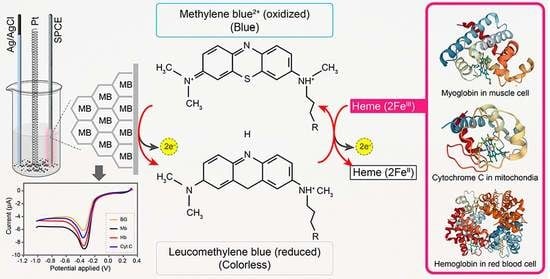
Bio-Electroanalysis Performance of Heme Redox-Center for π-π Interaction Bonding of a Methylene Blue-Graphene Modified Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Nanohybrid Materials
2.3. Fabrication of Electrode
2.4. Instrumentation and Procedure
3. Results
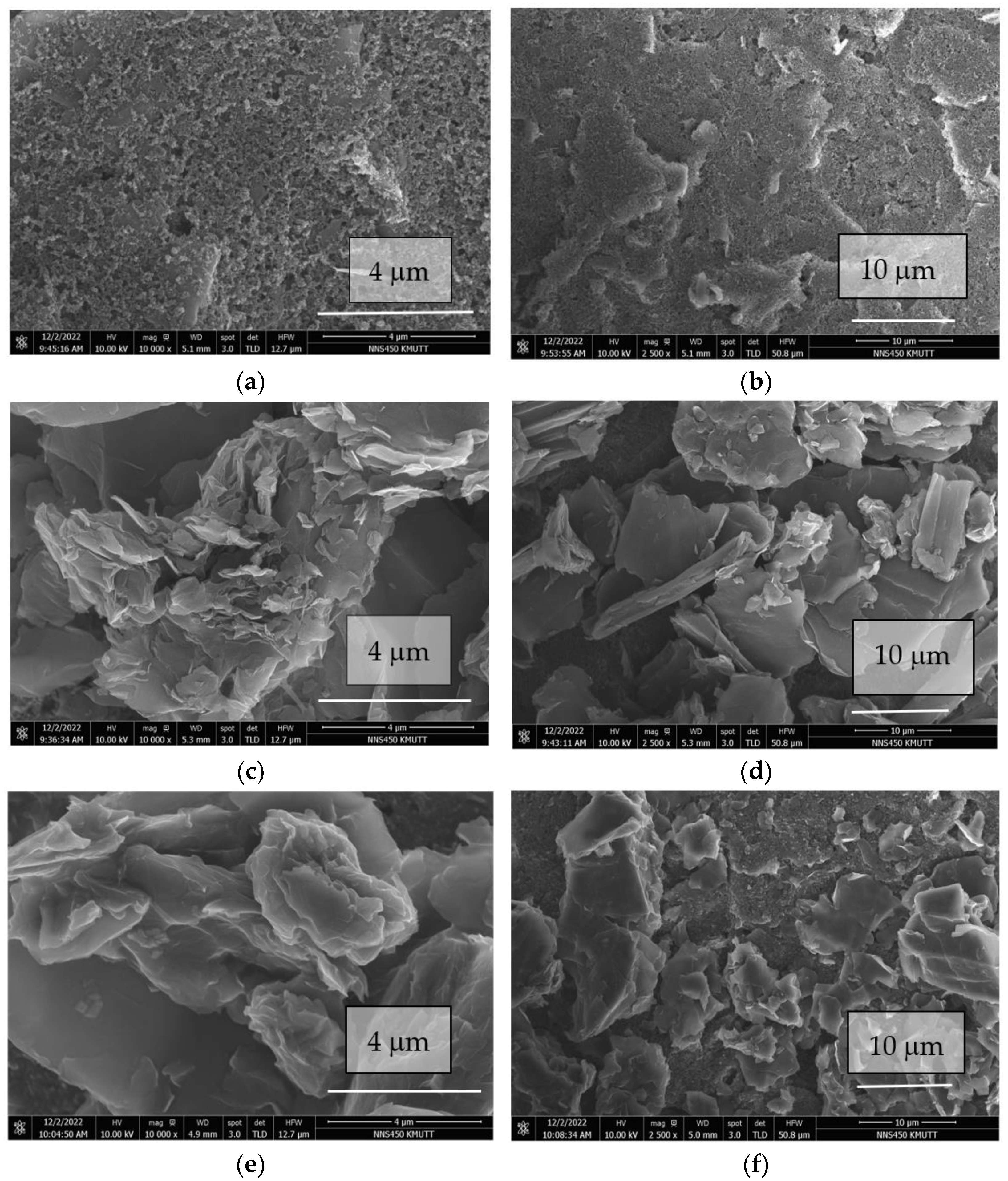
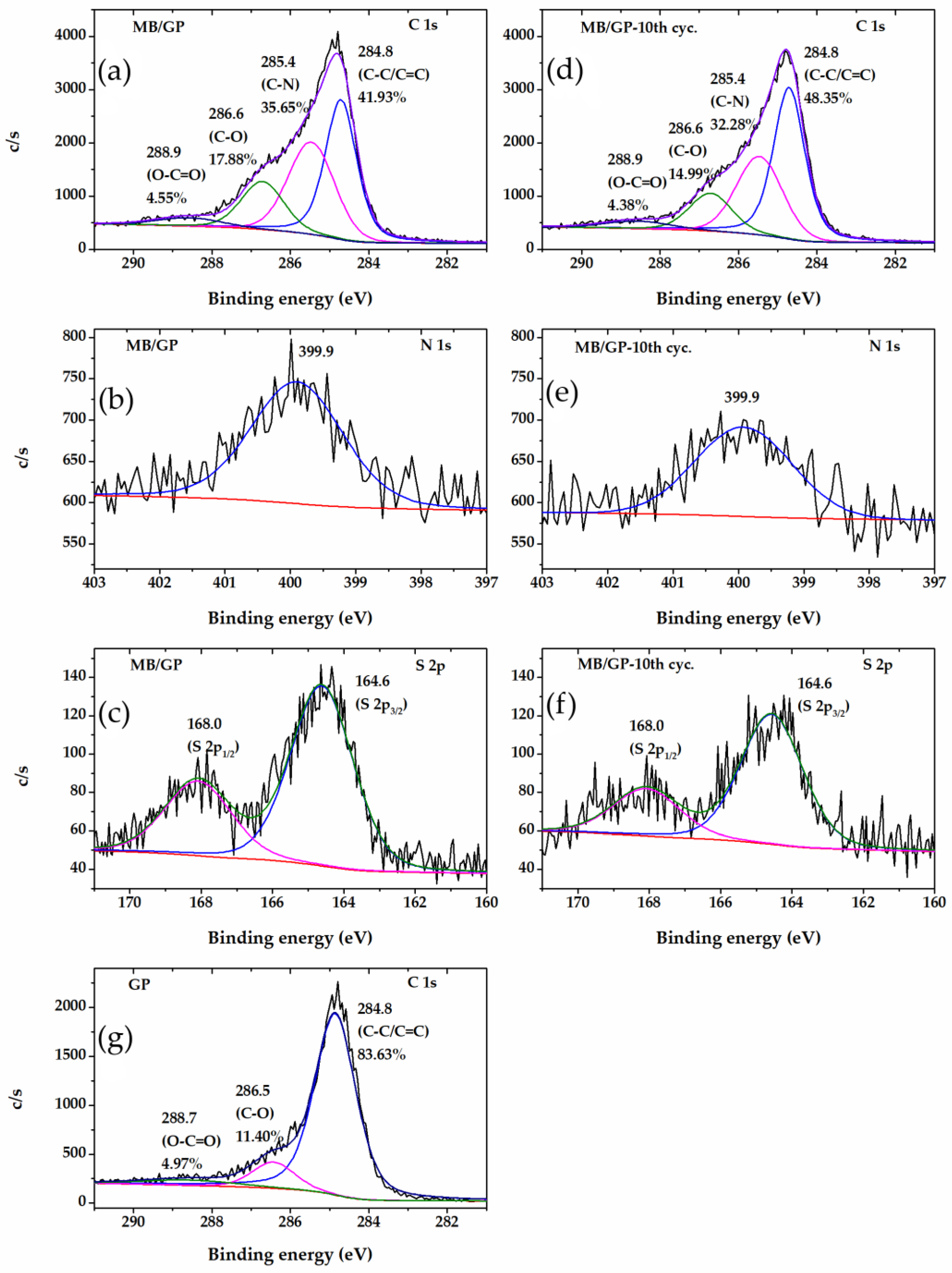
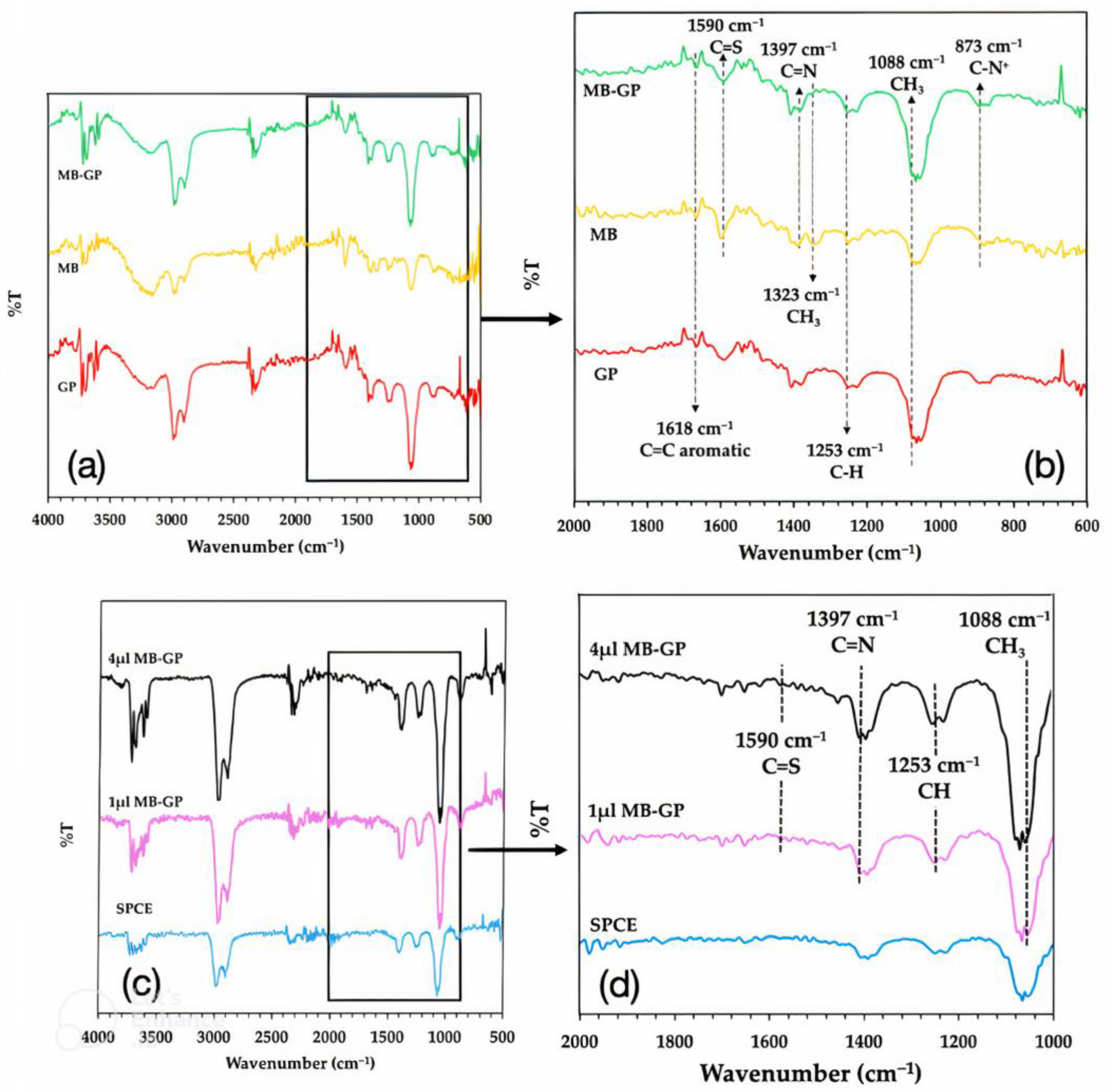
3.1. Characterization of MB/GP on Modified Electrode
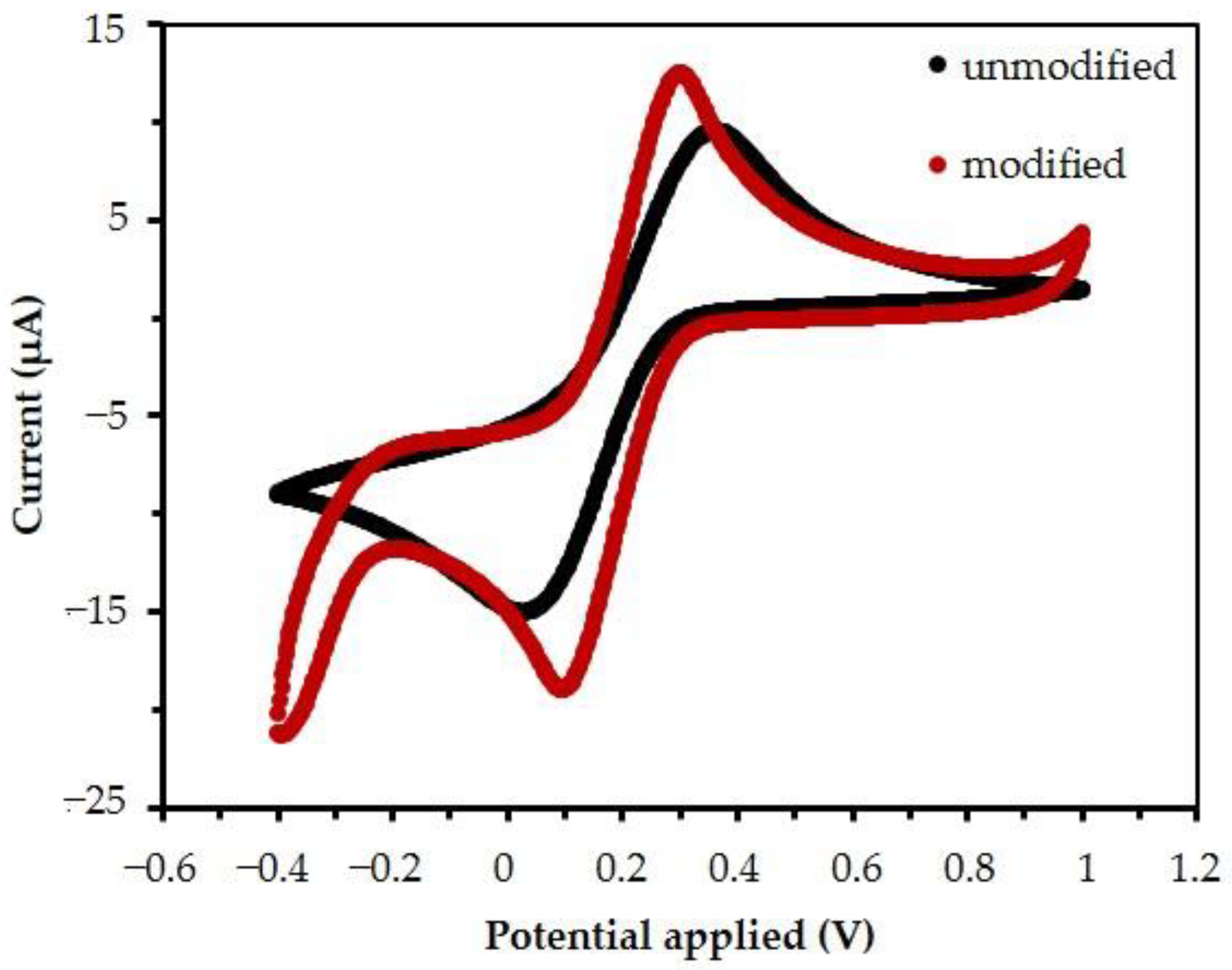
3.2. The Electrochemical Behavior of the Electrode
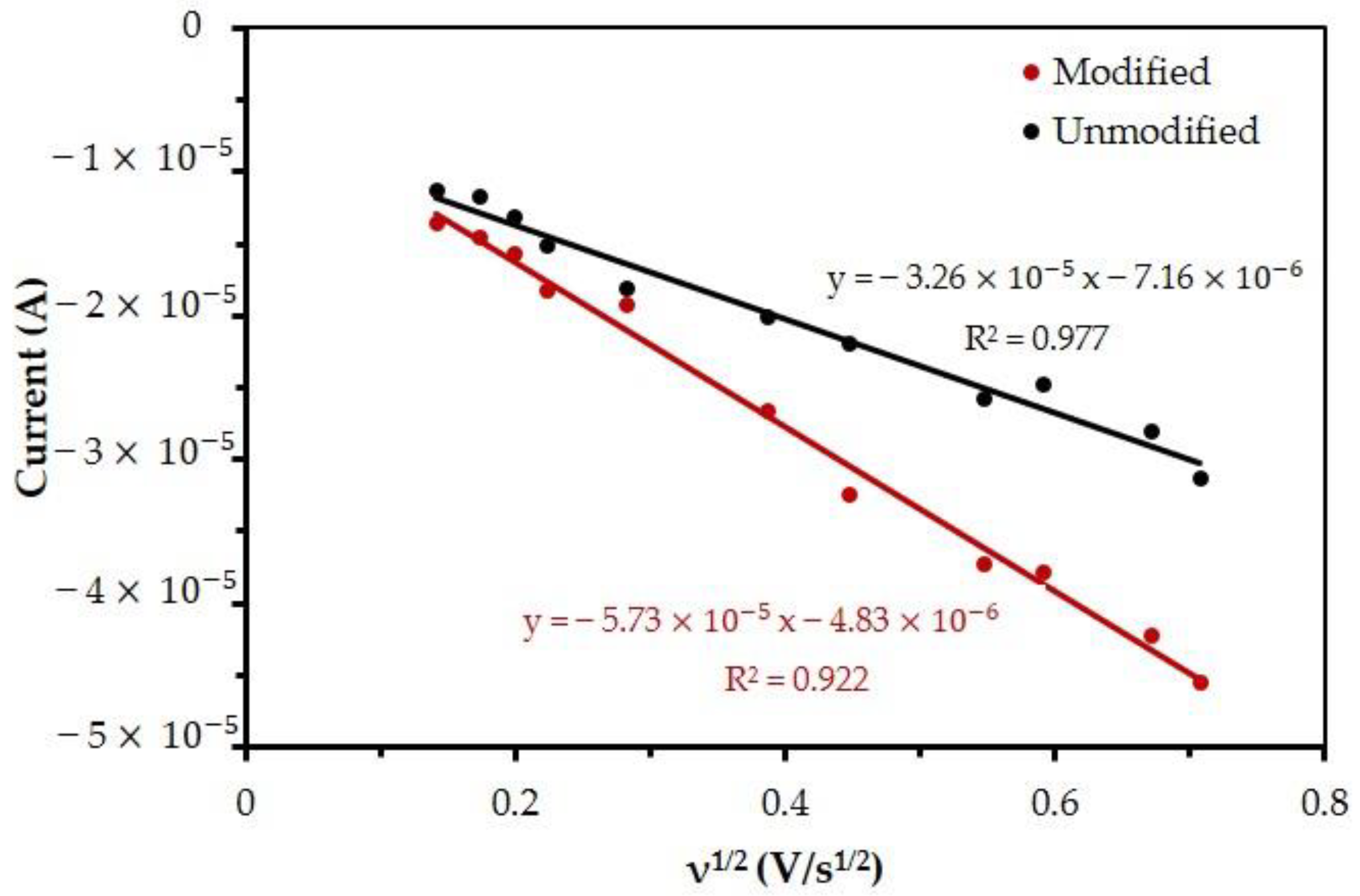
3.3. Total Active Area
3.4. The Surface Coverage
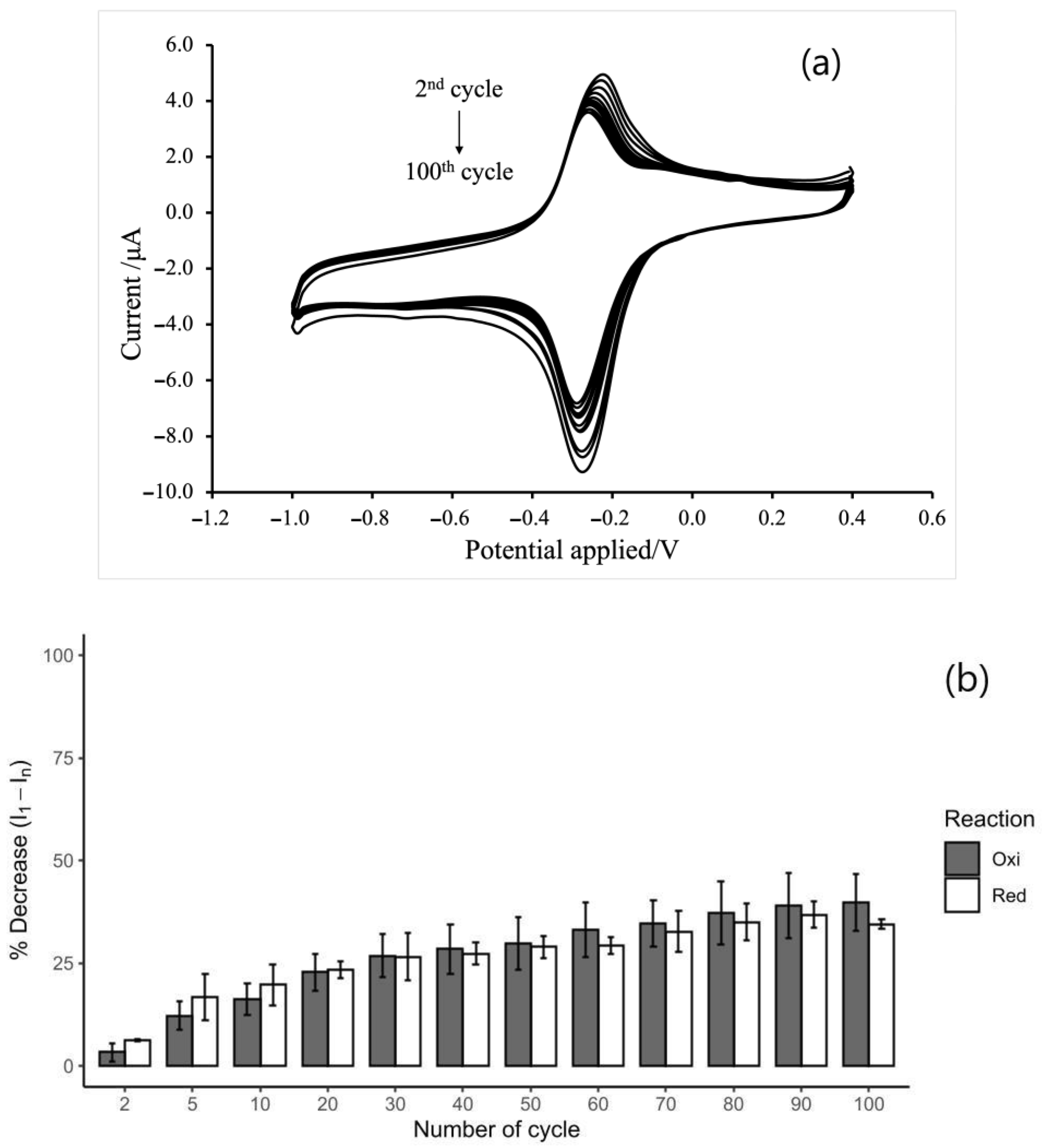
3.5. Stability of MB/GP Film on SPCE
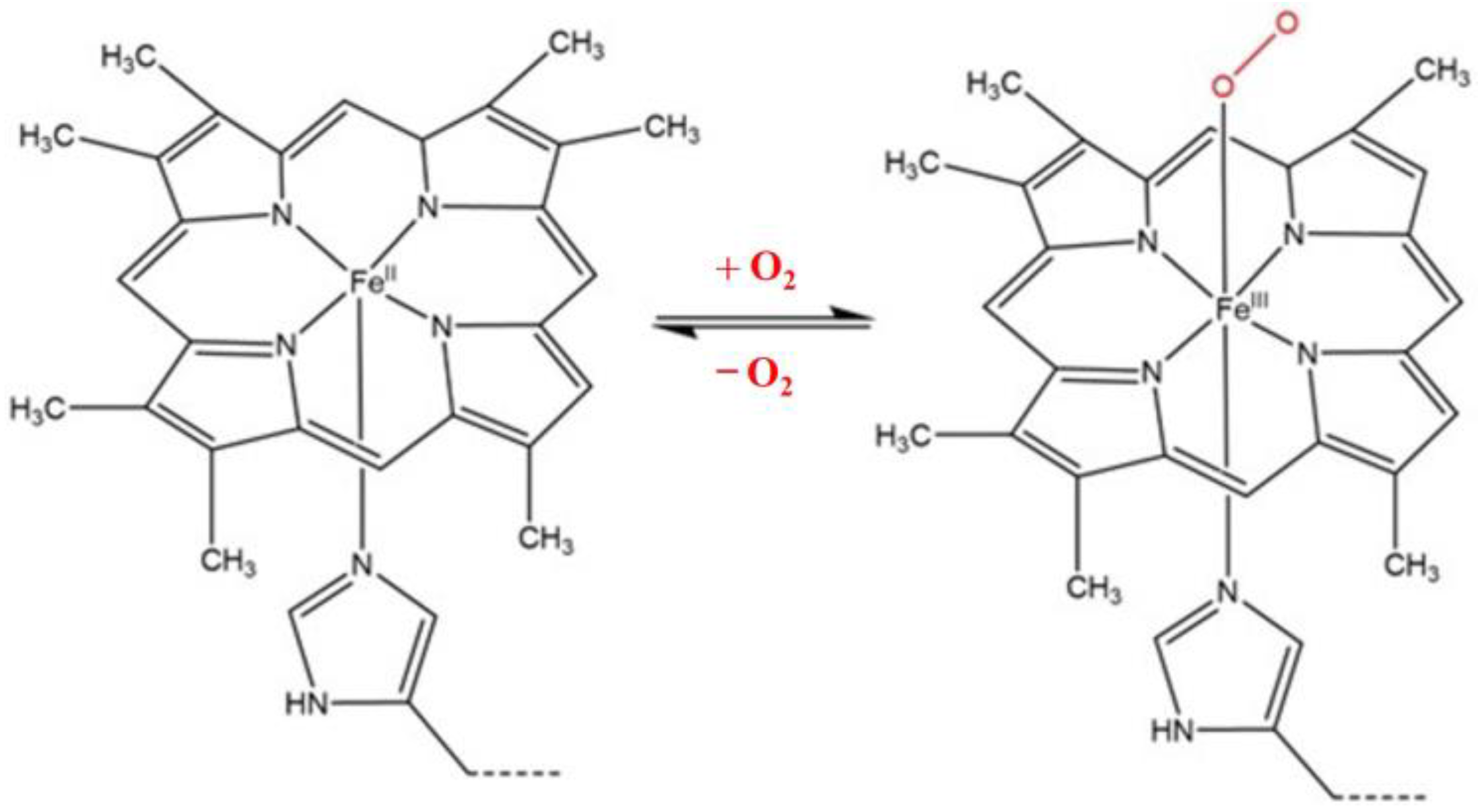
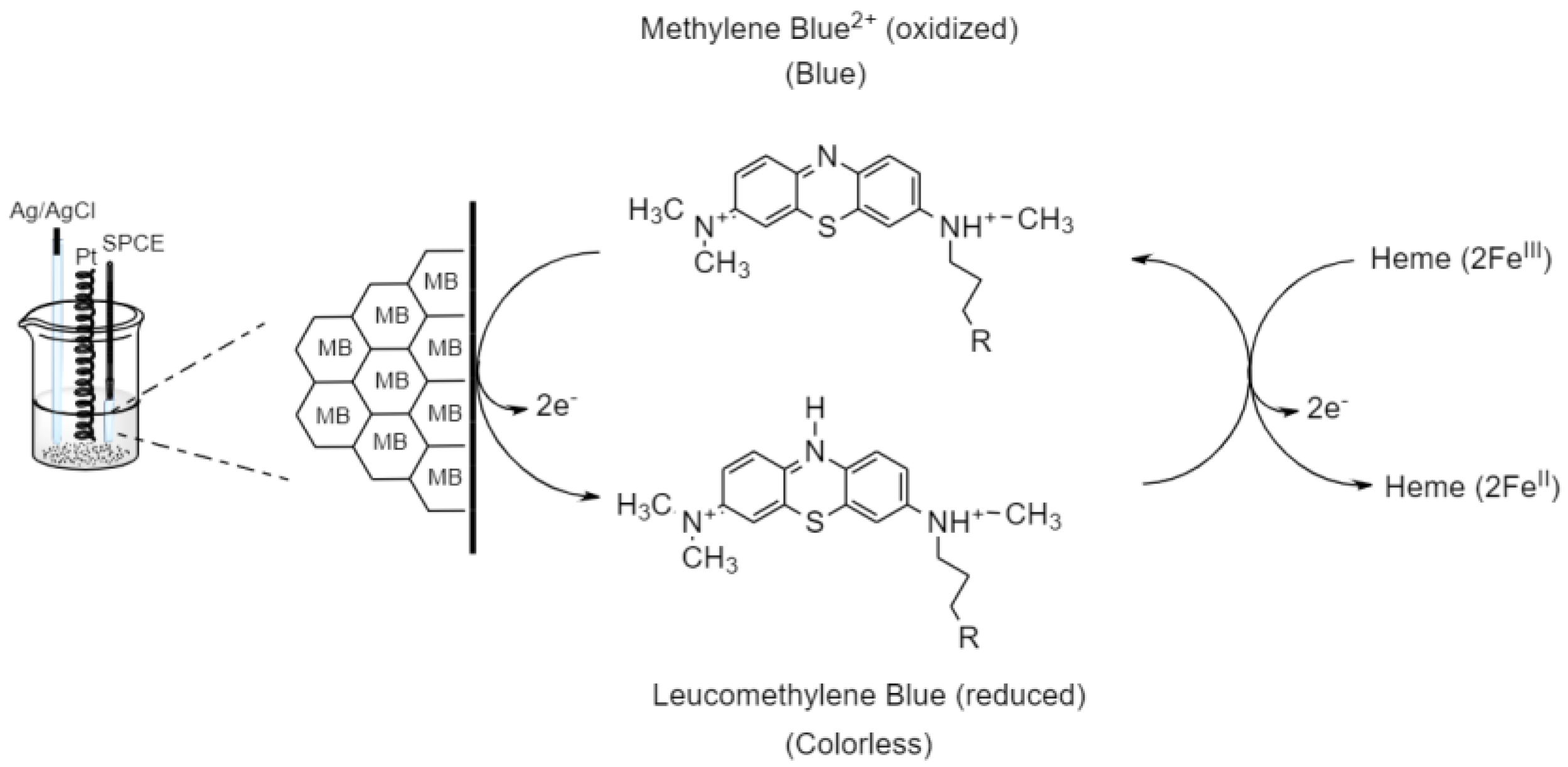
3.6. Electrocatalytic Reaction of Hemeproteins on MB/GP Modified Electrode
3.7. Performance of MB/GP Modified Electrode
3.8. Storage Stability of MB/GP Film on SPCE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodish, H.; Berk, A.; Kaiser, C.-A.; Krieger, M.; Scott, M.-P.; Bretscher, A.; Matsudaira, P. Molecular Cell Biology, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Nelson, D.-L.; Cox, M.-M. Principle of Biochemistry; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Pough, F.-H.; Janis, C.-M.; Heiser, J.-B. Vertebrate Life Boston; Pearson Benjamin Cummings: San Francisco, CA, USA, 2009. [Google Scholar]
- Baker, H.M.; Anderson, B.F.; Baker, E.N. Dealing with iron: Common structural principles in proteins that transport iron and heme. Proc. Natl. Acad. Sci. USA 2003, 58, 3579–3583. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Woollenberger, U.; Bistolas, N.; Guiseppi-Elis, A.; Scheller, F.W. Analytical Bioanalytical. Chemistry 2002, 372, 235–239. [Google Scholar]
- Weissbluth, M. Hemoglobin; Springer: Heidelberg, Germany, 1974. [Google Scholar]
- Berg, J.-M.; Tymoczko, J.-L.; Stryer, T. Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2002. [Google Scholar]
- Pratt, C.-W.; Cornely, K. Essential Biochemistry; Wiley Global Education: Hoboken, NJ, USA, 2012. [Google Scholar]
- Schmidt, M.; Gerlach, F.; Avivi, A.; Laufs, T.; Wystub, S.; Simpson, J.C.; Nevo, E.; Saaler-Reinhardt, S.; Reuss, S.; Hankeln, T.; et al. Cytoglobin Is a Respiratory Protein in Connective Tissue and Neurons, Which Is Up-regulated by Hypoxia. J. Biol. Chem. 2004, 279, 8063–8069. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Reedijk, J. Nomenclature Committee of the International Union of Biochemistry (NC-IUB). Nomenclature of electron-transfer proteins. Recommendations 1989. Biochim. Biophys. Acta 1991, 267, 665–677. [Google Scholar]
- Sankaran, V.G.; Weiss, M.J. Anemia: Progress in molecular mechanisms and therapies. Nat. Med. 2015, 21, 221–230. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Barkun, A.N.; Bardou, M. Management of Acute Bleeding from a Peptic Ulcer. N. Engl. J. Med. 2008, 359, 928–937. [Google Scholar] [CrossRef]
- Landefeld, C.; Beyth, R.J. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am. J. Med. 1993, 95, 315–328. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma Hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef]
- Mayer, T.K.; Freedman, Z.R. Protein glycosylation in diabetes mellitus: A review of laboratory measurements and of their clinical utility. Clin. Chim. Acta 1983, 127, 147–184. [Google Scholar] [CrossRef]
- The Thalassaemia Working Party of the BCSH General Haematology Task Force. Guidelines for investigation of the α and β thalassemia traits. J. Clin. Pathol. 1994, 47, 289–295. [Google Scholar] [CrossRef]
- Working Party of the General Hematology Task Force of the British Committee for Standards in Hematology. The Laboratory Diagnosis of Haemoglobinopathies. Br. J. Haematol. 1998, 101, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Silvestroni, E.; Bianco, I. A highly cost effective method of mass screening for thalassaemia. BMJ 1983, 286, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, C.; Efremov, G.; Pootrakul, S. Effectiveness of one tube osmotic fragility screening in detecting beta-thalassaemia trait. J. Med Genet. 1981, 18, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.H. Treatment of Ulcers on the Legs with Hyperbaric Oxygen. J. Dermatol. Surg. Oncol. 1975, 1, 55–58. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric Studies: II. Preparations from washed Blood cells; Nitric oxide Hemoglobin and Self hemoglobin. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Kumar, A.V.N.; Sivakumar, C.; Joseph, J. Photoluminescence of oligomers of aniline-2-sulfonic acid formed in the presence of AuCl4− and sodium citrate: Application in the optical detection of hemoglobin. Sens. Actuators B Chem. 2015, 209, 883–888. [Google Scholar] [CrossRef]
- Pourreza, N.; Golmohammadi, H. Hemoglobin detection using curcumin nanoparticles as a colorimetric chemosensor. RSC Adv. 2015, 5, 1712–1717. [Google Scholar] [CrossRef]
- Wajcman, H. Hemoglobin Disorders: Molecular Methods and Protocols; Humana Press Inc.: Totowa, NJ, USA, 2003; pp. 21–29. [Google Scholar]
- Traore, Z.S.; Shah, S.M.; Su, X. Flow-injection chemiluminescence determination of haemoglobin in the blood. Luminescence 2013, 28, 56–62. [Google Scholar] [CrossRef]
- Gabriel, L.; Soizic, C.; Cyrill, P.; Grard, S.; Corinne, L.; Joelle., R.-B. Direct Electron Transfer of Hemoglobin and Myoglobin at the Bare Glassy Carbon Electrode in an Aqueous BMI.BF4 Ionic-Liquid Mixture. ChemPhysChem 2011, 12, 411–418. [Google Scholar] [CrossRef]
- Handin, R.I.; Lux, S.E.; Stossel, T.P. Blood: Principles & Practica of Hematology, 1st ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Murray, S.S.; McKinney, E.S. Foundations of Maternal-Newborn Nursing, 4th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2006; p. 919. [Google Scholar]
- Wu, A.H.; Laios, I.; Green, S.; Gornet, T.G.; Wong, S.S.; Parmley, L.; Tonnesen, A.S.; Plaisier, B.; Orlando, R. Immunoassays for serum and urine myoglobin: Myoglobin clearance assessed as a risk factor for acute renal failure. Clin. Chem. 1994, 440, 796–802. [Google Scholar] [CrossRef]
- Goldstein, J.C.; Muñoz-Pinedo, C.; Ricci, J.E.; Adams, S.R.; Kelekar, A.; Schuler, M.; Tsien, R.Y.; Green, D.R. Cytochrome c is released in a single step during apoptosis. Cell Death Differ. 2005, 12, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Dejean, L.M.; Martinez-Caballero, S.; Guo, L.; Hughes, C.; Teijido, O.; Ducret, T.; Ichas, F.; Korsmeyer, S.J.; Antonsson, B.; Jonas, E.A.; et al. Oligomeric Bax Is a Component of the Putative Cytochrome c Release Channel MAC, Mitochondrial Apoptosis-induced Channel. Mol. Biol. Cell 2005, 16, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Jow, G.-M.; Chou, C.-J.; Chen, B.-F.; Tsai, J.-H. Beauvericin induces cytotoxic effects in human acute lymphoblastic leukemia cells through cytochrome c release, caspase 3 activation: The causative role of calcium. Cancer Lett. 2004, 216, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.F.; Dudgeon, P. A systemic approach to occupational stress. Syst. Res. 1988, 5, 101–106. [Google Scholar] [CrossRef]
- Sun, H.; Hu, N.; Ma, H. Direct Electrochemistry of Hemoglobin in Polyacrylamide Hydrogel Films on Pyrolytic Graphite Elec-trodes. Electroanalysis 2000, 12, 1064–1070. [Google Scholar] [CrossRef]
- Hu, Y. Electrochemistry and electrocatalysis with myoglobin in biomembrane-like surfactant-polymer 2C12N+PA− composite films. Talanta 2000, 50, 1183–1195. [Google Scholar] [CrossRef]
- Chen, X.; Hu, N.; Zeng, Y.; Rusling, J.F.; Yang, J. Ordered Electrochemically Active Films of Hemoglobin, Didodecyldimethylammonium Ions, and Clay. Langmuir 1999, 15, 7022–7030. [Google Scholar] [CrossRef]
- Merino, M.; Nuñez-Vergara, L.-J.; Squella, J.-A. Cytochrome C reductase immobilized on carbon paste electrode and its electro-catalytic effect on the reduction of cytochrome C. Boletín De La Soc. Chil. De Química 2000, 45, 1–7. [Google Scholar]
- Feng, J.-J.; Zhao, G.; Xu, J.-J.; Chen, H.-Y. Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Anal. Biochem. 2005, 342, 280–286. [Google Scholar] [CrossRef]
- Zhao, G.-C.; Zhang, L.; Wei, X.-W. An unmediated H2O2 biosensor based on the enzyme-like activity of myoglobin on multi-walled carbon nanotubes. Anal. Biochem. 2004, 329, 160–161. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Palangsuntikul, R.; Surareungchai, W. Electrochemical sensors for hemoglobin and myoglobin detection based on methylene blue-multiwalled carbon nanotubes nanohybrid-modified glassy carbon electrode. Electrochim. Acta 2011, 56, 6831–6836. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, X.; Sun, X. A Novel Label-Free Amperometric Immunosensor Based on Graphene Sheets-Methylene Blue Nanocomposite/Gold Nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 1399–1414. [Google Scholar]
- Li, Y.; Yang, W.-K.; Fan, M.-Q.; Liu, A. A Sensitive Label-free Amperometric CEA Immunosensor Based on Graphene-Nafion Nanocomposite Film as an Enhanced Sensing Platform. Anal. Sci. 2011, 27, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Hassan, G.; Shaukat, R.A.; Saqib, Q.M.; Chougale, M.Y.; Kim, J.; Bae, J. Wide range and highly linear signal processed systematic humidity sensor array using Methylene Blue and Graphene composite. Sci. Rep. 2021, 11, 16665. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Poo-Arporn, Y.; Pakapongpan, S.; Chanlek, N.; Poo-Arporn, R.P. The development of disposable electrochemical sensor based on Fe3O4-doped reduced graphene oxide modified magnetic screen-printed electrode for ractopamine determination in pork sample. Sens. Actuators B Chem. 2018, 284, 164–171. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Jiang, Y.; Wang, Y.; Chen, X. One-pot synthesis of highly greenish-yellow fluorescent nitrogen-doped graphene quantum dots for pyrophosphate sensing via competitive coordination with Eu3+ions. Nanoscale 2015, 7, 15427–15433. [Google Scholar] [CrossRef]
- Poo-Arporn, R.P.; Pakapongpan, S.; Khownarumit, P.; Waraho-Zhmayev, D.; Poo-Arporn, Y.; Surareungchai, W. Development of Mevalonic Acid Biosensor Using Amperometric Technique Based on Nanocomposite of Nicotinamide Adenine Dinucleotide and Carbon Nanotubes. J. Electrochem. Soc. 2017, 164, B349–B355. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, M.; Wang, L.; Liu, X. The Adsorption of Methylene Blue by an Amphiphilic Block Co-Poly(Arylene Ether Nitrile) Microsphere-Based Adsorbent: Kinetic, Isotherm, Thermodynamic and Mechanistic Studies. Nanomaterials 2019, 9, 1356. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, H.; Yang, X.; Zang, L. Tailoring Electronic Properties of Graphene by π–π Stacking with Aromatic Molecules. J. Phys. Chem. Lett. 2011, 2, 2897–2905. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, M.; Gong, K.; Su, L.; Guo, Z.; Mao, L. Adsorption of Methylene Blue Dye onto Carbon Nanotubes: A Route to an Electrochemically Functional Nanostructure and Its Layer-by-Layer Assembled Nanocomposite. Chem. Mater. 2005, 17, 3457–3463. [Google Scholar] [CrossRef]
- Yang, S.; Liu, D.; Meng, Q.B.; Wu, S.; Song, X.-M. Reduced graphene oxide-supported methylene blue nanocomposite as a glucose oxidase-mimetic for electrochemical glucose sensing. RSC Adv. 2018, 8, 32565–32573. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.; Hashim, M.A. Functionalization of graphene using deep eutectic solvents. Nanoscale Res. Lett. 2015, 10, 3–24. [Google Scholar] [CrossRef]
- Allen, J.-B.; Larry, R.-F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; David, H., Elizabeth, S., Charity, R., Eugene, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Adams, R. Electrochemistry at Solid Electrodes; Marcel Dekker: New York, NY, USA, 1969; p. 1098. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; Royal Society of Chemistry: London, UK, 2003. [Google Scholar]
- Stevens, N.P.C.; Rooney, M.B.; Bond, A.M.; Feldberg, S.W. A Comparison of Simulated and Experimental Voltammograms Obtained for the [+Fe(CN)6]3-/4- Couple in the Absence of Added Supporting Electrolyte at a Rotating Disk Electrode. J. Phys. Chem. A 2001, 105, 9085–9093. [Google Scholar] [CrossRef]
- Khownarumit, P.; Phanthong, C.; Surareungchai, W. Efficiency of Nanomaterials Modified Screen-Printed Electrode for Sudan I Detection Based on Electrochemical Experiments. KMUTT Res. Dev. J. 2017, 40, 473–483. [Google Scholar]
- Mechanism of Action. Available online: http://www.provayblue.com/MOA (accessed on 17 December 2019).
- Ju, H.; Xun, Y.; Chen, H. Heterogeneous catalytic reaction at a methylene blue/Nafion® modified carbon fiber microcylinder electrode. J. Electroanal. Chem. 1995, 380, 283–285. [Google Scholar] [CrossRef]
- Galus, Z. Fundamentals of Electrochemical Analysis; Eills Horwood: NewYork, NY, USA, 1994. [Google Scholar]
- Shen, L.; Hu, N. Heme protein films with polyamidoamine dendrimer: Direct electrochemistry and electrocatalysis. Biochim. Biophys. Acta BBA 2000, 1608, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Royer, W.E., Jr. High-Resolution Crystallographic Analysis of a Co-operative Dimeric Hemoglobin. J. Mol. Biol. 1994, 235, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.E. Structure and refinement of oxymyoglobin at 1·6 Å resolution. J. Mol. Biol. 1980, 142, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Than, M.E.; Hof, P.; Huber, R.; Bourenkov, G.; Bartunik, H.D.; Buse, G.; Soulimane, T. Thermus thermophilus cytochrome-c552: A new highly thermostable cytochrome-c structure obtained by MAD phasing. J. Mol. Biol. 1997, 271, 629–644. [Google Scholar] [CrossRef] [PubMed]




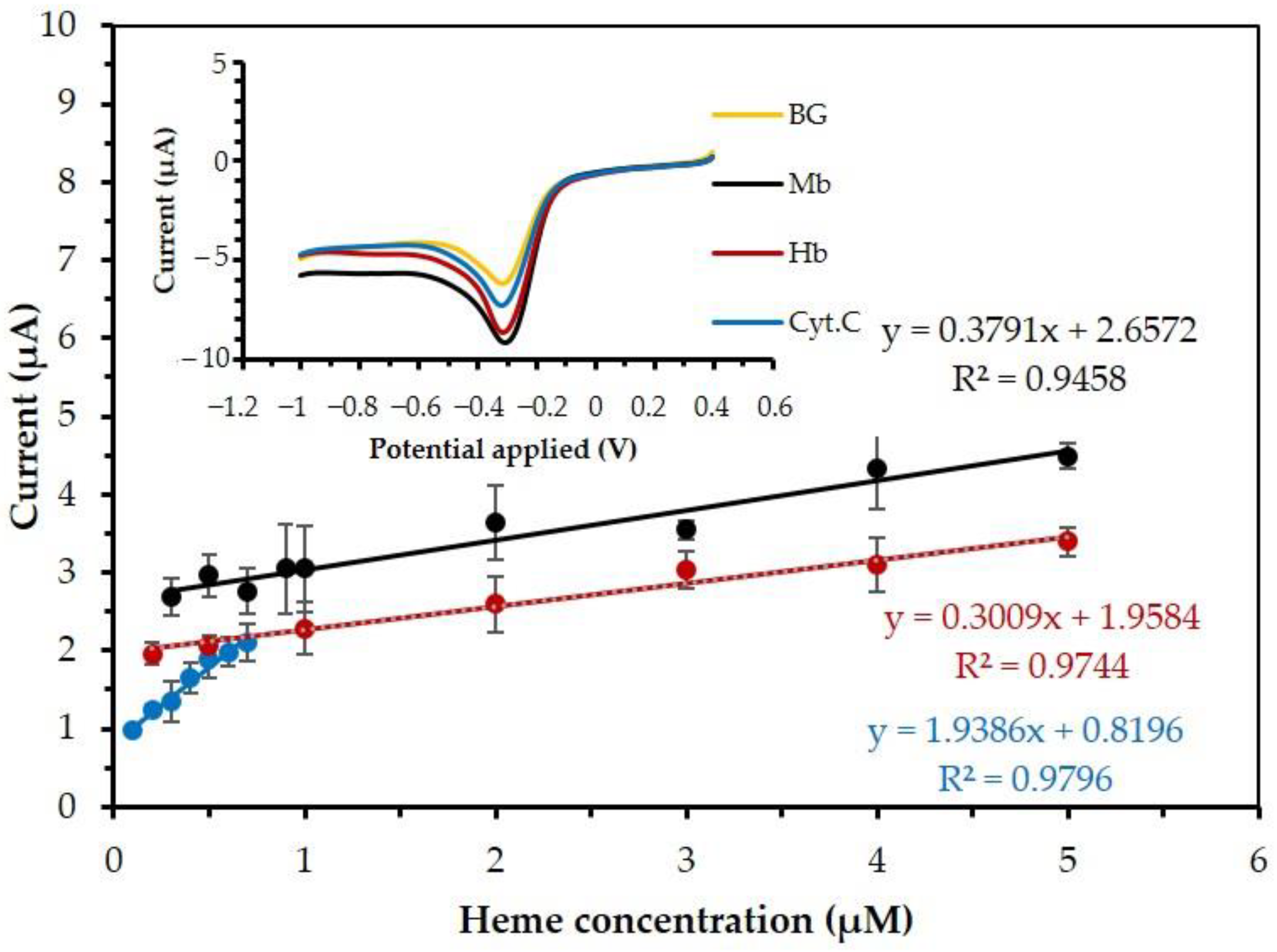
| Sample | Calibration Equation | Linear Range (µM) | Correlation Coefficients (R2) | LOD (µM) | Sensitivity (µA/µM) |
|---|---|---|---|---|---|
| Hb | Y = 0.307x + 1.95 | 0.2 to 5 | 0.98 | 0.2 | 0.307 |
| Mb | Y = 0.363x + 2.582 | 0.3 to 5 | 0.95 | 0.3 | 0.363 |
| Cyt. C | Y = 2.047x + 0.787 | 0.1 to 0.7 | 0.98 | 0.1 | 2.047 |
| Hemeproteins | The Catalytic Reaction Rate Constant (kcat, (Ms)−1) | Sensitivity (µA/µM) | The Structure of Hemeproteins | The 3D Feature of Hemeproteins [65,66,67] |
|---|---|---|---|---|
| Hb | 2.10 × 10−6 | 0.307 | polypeptide chains 4 heme 4 Fe (3+) | 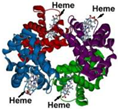 |
| Mb | 1.55 × 10−6 | 0.363 | polypeptide chain 1 heme 1 Fe (3+) | 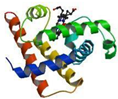 |
| Cyt. C | 1.50 × 10−6 | 2.047 | polypeptide chain 1 Fe (3+) 1 heme (the smallest protein molecule of heme) | 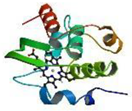 |
| The Item of the Electrode | The Number of Days | The Current Response (Ip (µA), (n = 3)) |
|---|---|---|
| #1 | 1st | −4.378 (SD ± 0.720) |
| #2 | 5th | −4.137 (SD ± 0.324) |
| #3 | 10th | −4.286 (SD ± 0.538) |
| #4 | 20th | −4.511 (SD ± 0.114) |
| #5 | 30th | −4.527 (SD ± 0.290) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khownarumit, P.; Choosang, K.; Poo-arporn, R.P.; Poo-arporn, Y.; Chanlek, N.; Surareungchai, W. Bio-Electroanalysis Performance of Heme Redox-Center for π-π Interaction Bonding of a Methylene Blue-Graphene Modified Electrode. Nanomaterials 2023, 13, 745. https://doi.org/10.3390/nano13040745
Khownarumit P, Choosang K, Poo-arporn RP, Poo-arporn Y, Chanlek N, Surareungchai W. Bio-Electroanalysis Performance of Heme Redox-Center for π-π Interaction Bonding of a Methylene Blue-Graphene Modified Electrode. Nanomaterials. 2023; 13(4):745. https://doi.org/10.3390/nano13040745
Chicago/Turabian StyleKhownarumit, Porntip, Kanmanee Choosang, Rungtiva P. Poo-arporn, Yingyot Poo-arporn, Narong Chanlek, and Werasak Surareungchai. 2023. "Bio-Electroanalysis Performance of Heme Redox-Center for π-π Interaction Bonding of a Methylene Blue-Graphene Modified Electrode" Nanomaterials 13, no. 4: 745. https://doi.org/10.3390/nano13040745
APA StyleKhownarumit, P., Choosang, K., Poo-arporn, R. P., Poo-arporn, Y., Chanlek, N., & Surareungchai, W. (2023). Bio-Electroanalysis Performance of Heme Redox-Center for π-π Interaction Bonding of a Methylene Blue-Graphene Modified Electrode. Nanomaterials, 13(4), 745. https://doi.org/10.3390/nano13040745