Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
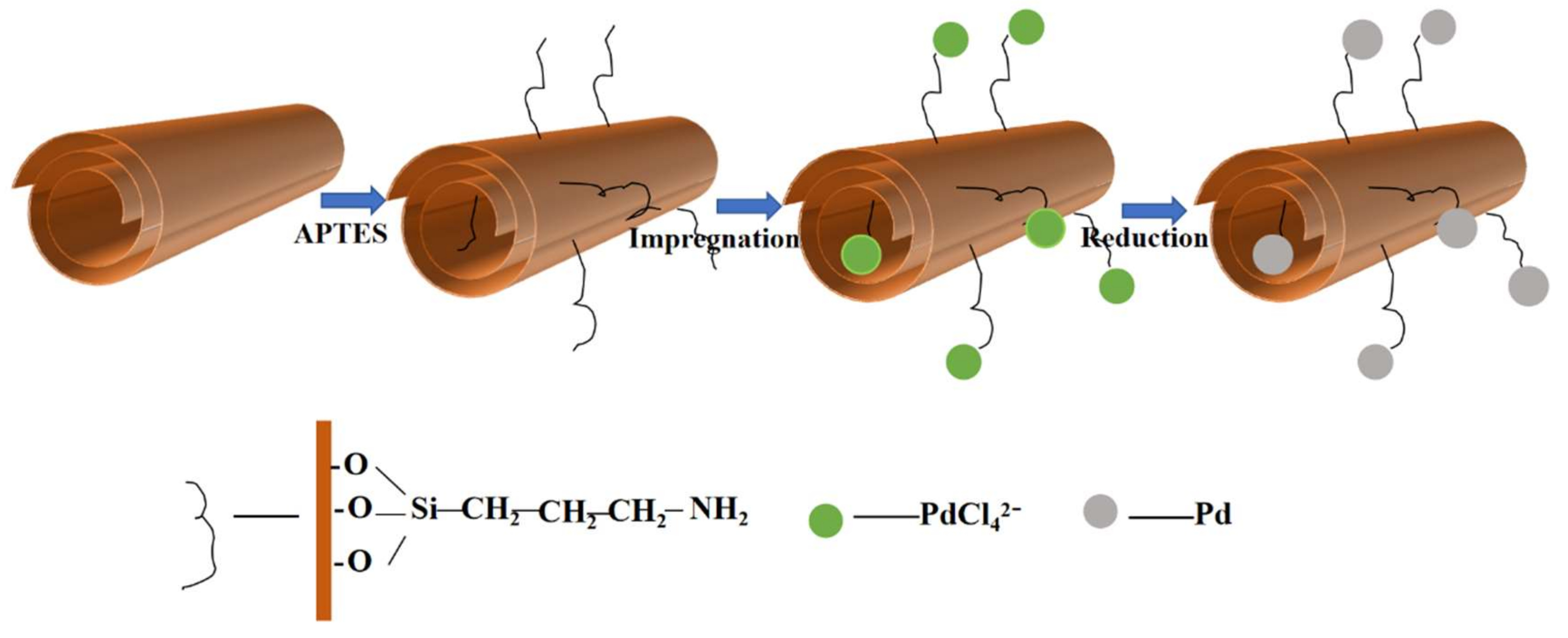
2.2. Synthesis of Catalysts
2.2.1. Amino-Functionalization of HNTs
2.2.2. Synthesis of Pd/NH2-HNTs
2.2.3. Synthesis of PdAu/NH2-HNTs
2.2.4. Synthesis of PdAg/NH2-HNTs
2.3. Characterization
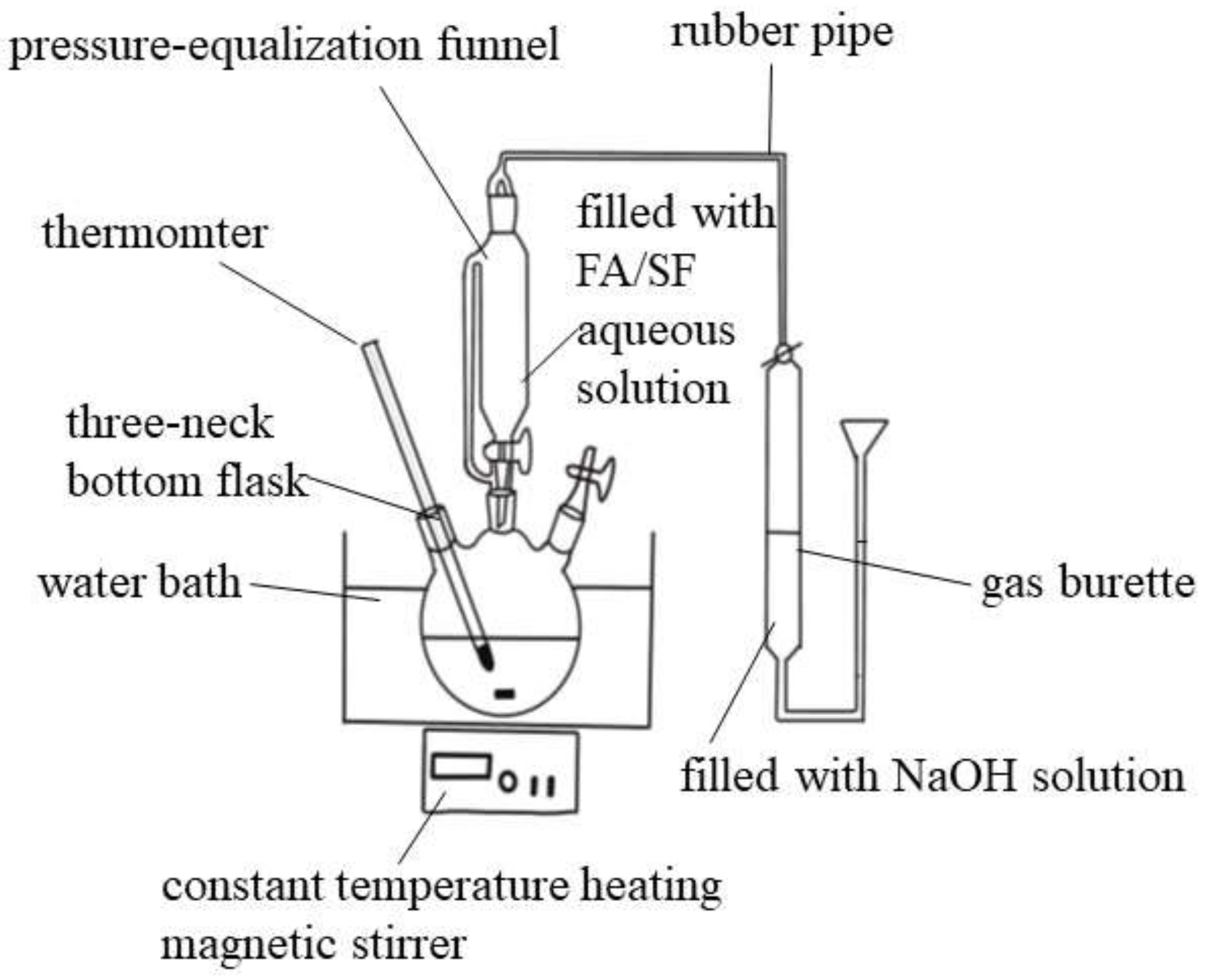
2.4. Evaluation of Catalytic Activity of Catalysts in DFA
2.5. Turn-Over-Frequency (TOF) Calculations
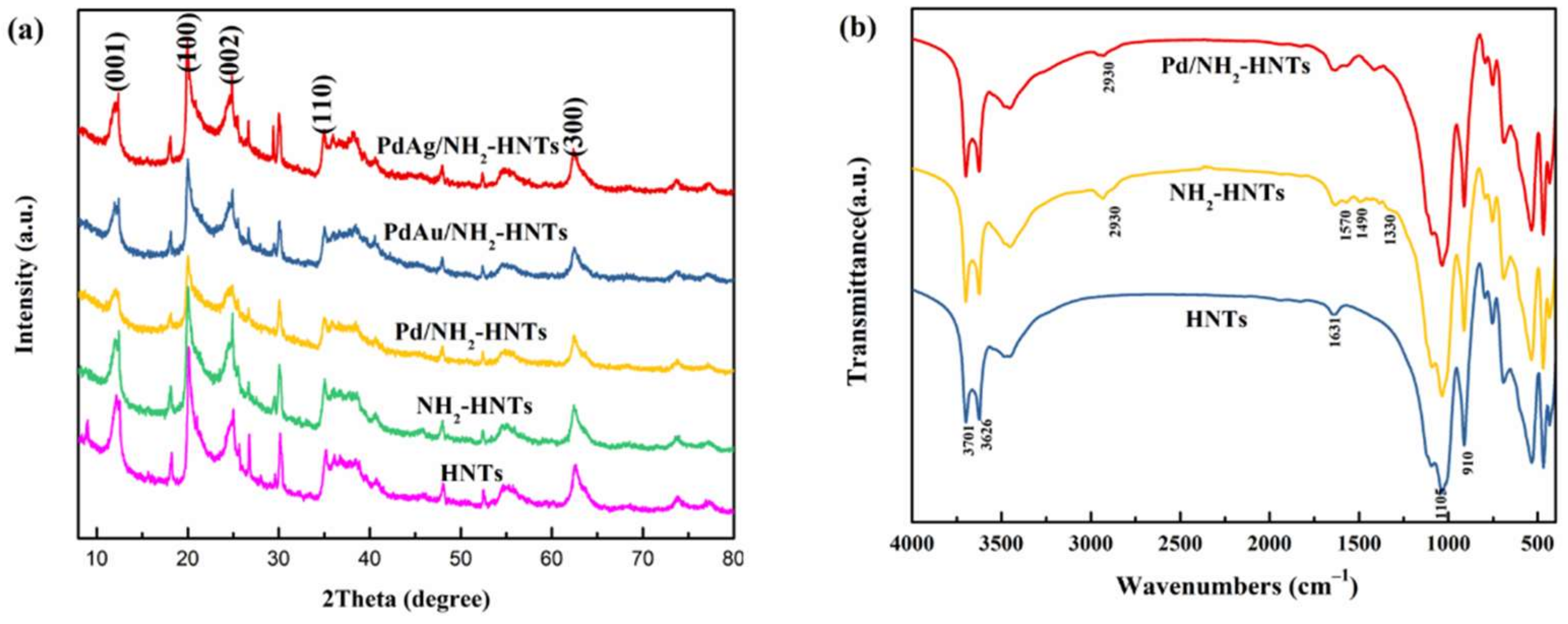
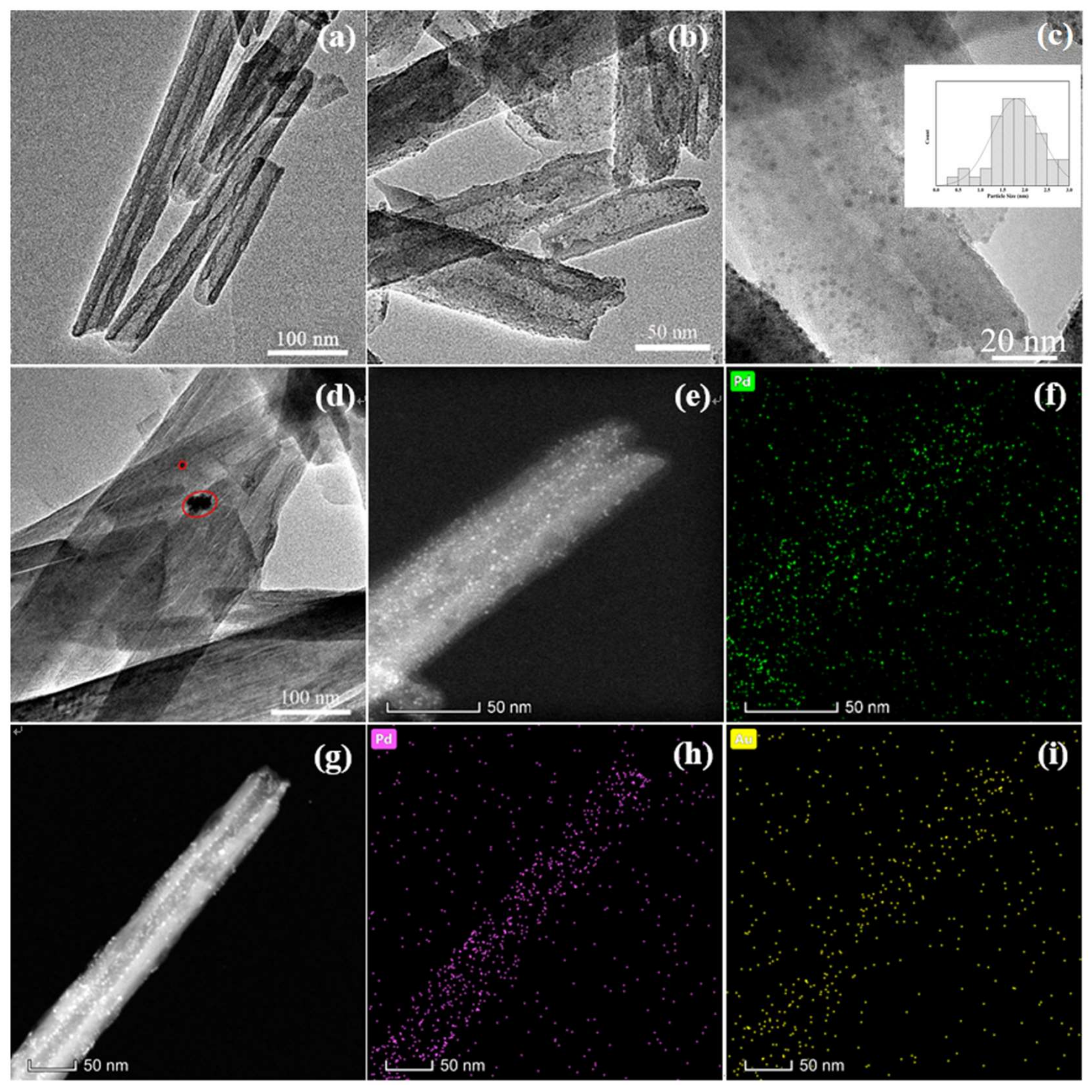
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steven, J.H.; Andrew, M.H.; Thomas, A.J. Fuel Cell Chemistry and Operation. J. Power Sources 2007, 172, 1. [Google Scholar]
- Brandon, N.P.; Kurban, Z. Clean energy and the hydrogen economy. Philos. Trans. R. Soc. A 2017, 375, 20160400. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Shi, W.; Feng, G.; Lu, Z.; Zhang, X.; Tao, D. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Enthaler, S. Carbon Dioxide—The Hydrogen-Storage Material of the Future. ChemSusChem 2008, 1, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.M. The hydrogen economy: Its history. Int. J. Hydrogen Energy 2013, 38, 2579–2588. [Google Scholar] [CrossRef]
- Jiang, Z.; Qi, R.; Huang, Z.; Shangguan, W.; Wong, R.; Lee, A. Impact of Methanol Photomediated Surface Defects on Photocatalytic H2 Production Over Pt/TiO2. Energy Environ. Mater. 2020, 3, 202–208. [Google Scholar] [CrossRef]
- Ferenc, J. Breakthroughs in hydrogen storage—Formic Acid as a sustainable storage material for hydrogen. ChemSusChem 2010, 1, 805–808. [Google Scholar]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Yadav, M.; Akita, T.; Tsumori, N.; Xu, Q. Strong metal–molecular support interaction (SMMSI): Amine-functionalized gold nanoparticles encapsulated in silica nanospheres highly active for catalytic decomposition of formic acid. J. Mater. Chem. 2012, 22, 12582–12586. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B Environ. 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Putten, R.; Schulpen, E.N.; Hensen, E.J.; Pidko, E.A. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP-Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Myers, T.W.; Berben, L.A. Aluminium–ligand cooperation promotes selective dehydrogenation of formic acid to H2 and CO2. Chem. Sci. 2014, 5, 2771–2777. [Google Scholar] [CrossRef]
- Mellone, I.; Gorgas, N.; Bertini, F.; Peruzzini, M.; Kirchner, K.; Gonsalvi, L. Selective Formic Acid Dehydrogenation Catalyzed by Fe-PNP Pincer Complexes Based on the 2,6-Diaminopyridine Scaffold. Organometallics 2016, 35, 3344–3349. [Google Scholar] [CrossRef]
- Bertini, F.; Mellone, I.; Ienco, A.; Peruzzini, M.; Gonsalvi, L. Iron(II) Complexes of the Linear rac-Tetraphos-1 Ligand as Efficient Homogeneous Catalysts for Sodium Bicarbonate Hydrogenation and Formic Acid Dehydrogenation. ACS Catal. 2015, 5, 1254–1265. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.H.; Ham, H.C.; Kim, D.H. Mechanistic insights on aqueous formic acid dehydrogenation over Pd/C catalyst for efficient hydrogen production. J. Catal. 2020, 389, 506–516. [Google Scholar] [CrossRef]
- Vatsa, A.; Padhi, S.K. Dehydrogenation of Formic Acid by RuII Half Sandwich Catalysts. Chem. Sel. 2021, 6, 9447–9452. [Google Scholar]
- Agapova, A.; Alberico, E.; Kammer, A.; Junge, H.; Beller, M. Catalytic Dehydrogenation of Formic Acid with Ruthenium-PNP-Pincer Complexes: Comparing N-Methylated and NH-Ligands. ChemCatChem 2019, 11, 1910–1914. [Google Scholar] [CrossRef]
- Zhou, W.; Wei, Z.; Spannenberg, A.; Jiao, H.; Junge, K.; Junge, H.; Beller, M. Cobalt-Catalyzed Aqueous Dehydrogenation of Formic Acid. Chem. Eur. J. 2019, 25, 8459–8464. [Google Scholar] [CrossRef]
- Amos, R.I.J.; Heinroth, F.; Chan, B.; Zheng, S.; Haynes, B.S.; Easton, C.J.; Masters, A.F.; Radom, L.; Maschmeyer, T. Hydrogen from Formic Acid through Its Selective Disproportionation over Sodium Germanate—A Non-Transition-Metal Catalysis System. Angew. Chem. Int. Ed. 2014, 53, 11275–11279. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Jiang, Z.; Fang, T. A review on liquid-phase heterogeneous dehydrogenation of formic acid: Recent advances and perspectives. Chem. Pap. 2018, 72, 2121–2135. [Google Scholar] [CrossRef]
- Ojeda, M.; Iglesia, E. Formic Acid Dehydrogenation on Au-Based Catalysts at Near-Ambient Temperatures. Angew. Chem. Int. Ed. 2009, 121, 4894–4897. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Upadhye, R.S. The catalytic decomposition of aqueous formic acid over suspended palladium catalysts. J. Catal. 1971, 21, 39–47. [Google Scholar] [CrossRef]
- Ye, W.; Huang, H.; Zou, W.; Ge, Y.; Lu, R.; Zhang, S. Controllable Synthesis of Supported PdAu Nanoclusters and Their Electronic Structure-Dependent Catalytic Activity in Selective Dehydrogenation of Formic Acid. ACS Appl. Mater. Interfaces 2021, 13, 34258–34265. [Google Scholar] [CrossRef]
- Dai, H.; Cao, N.; Yang, L.; Su, J.; Luo, W.; Cheng, G. AgPd nanoparticles supported on MIL-101 as high performance catalysts for catalytic dehydrogenation of formic acid. J. Mater. Chem. A 2014, 2, 11060–11064. [Google Scholar] [CrossRef]
- Bi, Q.; Lin, J.; Liu, Y.; He, H.; Huang, F.; Cao, Y. Gold supported on zirconia polymorphs for hydrogen generation from formic acid in base-free aqueous medium. J. Power Sources 2016, 328, 463–471. [Google Scholar] [CrossRef]
- Bi, Q.; Lin, J.; Liu, Y.; He, H.; Huang, F.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew. Chem. Int. Ed. 2016, 55, 11849–11853. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Tang, Y.; Ziaee, M.A.; Tian, D.; Wang, R. Highly Dispersed Ultrafine Palladium Nanoparticles Enabled by Functionalized Porous Organic Polymer for Additive-free Dehydrogenation of Formic Acid. ChemCatChem 2017, 10, 1431–1437. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Mao, S.; Gong, Y.; Chen, Y.; Wang, Y. Pd nanoparticles anchored on amino-functionalized hierarchically porous carbon for efficient dehydrogenation of formic acid under ambient conditions. J. Mater. Chem. A 2019, 7, 25791–25795. [Google Scholar] [CrossRef]
- Halasi, G.; Schubert, G.; Solymosi, F. Photolysis of HCOOH over Rh Deposited on Pure and N-Modified TiO2. Catal. Lett. 2012, 142, 218–223. [Google Scholar] [CrossRef][Green Version]
- Mori, K.; Masuda, S.; Tanaka, H.; Yoshizawa, K.; Che, M.; Yamashita, H. Phenylamine-functionalized mesoporous silica supported PdAg nanoparticles: A dual heterogeneous catalysts for formic acid/CO2-mediated chemical hydrogen delivery/storage. Chem. Comm. 2017, 53, 4677–4680. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Chi, Y.; Wang, M.; Zhang, J.; Wu, C.; Gu, Y.; Wang, H.; et al. CrPd nanoparticles on NH2-functionalized metal-organic framework as a synergistic catalyst for efficient hydrogen evolution from formic acid. Chem. Eng. J. 2019, 361, 953–959. [Google Scholar] [CrossRef]
- Liu, X.; Su, P.; Chen, Y.; Zhu, B.; Zhang, S.; Huang, W. g-C3N4 supported metal (Pd, Ag, Pt) catalysts for hydrogen-production from formic acid. New J. Chem. 2018, 42, 9449–9454. [Google Scholar] [CrossRef]
- Riela, S.; Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef]
- Bates, T.F.; Hildebrand, F.A.; Swineford, A. Morphology and structure of endellite and halloysite. Am. Mineral. 1950, 35, 463–484. [Google Scholar]
- Saif, M.J.; Asif, H.M.; Naveed, M. Properties and modification methods of halloysite nanotubes: A state-of-the-art review. J. Chil. Chem. Soc. 2018, 63, 4109–4125. [Google Scholar] [CrossRef]
- Yu, L.; Bing, Y.; Ma, J. Epoxidation of alkenes efficiently catalyzed by Mo salen supported on surface-modified halloysite nanotubes. Chin. J. Catal. 2015, 36, 348–354. [Google Scholar]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review-ScienceDirect. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Zhong, F.; Cai, G.; Xiao, Y.; Jiang, L. Site-Oriented Design of High-Performance Halloysite-Supported Palladium Catalysts for Methane Combustion. Ind. Eng. Chem. Res. 2020, 59, 5636–5647. [Google Scholar] [CrossRef]
- Abu El-Soad, A.M.; Pestov, A.V.; Tambasova, D.P.; Osipova, V.A.; Martemyanov, N.A.; Cavallaro, G.; Kovaleva, E.G.; Lazzara, G.; Organomet, J. Insights into grafting of (3-Mercaptopropyl) trimethoxy silane on halloysite nanotubes surface. J. Organomet. Chem. 2020, 915, 121224. [Google Scholar] [CrossRef]
- Osipova, V.A.; Pestov, A.V.; Mekhaev, A.V.; Abuelsoad, A.M.A.; Tambasova, D.P.; Antonov, D.O.; Kovaleva, E.G. Functionalization of Halloysite by 3-Aminoproryltriethoxysilane in Various Solvents. Petrol. Chem. 2020, 60, 597–600. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Ramesh, G.V.; Tanabe, T.; Koudelkova, E.; Liu, L.; Shrestha, K.; Lvov, Y.; Hill, J.P.; Ariga, K.; Abe, H. Activated interiors of clay nanotubes for agglomeration-tolerant automotive exhaust remediation. J. Mater. Chem. A 2015, 3, 6614–6619. [Google Scholar] [CrossRef]
- Das, S.; Jana, S. A tubular nanoreactor directing the formation of in situ iron oxide nanorods with superior photocatalytic activity. Environ. Sci. Nano 2017, 4, 596–603. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Guan, H.; Zhao, Y.; Yang, J.; Zhang, B. Preparation of bimetallic Cu-Co nanocatalysts on poly (diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2018, 427, 106–113. [Google Scholar] [CrossRef]
- Yuan, P.; Peter, D.S.; Liu, Z.; Green, M.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Koh, K.; Jeon, M.; Yoon, C.W.; Asefa, T. Formic acid dehydrogenation over Pd NPs supported on amine-functionalized SBA-15 catalysts: Structure-activity relationships. J. Mater. Chem. A 2017, 5, 16150–16161. [Google Scholar] [CrossRef]
- Koh, K.; Seo, J.E.; Lee, J.H.; Goswami, A.; Yoon, C.W.; Asefa, T. Untrasmall palladium nanoparticles supported on amine-functionalized SBA-15 efficiently catalyze hydrogen evolution from formic acid. J. Mater. Chem. A 2014, 2, 20444–20449. [Google Scholar] [CrossRef]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd–Ag Nanoparticles within a Macroreticular Basic Resin: An Efficient Catalyst for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Kepert, C.J. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release. Nanotechnology 2012, 23, 375705. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, B.; Jiang, K.; Cai, W. Surfactant-Free Synthesis of Carbon-Supported Palladium Nanoparticles and Size-Dependent Hydrogen Production from Formic Acid-Formate Solution. ACS Appl. Mater. Interfaces 2017, 9, 24678–24687. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline cobalt–nickel–boron (metal boride) catalysts for efficient hydrogen production from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Ngnie, G.; Detallier, C. PdNP Decoration of Halloysite Lumen via Selective Grafting of Ionic Liquid onto the Aluminol Surfaces and Catalytic Application. ACS Appl. Mater. Interfaces 2016, 8, 4862–4869. [Google Scholar] [CrossRef]
- Pena-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of γ-Aminopropyltriethoxysilane by FT-IR spectroscopy. J. Mater. Sci. 2007, 42, 595–603. [Google Scholar] [CrossRef]
- Yang, J.; Stargent, E.; Kelley, S.; Ying, J.Y. A general phase-transfer protocol for metal ions and its application in nanocrystal synthesis. Nat. Mater. 2009, 8, 683–689. [Google Scholar] [CrossRef]
- Dong, C.; Gao, Z.; Li, Y.; Peng, M.; Wang, M.; Xu, Y.; Li, C.; Xu, M.; Deng, Y.; Qin, X.; et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 2022, 5, 485–493. [Google Scholar] [CrossRef]
- Hu, C.; Pulleri, J.K.; Ting, S.W.; Chan, K. Activity of Pd/C for hydrogen generation in aqueous formic acid solution. Inter. J. Hydrogen Energy 2014, 39, 381–390. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, W.; Yang, W.; Li, Q. Formic acid as the in-situ hydrogen source for catalytic reduction of nitrate in water by PdAg alloy nanoparticles supported on amine-functionalized SiO2. Appl. Catal. B Environ. 2017, 203, 372–380. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Bai, R.; Li, X.; Guo, G.; Yu, J. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, X.; Liu, P.; Zhang, H.; Ma, L.; Su, H. Achieving efficient room-temperature catalytic H2 evolution from formic acid through atomically controlling the chemical environment of bimetallic nanoparticles immobilized by isoreticular amine-functionalized metal-organic frameworks. Appl. Catal. B Environ. 2017, 45, 1953–1958. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, J.; Kim, J.Y.; Nam, S.W.; Han, J.H.; Lim, T.H.; Gautam, S.; Chae, K.H.; Yoon, C.W. Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride. J. Mater. Chem. A 2014, 2, 9490–9495. [Google Scholar] [CrossRef]
- Lee, D.W.; Jin, M.H.; Park, J.H.; Lee, Y.J.; Choi, Y.C.; Chan Park, J.; Chun, D.H. Alcohol and water free synthesis of mesoporous silica using deep eutectic solvent as a template and solvent and its application as a catalyst support for formic acid dehydrogenation. ACS Sustain. Chem. Eng. 2018, 6, 12241–12250. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, H.; Cao, W.; Fu, H.; Wan, H.; Xu, Z.; Zheng, S. Ultrafine Pd particles embedded in nitrogen-enriched mesoporous carbon for efficient H2 production from formic acid decomposition. ACS Sustain. Chem. Eng. 2019, 7, 1963–1972. [Google Scholar] [CrossRef]


| Sample | SSA (m2·g−1) | Vp (cm3 ·g−1) | Particle * Size (nm) | Content (wt.%) | ||
|---|---|---|---|---|---|---|
| Pd | Au | Ag | ||||
| HNTs | 62.3 | 0.41 | —— | —— | ||
| NH2-HNTs | 34.8 | 0.49 | —— | —— | ||
| Pd/HNTs | 55.9 | 0.40 | 3.5 | 0.60 | —— | —— |
| Pd/NH2-HNTs | 54.9 | 0.45 | 1.2 | 2.50 | —— | —— |
| PdAu/NH2-HNTs | 50.2 | 0.51 | —— | 1.31 | 1.31 | —— |
| PdAg/NH2-HNTs | 51.1 | 0.55 | —— | 1.30 | —— | 1.20 |
| Sample | Pd5/2 | Pd3/2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pd2+ | Pd0 | Pd2+ | Pd0 | Pd2+/Pd0 | |||||
| BE | % | BE | % | BE | % | BE | % | ||
| Pd/NH2-HNTs | 338.0 | 23.6 | 335.8 | 36.3 | 343.3 | 15.8 | 341.0 | 24.3 | 0.65 |
| Pd/HNTs | 337.2 | 21.4 | 335.2 | 38.5 | 342.2 | 14.3 | 340.4 | 25.8 | 0.55 |
| Entry | Catalysts | Pd (wt.%) | V b (mL) | TOF (h−1) Initial c |
|---|---|---|---|---|
| 1 | Pd/HNTs | 0.60 | 16 | 126.2 |
| 2 | Pd/HNTs | 1.28 | 47.6 | 136.0 |
| 3 | Pd/NH2-HNTs | 2.50 | 154.5 | 412.9 |
| 4 | Pd/NH2-HNTs | 1.30 | 84.6 | 439.2 |
| 5 | PdAu/NH2-HNTs | 1.31 | 133.9 | 701.6 |
| 6 | PdAg/NH2-HNTs | 1.30 | 65 | 370.3 |
| 7 | Au/NH2-HNTs | —— | 0 | 0 |
| 8 | Ag/NH2-HNTs | —— | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Tan, K.; Ye, Y.; Zhu, B.; Zhang, S.; Huang, W. Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid. Nanomaterials 2022, 12, 2414. https://doi.org/10.3390/nano12142414
Song L, Tan K, Ye Y, Zhu B, Zhang S, Huang W. Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid. Nanomaterials. 2022; 12(14):2414. https://doi.org/10.3390/nano12142414
Chicago/Turabian StyleSong, Limin, Kaiyuan Tan, Yingyue Ye, Baolin Zhu, Shoumin Zhang, and Weiping Huang. 2022. "Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid" Nanomaterials 12, no. 14: 2414. https://doi.org/10.3390/nano12142414
APA StyleSong, L., Tan, K., Ye, Y., Zhu, B., Zhang, S., & Huang, W. (2022). Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid. Nanomaterials, 12(14), 2414. https://doi.org/10.3390/nano12142414






