5. STB-MP Treatment Mechanism of Action in AD Cortical Brain Organoids
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Papaspyropoulos, A.; Tsolaki, M.; Foroglou, N.; Pantazaki, A.A. Modeling and targeting Alzheimer’s disease with organoids. Front. Pharmacol. 2020, 11, 396. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. The Genetics of Alzheimer’s Disease. J. Geriatr. Psychiatry Neurol. 2020, 23, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.D.; Pasca, S.P. Building models of brain disorders with three-dimensional organoids. Neuron 2018, 100, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef]
- Ohno, M. Alzheimer’s therapy targeting the β-secretase enzyme BACE1: Benefits and potential limitations from the perspective of animal model studies. Brain Res. Bull. 2016, 126, 183–198. [Google Scholar] [CrossRef]
- Manger, P.; Cort, J.; Ebrahim, N.; Goodman, A.; Henning, J.; Karolia, M.; Rodrigues, S.L.; Strkalj, G. Is 21st century neuroscience too focused on the rat/mouse model of brain function and dysfunction? Front. Neuroanat. 2008, 2, 5. [Google Scholar] [CrossRef]
- Lee, C.T.; Bendriem, R.M.; Wu, W.W.; Shen, R.F. 3D brain Organoids derived from pluripotent stem cells: Promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017, 24, 59. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, J.; Park, H.; Choi, H.; Kim, J. Modelling neurodegenerative diseases with 3D brain organoids. Biol. Rev. 2020, 95, 1497–1509. [Google Scholar] [CrossRef]
- Grenier, K.; Kao, J.; Diamandis, P. Three-dimensional modeling of human neurodegeneration: Brain organoids coming of age. Mol. Psychiatry 2020, 25, 254–274. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, H.J.; Cho, S.M.; Kim, B.; Jung, Y.K.; Kim, S.H. Particled Mica, STB-HO has chemopreventive potential via G1 arrest, and inhibition of proliferation and vascular endothelial growth factor receptor 2 in HCT colorectal cancer cells. BMC Complement. Altern. Med. 2013, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Kim, H.S.; Lee, B.C.; Shin, T.H.; Choi, S.W.; Kim, Y.J.; Lee, H.Y.; Jung, Y.K.; Seo, K.W.; Kang, K.S. Mica Nanoparticle, STB-HO Eliminates the Human Breast Carcinoma Cells by Regulating the Interaction of Tumor with its Immune Microenvironment. Sci. Rep. 2015, 5, 17515. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.A.; Andersen, J.; Pașca, A.M.; Birey, F.; Pașca, S.P. Generation and Assembly of Human Brain Region-Specific Three-Dimensional Cultures. Nat. Protoc. 2018, 13, 2062–2085. [Google Scholar] [CrossRef]
- Yu, K.R.; Shin, J.H.; Kim, J.J.; Koog, M.G.; Lee, J.Y.; Choi, S.W.; Kim, H.S.; Seo, Y.; Lee, S.; Shin, T.H.; et al. Rapid and Efficient Direct Conversion of Human Adult Somatic Cells into Neural Stem Cells by HMGA2/let-7b. Cell Rep. 2015, 10, 441–452. [Google Scholar] [CrossRef]
- Lee, S.E.; Shin, N.; Kook, M.G.; Kong, D.; Kim, N.G.; Choi, S.W.; Kang, K.S. Human iNSC-derived brain organoid model of lysosomal storage disorder in Niemann–Pick disease type C. Cell Death Dis. 2020, 11, 1059. [Google Scholar] [CrossRef]
- Hur, J.Y.; Frost, G.R.; Wu, X.; Crump, C.; Pan, S.J.; Wong, E.; Barros, M.; Li, T.; Nie, P.; Zhai, Y.; et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 2020, 586, 735–740. [Google Scholar] [CrossRef]
- Hung, S.Y.; Huang, W.P.; Liou, H.C.; Fu, W.M. Autophagy protects neuron from Aβ-induced cytotoxicity. Autophagy 2009, 5, 502–510. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer’s disease. Nat. Rev. Neurosci. 2006, 11, 361–370. [Google Scholar] [CrossRef]
- Marchesi, V.T. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J. 2011, 25, 5–13. [Google Scholar] [CrossRef]
- Roberson, E.D.; Mucke, L. 100 Years and Counting: Prospects for Defeating Alzheimer’s Disease. Science 2006, 314, 781–784. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Cho, S.W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Loganathan, K.; Sekiguchi, M.; Matsuba, Y.; Hui, K.; Tsubuki, S.; Tanaka, M.; Iwata, N.; Saito, T.; Saido, T.C. Aβ Secretion and Plaque Formation Depend on Autophagy. Cell Rep. 2013, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Asil, S.M.; Ahlawat, J.; Barroso, G.G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Choi, S.W.; Shin, T.H.; Uddin, M.H.; Shin, J.H.; Kang, T.W.; Lee, B.C.; Kim, H.S.; Seo, Y.; Shams, S.; Jung, Y.K.; et al. STB-HO, a novel mica fine particle, inhibits the teratoma-forming ability of human embryonic stem cells after in vivo transplantation. Oncotarget 2016, 7, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.G.; Jung, D.J.; Jung, Y.-K.; Kang, K.-S. The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model. Nanomaterials 2023, 13, 893. https://doi.org/10.3390/nano13050893
Kim NG, Jung DJ, Jung Y-K, Kang K-S. The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model. Nanomaterials. 2023; 13(5):893. https://doi.org/10.3390/nano13050893
Chicago/Turabian StyleKim, Nam Gyo, Dong Ju Jung, Yeon-Kwon Jung, and Kyung-Sun Kang. 2023. "The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model" Nanomaterials 13, no. 5: 893. https://doi.org/10.3390/nano13050893
APA StyleKim, N. G., Jung, D. J., Jung, Y.-K., & Kang, K.-S. (2023). The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model. Nanomaterials, 13(5), 893. https://doi.org/10.3390/nano13050893





