Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Fruit Extract
2.3. Total Phenolic Content Evaluation
2.4. Synthesis of Silver Nanoparticles Using Viburnum opulus Fruit Extract
2.5. Characterization of Silver Nanoparticles
2.6. Catalytic Activity of Silver Nanoparticles
3. Results and Discussion
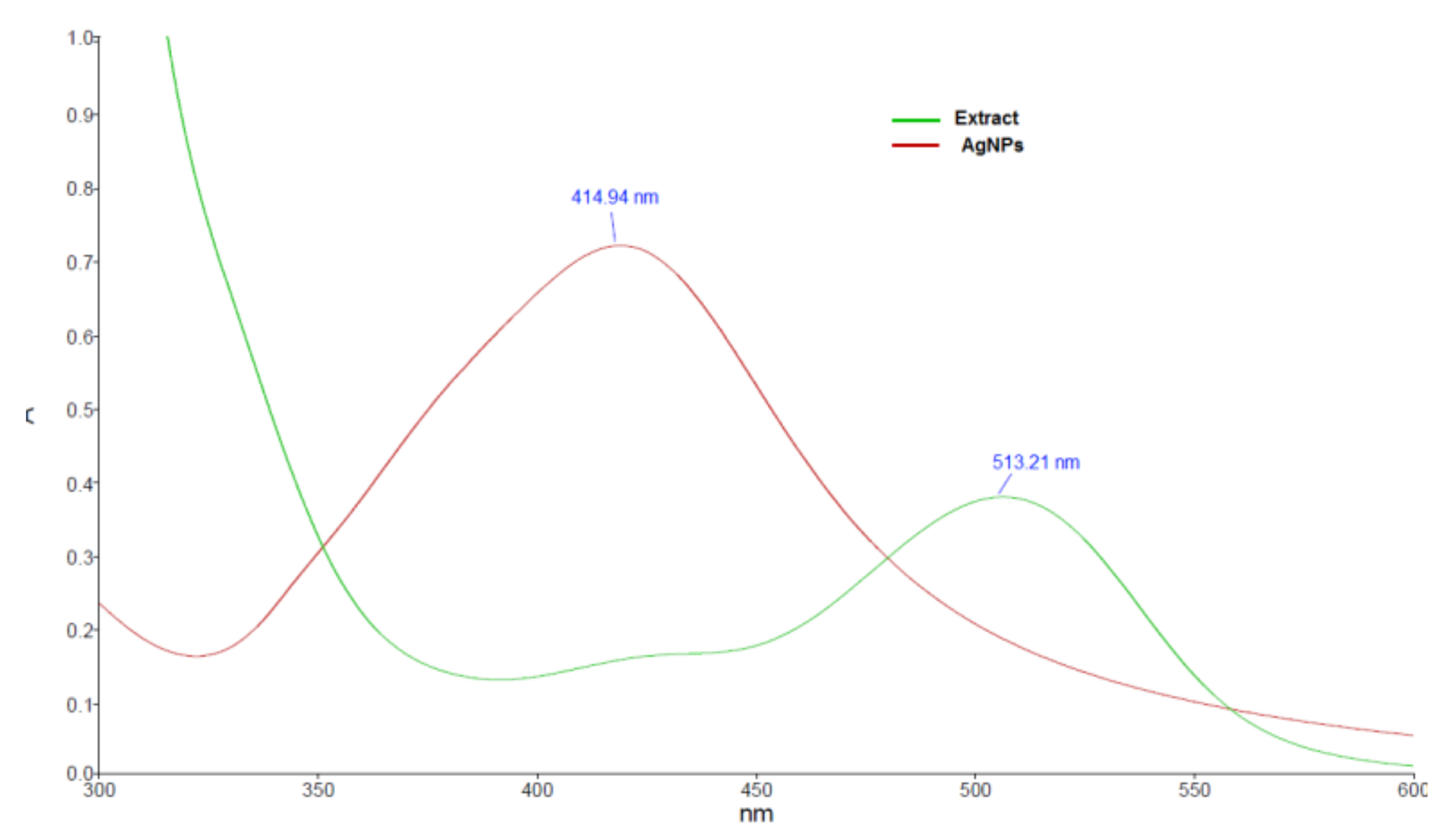
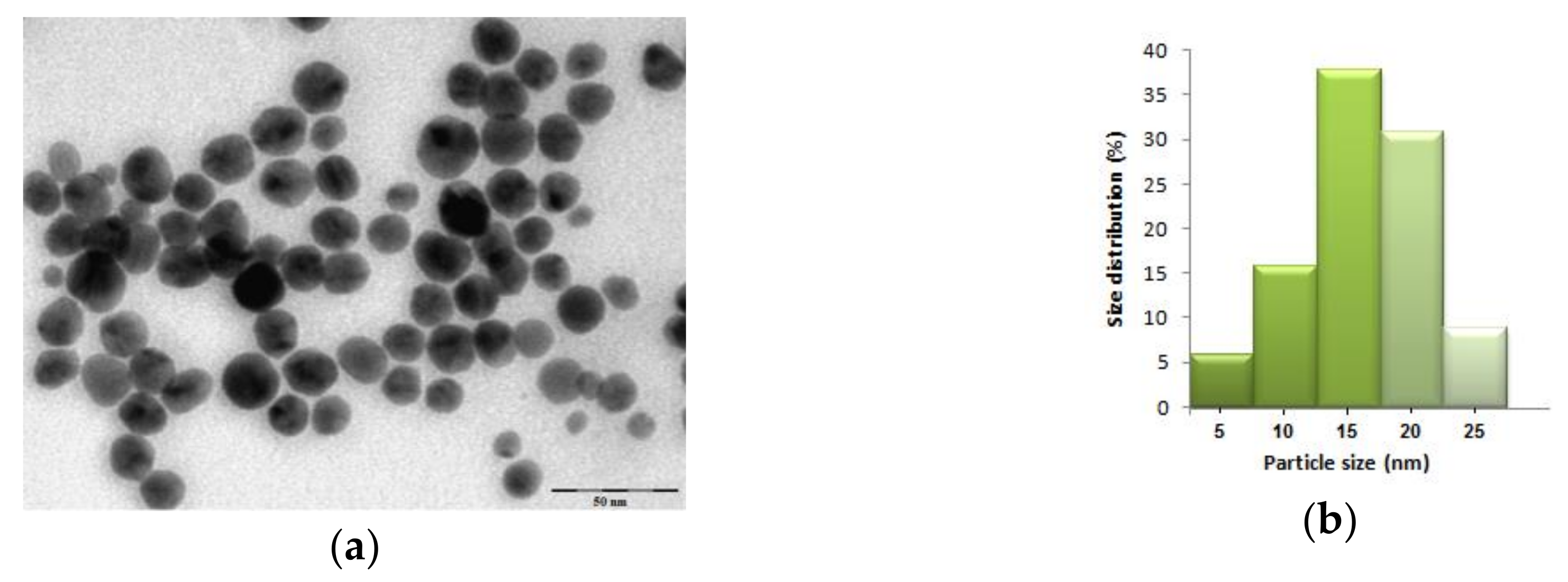
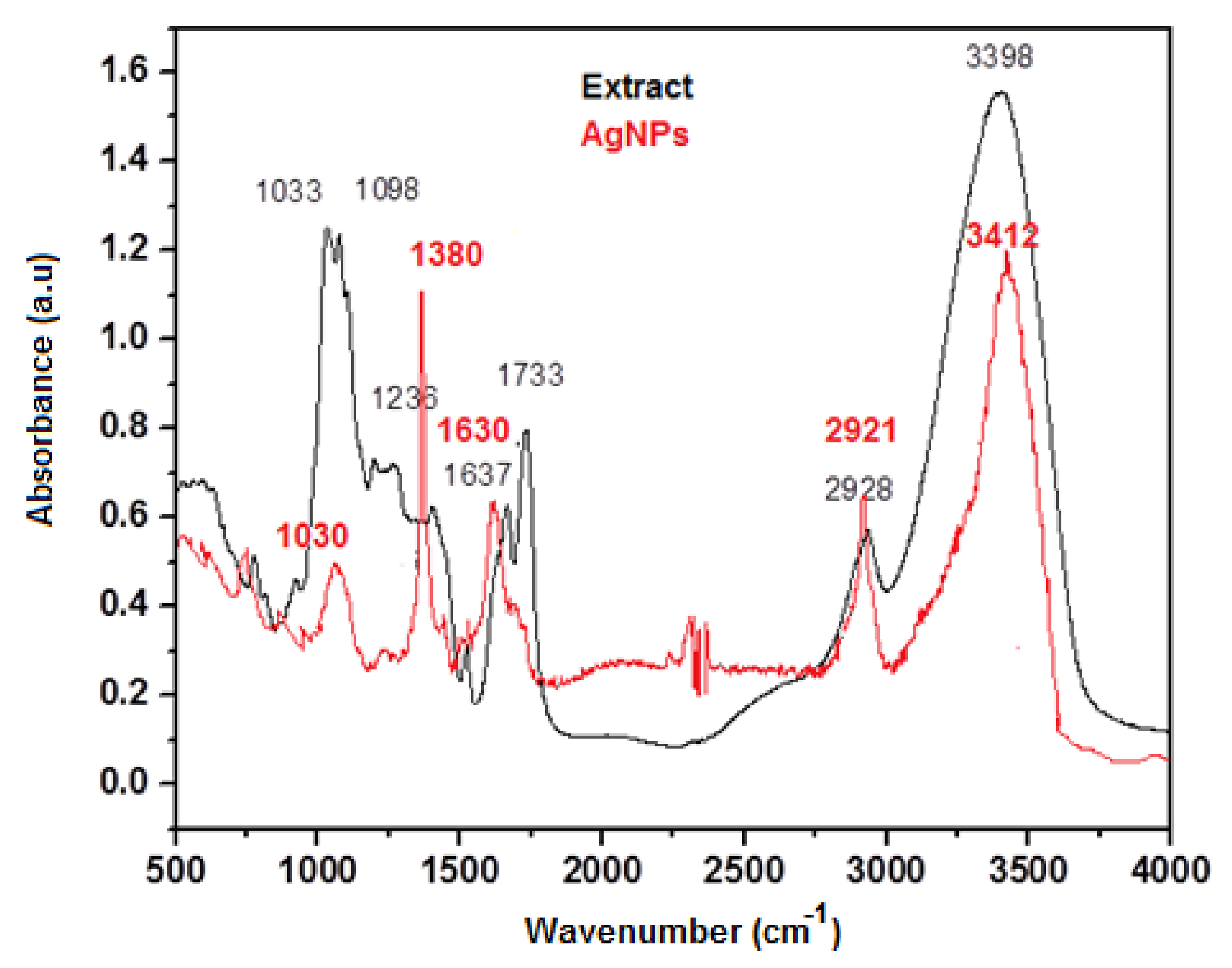
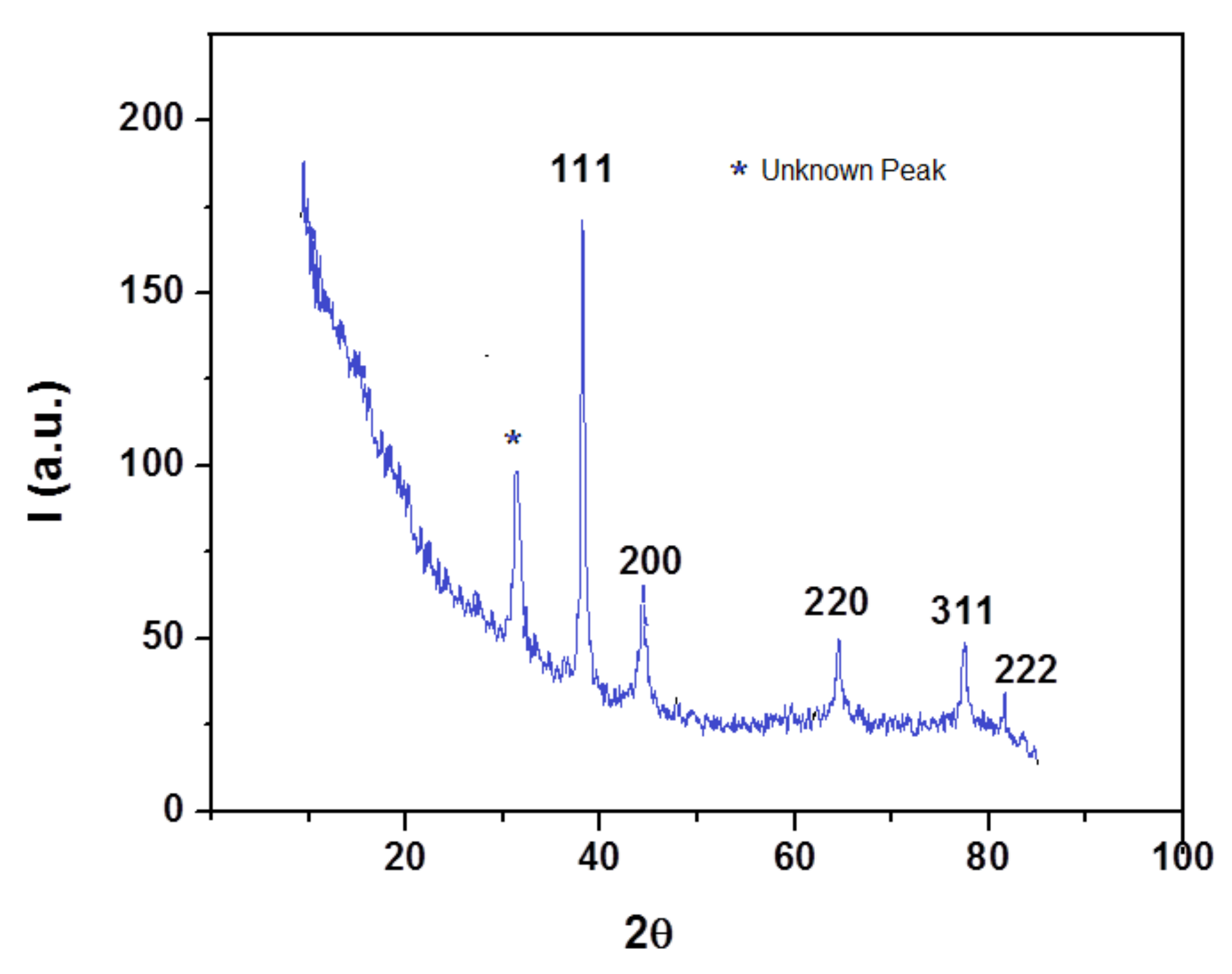
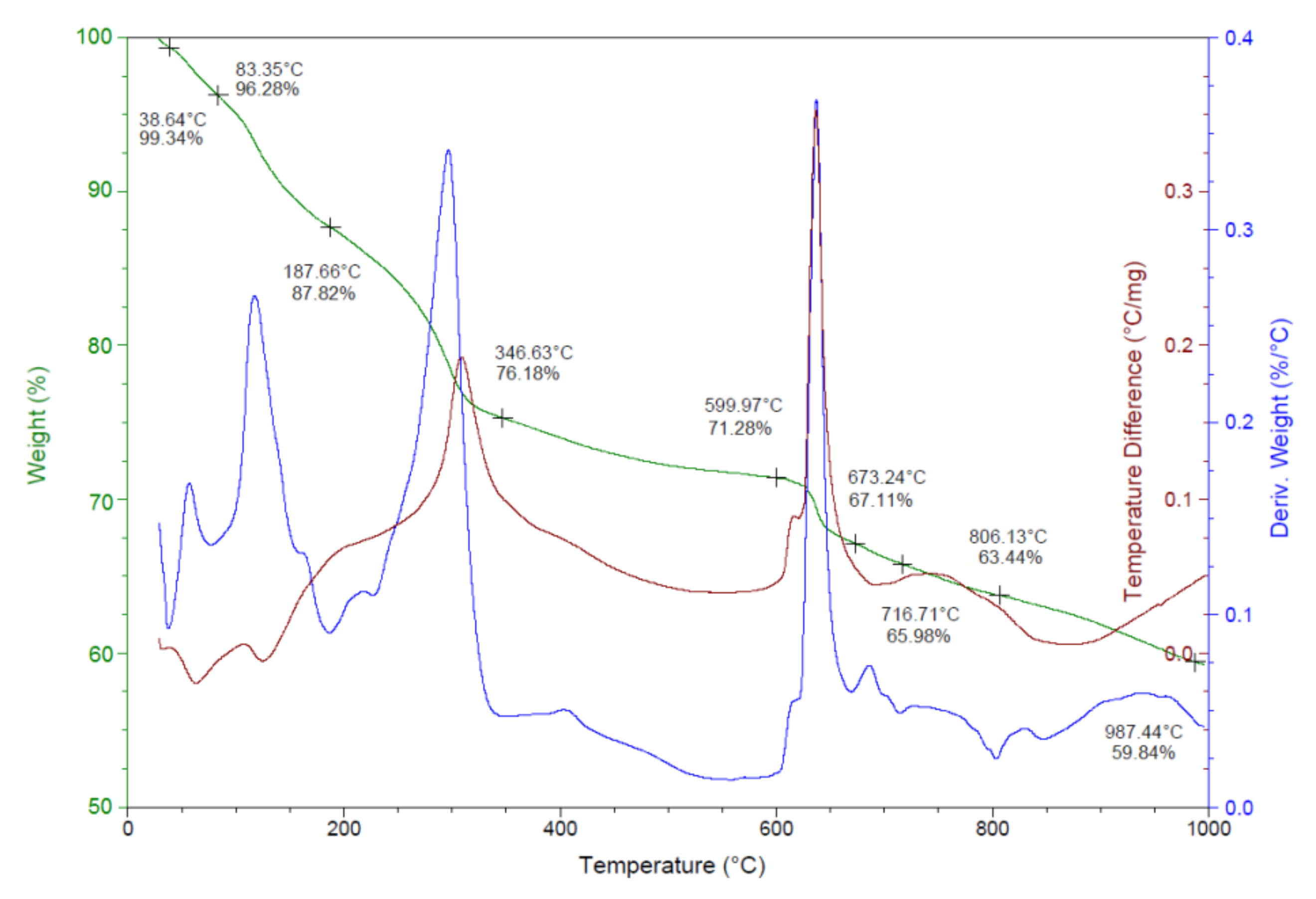
3.1. Synthesis and Characterization of Silver Nanoparticles
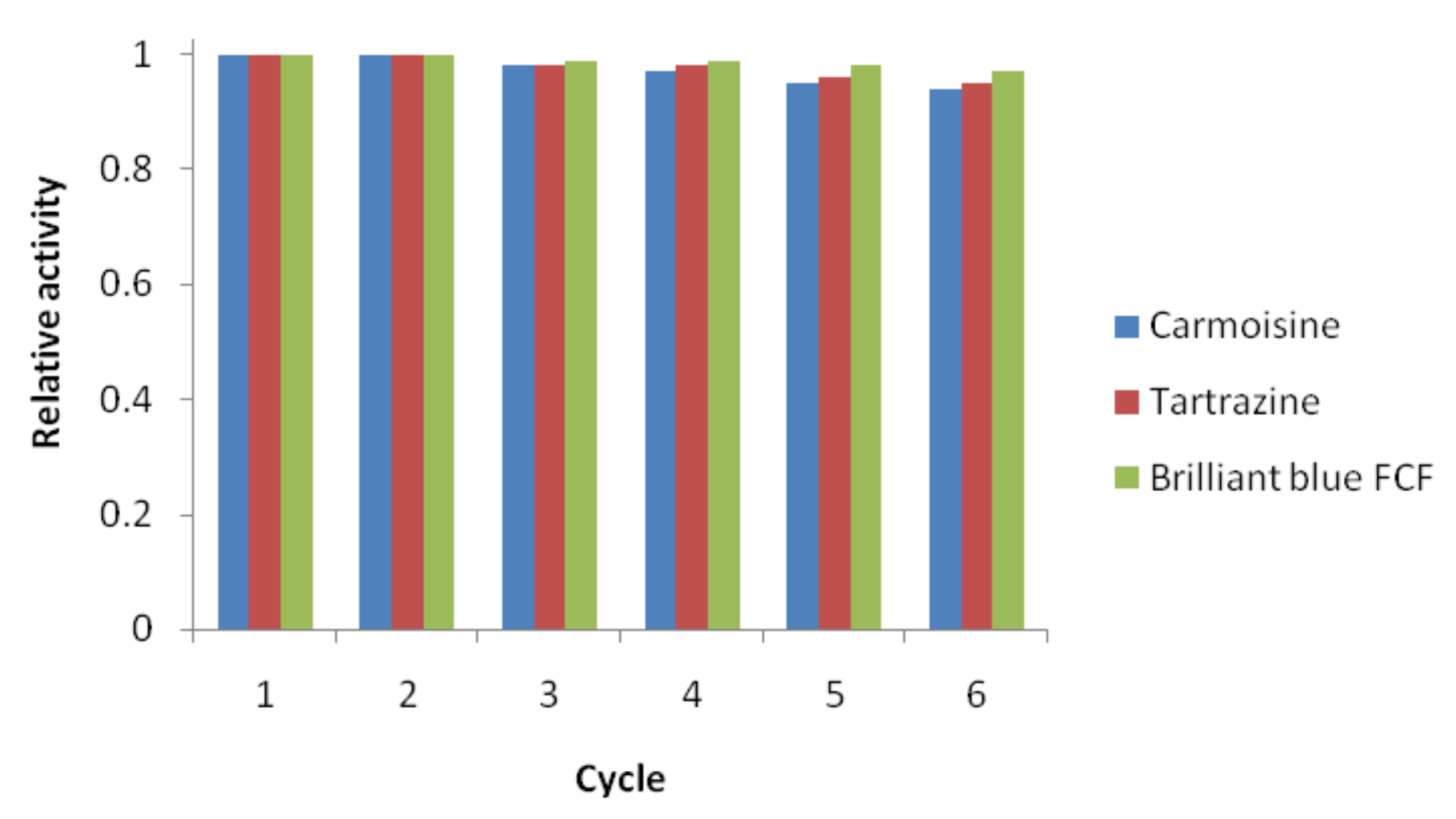
3.2. Dye Reducing Catalytic Activity of AgNPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Curtis, A.; Wilkinson, C. Nanotechniquesand approaches in biotechnology. Trends Biotechnol. 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their anti-inflammatory activity. J. Photochem. Photobiol. B 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Danila, O.; Berghian-Sevastre, A.; Dionisie, V.; Gheban, D.; Olteanu, D.; Tabaran, F.; Baldea, I.; Katona, G.; Moldovan, B.; Clichici, S.; et al. The effects of silver nanoparticles on behavior, apoptosisand nitro-oxidative stress in offspring Wistar rats. Nanomedicine 2017, 12, 1455–1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Vizuete, K.S.; Sharma, V.; Debut, A.; Cumbal, L. Ecofriendly synthesis of monodispersed silver nanoparticles using Andean mortiñoberry as reductantand its photocatalytic activity. Vacuum 2019, 160, 272–278. [Google Scholar] [CrossRef]
- Filip, A.G.; Potara, M.; Florea, A.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; David, L.; Moldovan, B.; Olenic, L.; et al. Comparative evaluation by scanning confocal Raman spectroscopy and transmission electron microscopy of therapeutic effects of noble metal nanoparticles in experimental acute inflammation. RSC Adv. 2015, 5, 67435–67448. [Google Scholar] [CrossRef]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosynthesized gold nanoparticles on diabetic rats. Coll. Surf. B 2017, 150, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Opris, R.; Toma, V.; Olteanu, D.; Baldea, I.; Baciu, A.; Imre-Lucaci, F.; Berghian Sevastre, A.; Tatomir, C.; Moldovan, B.; Clichici, S.; et al. Effects of silver nanoparticles functionalized with Cornus mas L. extract on architecture and apoptosis in rat testicle. Nanomedicine 2019, 14, 275–299. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataram, D.; Vilai, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus Licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.-K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolummajus L. and its enhanced in vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B 2019, 191, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; Sincari, V.; Perde-Schrepler, M.; David, L. Biosynthesis of silver nanoparticles using Ligustrum ovalifolium fruits and their cytotoxic effects. Nanomaterials 2018, 8, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldovan, B.; Ghic, O.; David, L.; Chişbora, C. The influence of storage on the total phenols content and antioxidant activity of the Cranberrybush (Viburnum opulus L.) fruits extract. Rev. Chim. Buchar. 2012, 63, 463–464. [Google Scholar]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Česoniene, L.; Daubaras, R. Antioxidant properties and phenolic compositions of fruits from different European cranberry bush (Viburnum opulus L.) genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Ekici, L.; Poyrazoglu, E.S. Phenolic composition of European cranberrybush (Viburnum opulus L.) berries and astringency removal of its commercial juice. Int. J. Food Sci. Technol. 2006, 41, 1011–1015. [Google Scholar] [CrossRef]
- Rop, O.; Resnicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var. Edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar] [CrossRef]
- Česoniene, L.; Daubaras, R.; Viškelis, P.; Šarkinas, A. Determination of the total phenolic and anthocyanin contents and antimicrobial activity of Viburnum opulus fruit juice. Plant Foods Hum. Nutr. 2012, 67, 256–261. [Google Scholar] [CrossRef]
- Ersoy, N.; Ercisli, S.; Gundogdu, M. Evaluation of European cranberrybush (Viburnum opulus L.) genotypes from agro-morphological, biochemical and bioactive characteristics in Turkey. Folia Hor. 2017, 29, 181–188. [Google Scholar] [CrossRef] [Green Version]
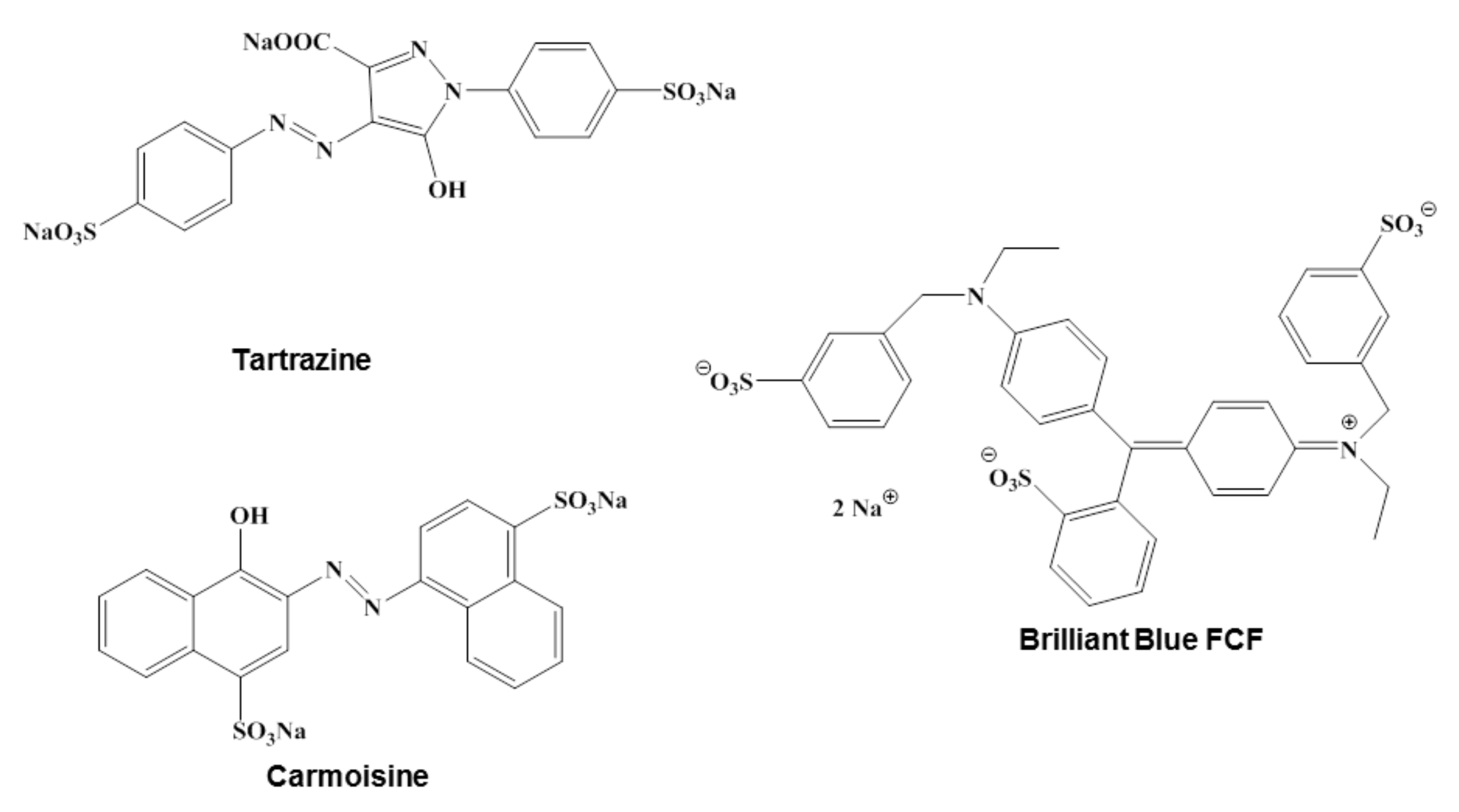
- Chung, K.T.; Cerniglia, C.E. Mutagenicity of azo dye: Structure-activity relationships. Mutat. Res. 1992, 277, 201–220. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.; Karmakar, S.; Arora, L.S.; Mishra, V. Ecotoxic potential of a presumably nontoxic azo dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. The mediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2011, 77, 247–255. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shriavastava, M. Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Han, F.; Kanbala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalyst for degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Phyto-synthesis of silver nanoparticles from Mussaendaerythophylla leaf extract and their application in catalytic degradation of methyl dye. J. Mol. Liq. 2016, 221, 1063–1070. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M.N. Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J. Photochem. Photobiol. B 2018, 181, 44–52. [Google Scholar] [CrossRef]
- Rupa, E.J.; Kaliraj, L.; Abin, S.; Yang, B.-C.; Jung, S.-K. Synthesis of a zinc oxide nanoflowerphotocatalyst from Sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles in the study on catalytic activity in the degradation of dyes. J. Mol. Liq. 2015, 204, 184–191. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Gen. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y. Reductive degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J. Photochem. Photobiol. B 2016, 162, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Borzelleca, J.F.; Hollazan, J.B. Chronic toxicity/carcinogenity studies of FD&C yellow no. 5 (tartrazine) in rats. Food Chem. Toxicol. 1988, 26, 175–190. [Google Scholar]
- Yousefi, M.; Ghanbari, F.; Zazouli, M.A.; Madihi-Bidgoli, S. Brilliant blue FCF degradation by persulfate/zero valent iron: The effect of influencing parameters and anions. Desalin. Water Treat. 2017, 70, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteureagent. Met. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Konarska, A.; Domaciuk, M. Differences in the fruit structure and the location and content of bioactive substances in Viburnum opulus and Viburnum lantana fruits. Protoplasma 2018, 255, 25–41. [Google Scholar] [CrossRef]
- Zaklos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziolkiewicz, M.; Podsedek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Chand, K.; Abro, M.I.; Aftab, U.; Shah, A.H.; Lakhan, M.N.; Cao, D.; Mehdi, G.; Mohamed, A.M.A. Green synthesis, characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extract of neem, onion and tomato. RSC Adv. 2019, 9, 17002–17015. [Google Scholar] [CrossRef] [Green Version]
- Ajidha, P.; Reddy, Y.A.K.; Reddy, P.S.; Suneetha, Y.; Jeon, H.-J.; Ahn, C.W. Instant biosynthesis of silver nanoparticles using Lawsonia inermis leaf extract: Innate catalytic, antimicrobial and antioxidant activity. J. Mol. Liq. 2016, 219, 474–481. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Coll. Surf. A 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Yallapa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au-Ag alloy nanoparticles using J. Sambac. leaves extract and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim. Acta A 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver nanoparticles mediated by Costusafer leaf extract: Synthesis, antibacterial, antioxidant and electrochemical properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Singh, H.P.; Sharma, R.K. Metal nanoparticles with high catalytic activity in degradation of methyl orange: An electron relay effect. J. Mol. Catal. A 2011, 335, 248–252. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalized redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Raja, K.; Chandra, V.K.; Sadras, S.R. Green synthesized silver nanoparticles: Catalytic dye degradation, in vitro anticancer activity and in vivo toxicity in rats. Mater. Sci. Eng. C 2018, 91, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Bello, B.A.; Khan, J.A.; Anwar, Y.; Mirza, M.B.; Qadri, F.; Farook, A.; Kadam, I.K.; Asiri, A.M.; Khan, S.B. Albizia chevalier based Ag nanoparticles: Antiproliferation, bactericidal and pollutants degradation performance. J. Photochem. Photobiol. B 2018, 182, 62–70. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H.; Arami, M.; Pajootan, E. Photocatalytic discoloration of Acid Red 14 aqueous solution using titania nanoparticles immobilized on graphene oxide fabricated plate. Chemosphere 2016, 159, 293–299. [Google Scholar] [CrossRef]
- Scott, R.; Mudimbi, P.; Miller, M.E.; Magnuson, M.; Willison, S.; Phillips, R.; Harper, W.F. Advanced oxidation of Tartrazine and Brilliant Blue with pulsed ultraviolet light emitting diodes. Water Environ. Res. 2017, 89, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Shahmoradi, B.; Maleki, A.; Byrappa, K. Photocatalytic degradation of Amaranth and Brilliant Blue FCF dyes using in situ modified tungsten doped TiO2 hybrid nanoparticles. Catal. Sci. Technol. 2011, 1, 1216–1223. [Google Scholar] [CrossRef]
- Shahmoradi, B.; Namratha, K.; Byrappa, K.; Soga, K.; Ananda, S.; Somashekar, R. Enhancement of the photocatalytic activity of modified ZnO nanoparticles with manganese additive. Res. Chem. Itermed. 2011, 37, 329–340. [Google Scholar] [CrossRef]
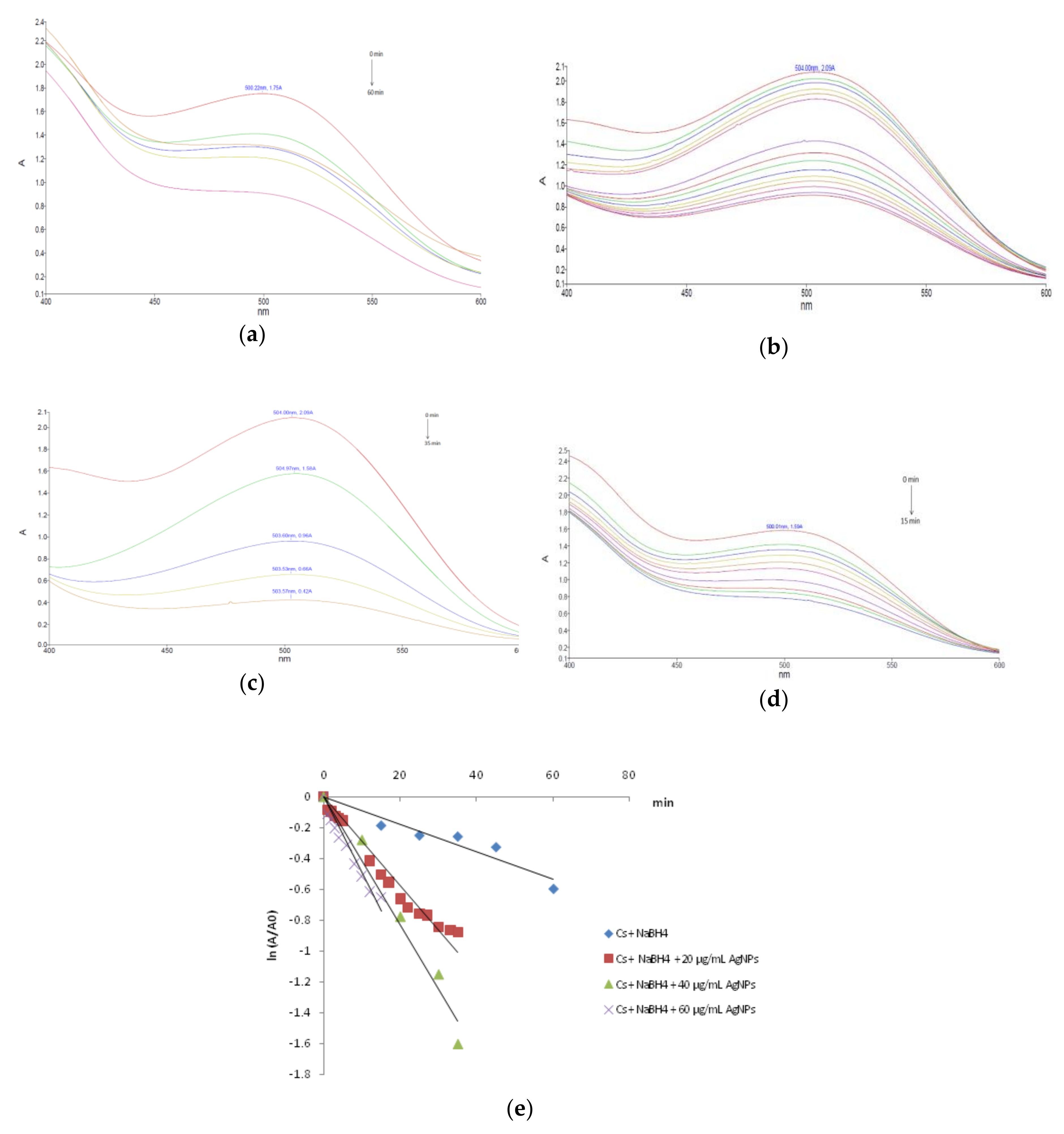
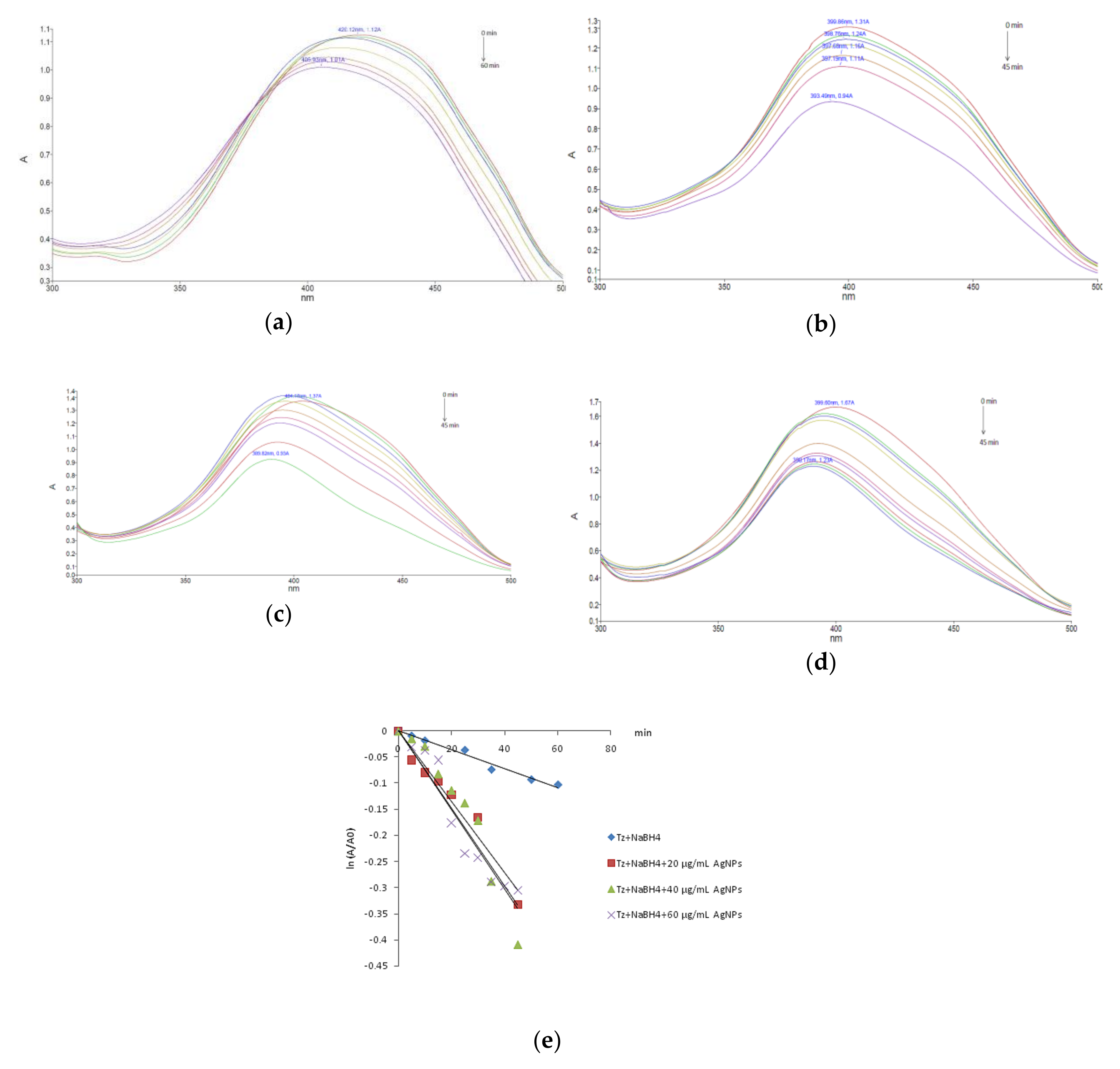
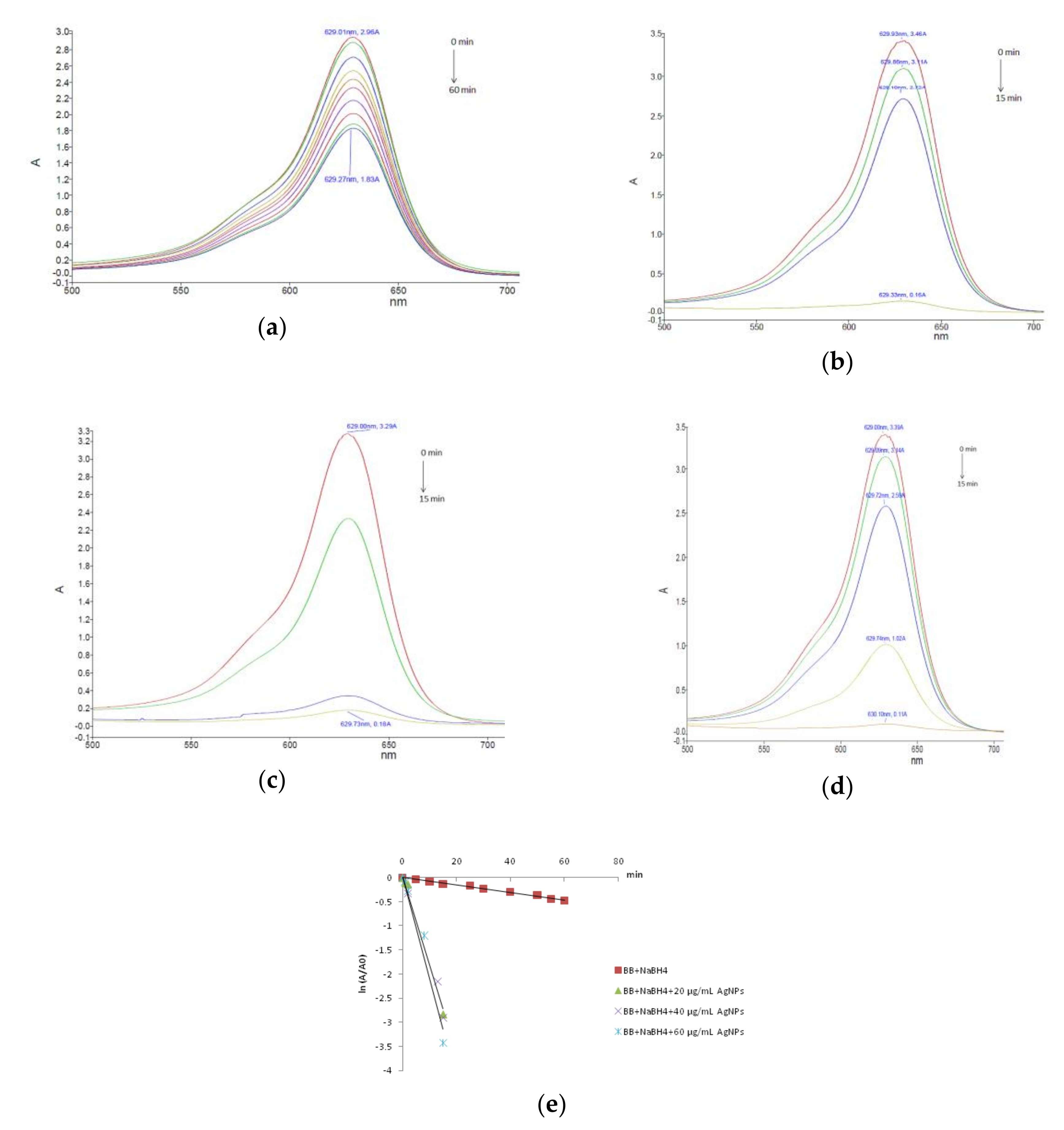
| Colorant/Catalyst Concentration | k (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| Brilliant Blue | |||
| No catalyst | 0.0077 | 90.00 | 0.988 |
| 20 µg/mL AgNPs | 0.1817 | 3.81 | 0.987 |
| 40 µg/mL AgNPs | 0.1864 | 3.71 | 0.987 |
| 60 µg/mL AgNPs | 0.2097 | 3.30 | 0.958 |
| Tartrazine | |||
| No catalyst | 0.0018 | 385 | 0.978 |
| 20 µg/mL AgNPs | 0.0068 | 101.92 | 0.956 |
| 40 µg/mL AgNPs | 0.0071 | 93.6 | 0.891 |
| 60 µg/mL AgNPs | 0.0076 | 91.18 | 0.929 |
| Carmoisine | |||
| No catalyst | 0.0090 | 77 | 0.916 |
| 20 µg/mL AgNPs | 0.0287 | 24.14 | 0.961 |
| 40 µg/mL AgNPs | 0.0416 | 16.81 | 0.969 |
| 60 µg/mL AgNPs | 0.0496 | 13.97 | 0.943 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. https://doi.org/10.3390/nano10020202
David L, Moldovan B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials. 2020; 10(2):202. https://doi.org/10.3390/nano10020202
Chicago/Turabian StyleDavid, Luminita, and Bianca Moldovan. 2020. "Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes" Nanomaterials 10, no. 2: 202. https://doi.org/10.3390/nano10020202




