Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the α-Ferrous Oxalate Dihydrate
2.2. Adsorption, Photocatalysis, and Photolysis
2.3. Effect of the Phenol Initial Concentration
2.4. Effect of the Solution pH
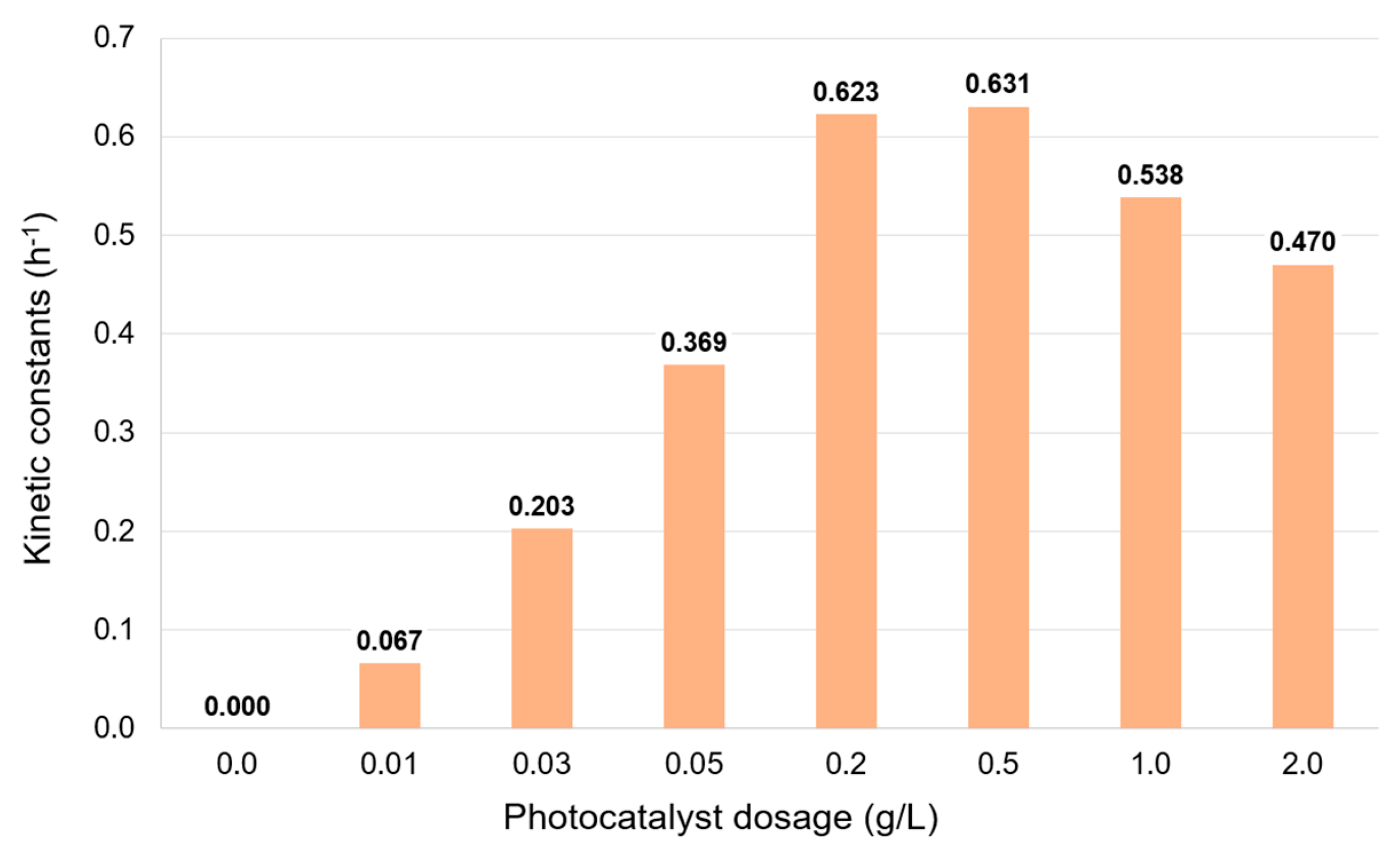
2.5. Effect of the Photocatalyst Dosage
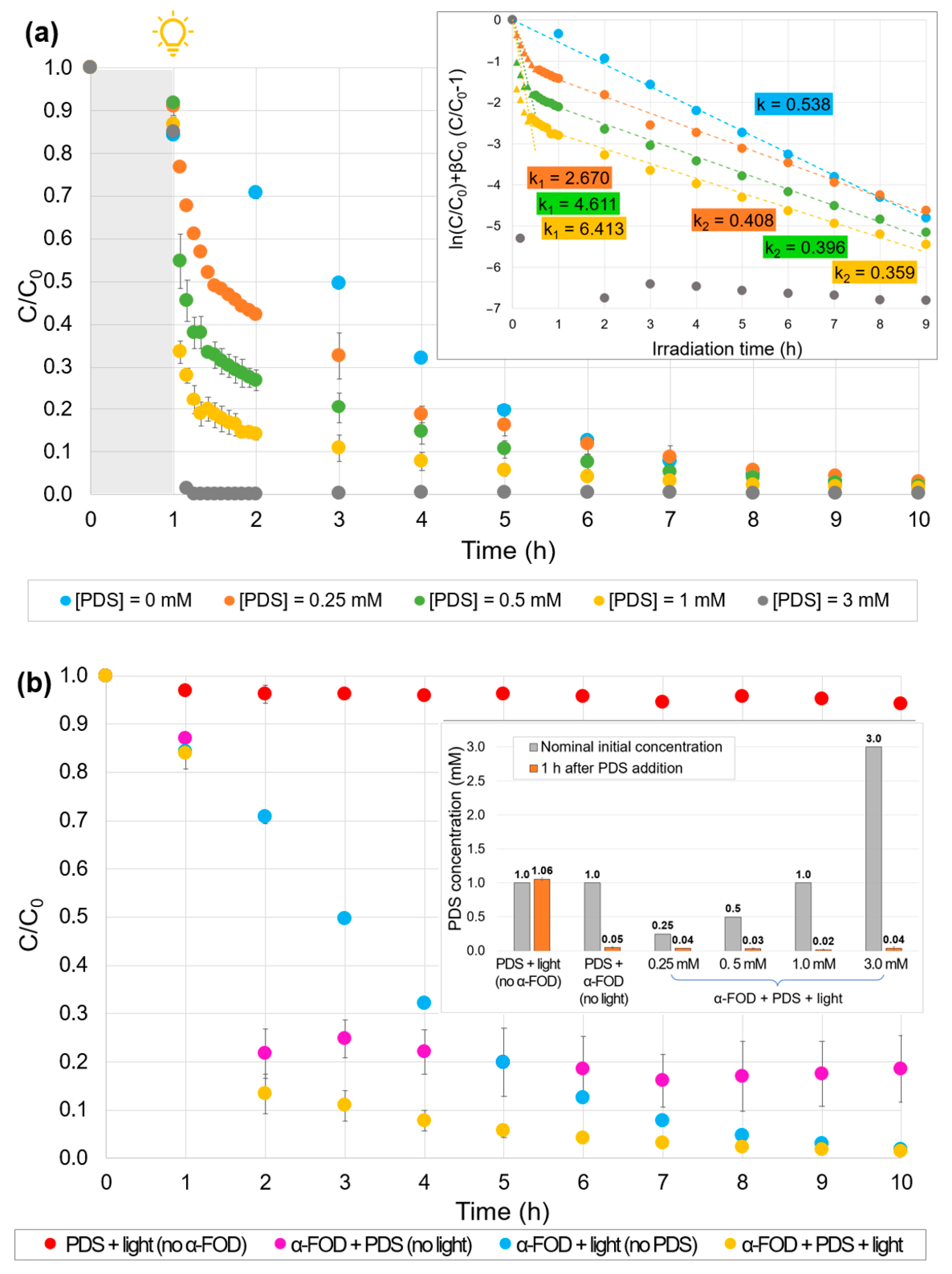
2.6. Effect of the Addition of PDS
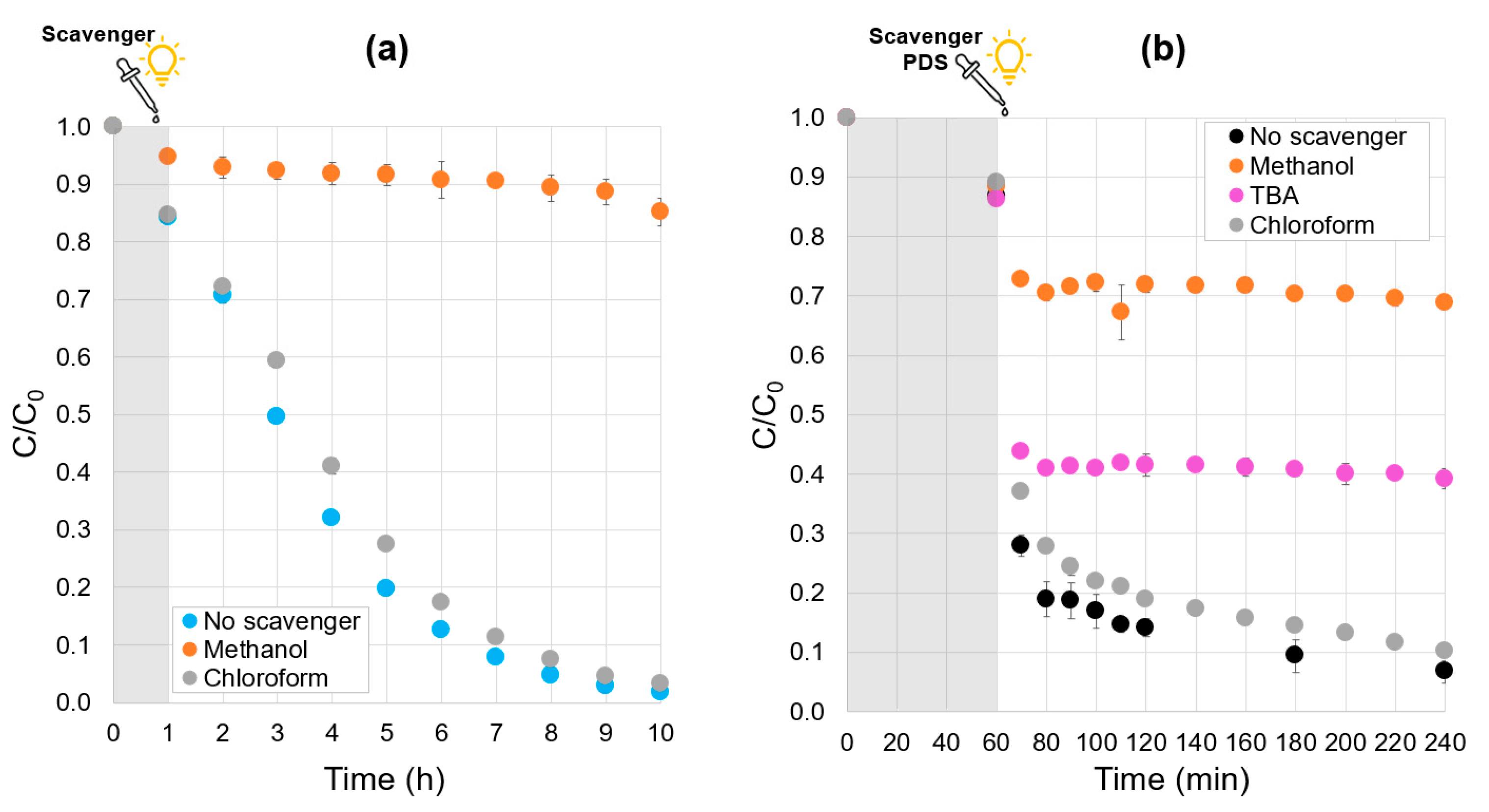
2.7. Quenching Tests
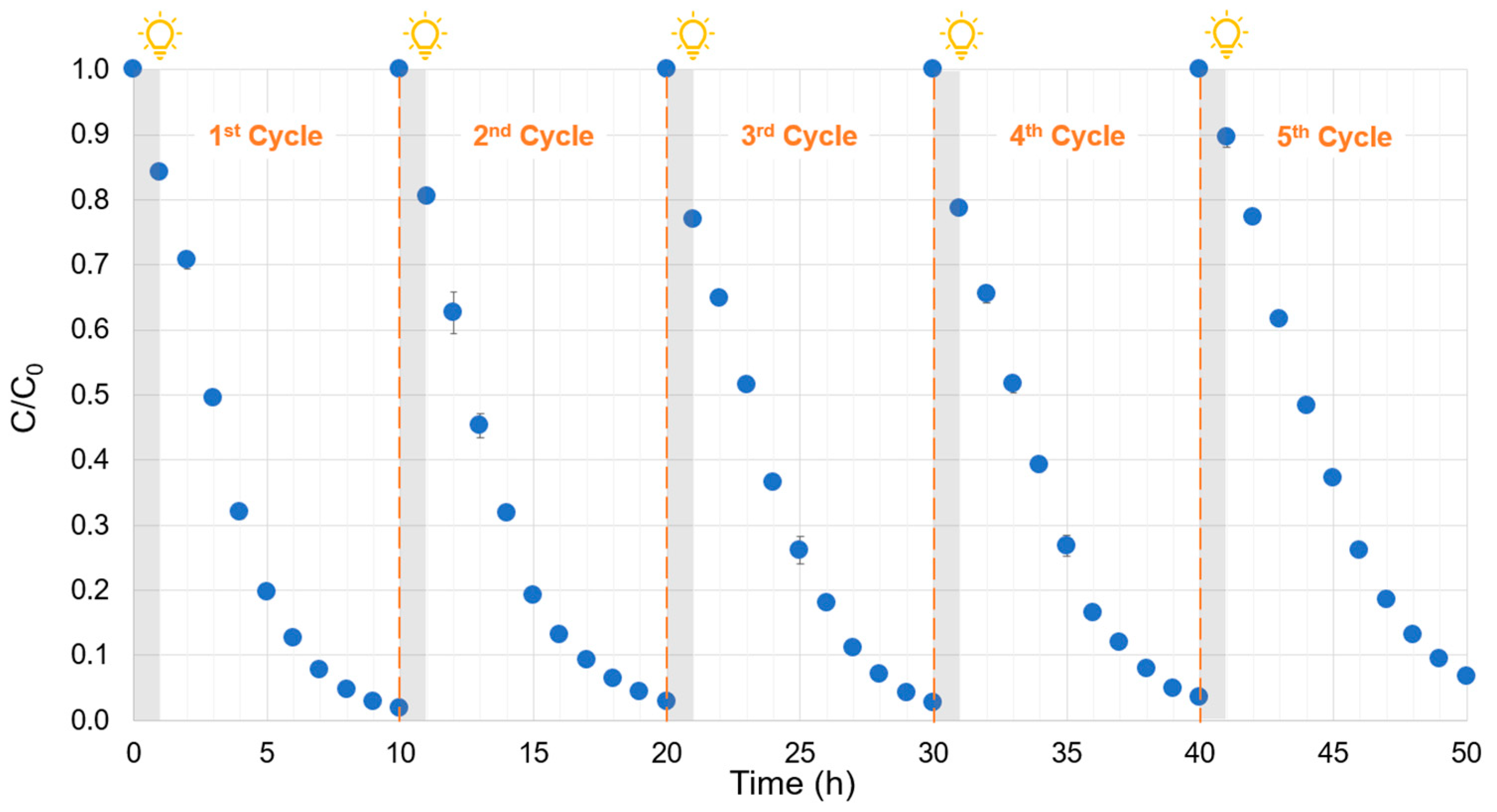
2.8. Photocatalytic Cycling Tests
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Photocatalyst
3.3. Characterization of the Photocatalyst
3.4. Point of Zero Charge
3.5. Phenol Removal Tests
3.5.1. Adsorption, Photolysis, and Photocatalysis
3.5.2. Effects of the Initial Phenol Concentration, Solution pH, and Photocatalyst Dosage
3.5.3. Effect of the Addition of PDS
3.5.4. Quenching Tests
3.5.5. Photocatalytic Cycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bustillo-Lecompte, C. Advanced Oxidation Processes—Applications, Trends, and Prospects, 1st ed.; Intech Open: London, UK, 2020; ISBN 9781789848908. [Google Scholar]
- Khare, P.; Patel, R.K.; Sharan, S.; Shankar, R. Chapter 08. Recent trends in advanced oxidation process for treatment of recalcitrant industrial effluents. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 137–160. ISBN 9780128210116. [Google Scholar]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Primo, A.; García, H. Chapter 06. Solar Photocatalysis for Environment Remediation. In New and Future Developments in Catalysis: Solar Photocatalysis; Elsevier B.V.: Amsterdam, The Netherlands, 2013; pp. 145–165. ISBN 9780444538727. [Google Scholar]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Li, M.; Wang, M.; Zhou, X.; Cheng, R.; Zhou, Y.; Shi, J. α-Ferrous oxalate dihydrate: A simple coordination polymer featuring photocatalytic and photo-initiated Fenton oxidations. Sci. China Mater. 2016, 59, 574–580. [Google Scholar] [CrossRef]
- Fu, L.; Huang, Z.; Zhou, X.; Deng, L.; Liao, M.; Yang, S.; Chen, S.; Wang, H.; Wang, L. Ferrous-Oxalate-Modified Aramid Nanofibers Heterogeneous Fenton Catalyst for Methylene Blue Degradation. Polymers 2022, 14, 3491. [Google Scholar] [CrossRef]
- Sarmah, K.; Pratihar, S. Synthesis, Characterization, and Photocatalytic Application of Iron Oxalate Capped Fe, Fe-Cu, Fe-Co, and Fe-Mn Oxide Nanomaterial. ACS Sustain. Chem. Eng. 2017, 5, 310–324. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- John, D.; Jose, J.; Bhat, S.G.; Achari, V.S. Integration of heterogeneous photocatalysis and persulfate based oxidation using TiO2-reduced graphene oxide for water decontamination and disinfection. Heliyon 2021, 7, e07451. [Google Scholar] [CrossRef]
- Galeas, S.; Valdivieso-Ramírez, C.S.; Pontón, P.I.; Guerrero, V.H.; Goetz, V. Photocatalytic degradation of phenol under visible light irradiation by using ferrous oxalate synthesized from iron-rich mineral sands via a green hydrothermal route. Environ. Technol. Innov. 2023, 32, 103325. [Google Scholar] [CrossRef]
- Allen, T. Powder Sampling and Particle Size Determination; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 1–660. ISBN 978-0-444-51564-3. [Google Scholar]
- Ogasawara, H.; Koga, N. Kinetic modeling for thermal dehydration of ferrous oxalate dihydrate polymorphs: A combined model for induction period-surface reaction-phase boundary reaction. J. Phys. Chem. A 2014, 118, 2401–2412. [Google Scholar] [CrossRef]
- Zelenskaya, M.S.; Izatulina, A.R.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. Iron oxalate humboldtine crystallization by fungus aspergillus niger. Crystals 2021, 11, 1591. [Google Scholar] [CrossRef]
- Dhal, J.P.; Mishra, B.G.; Hota, G. Ferrous oxalate, maghemite and hematite nanorods as efficient adsorbents for decontamination of Congo red dye from aqueous system. Int. J. Environ. Sci. Technol. 2015, 12, 1845–1856. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Vieira, Y.; da Boit Martinello, K.; Ribeiro, T.H.; Silveira, J.P.; Salla, J.S.; Silva, L.F.O.; Foletto, E.L.; Dotto, G.L. Photo-assisted degradation of organic pollutant by CuFeS2 powder in RGB-LED reactors: A comprehensive study of band gap values and the relation between wavelength and electron-hole recombination. Adv. Powder Technol. 2022, 33, 103368. [Google Scholar] [CrossRef]
- Conde-Morales, I.I.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Hernández-Ramírez, A.; Sáenz-Tavera, I.d.C.; Villanueva-Rodríguez, M. Different Iron Oxalate Sources as Catalysts on Pyrazinamide Degradation by the Photo-Fenton Process at Different pH Values. Water. Air. Soil Pollut. 2020, 231, 425. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhang, J. Fenton-like reaction and photocatalysis using ferrous oxalate and G-C3 N4 enhancing reactive oxygen species for dye wastewater degradation under visible-light irradiation. Desalin. Water Treat. 2020, 193, 359–368. [Google Scholar] [CrossRef]
- Li, K.; Liang, Y.; Yang, J.; Yang, G.; Xu, R.; Xie, X. α-Ferrous oxalate dihydrate: An Fe-based one-dimensional metal organic framework with extraordinary photocatalytic and Fenton activities. Catal. Sci. Technol. 2018, 8, 6057–6061. [Google Scholar] [CrossRef]
- Chuaicham, C.; Shenoy, S.; Trakulmututa, J.; Balakumar, V.; Santawaja, P.; Kudo, S.; Sekar, K.; Sasaki, K. Preparation of Iron Oxalate from Iron Ore and Its Application in Photocatalytic Rhodamine B Degradation. Separations 2023, 10, 378. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Jian, Q.; Jin, Z. Ferrous oxalate dehydrate over CdS as Z-scheme photocatalytic hydrogen evolution. J. Solid State Chem. 2019, 274, 286–294. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, W.; Wang, Y.; Guo, M.L. Preparation of β-ferrous oxalate dihydrate layered nanosheets by mechanochemical method and its visible-light-driven photocatalytic performance. Mater. Lett. 2016, 178, 83–86. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Zhou, H.; Jin, P.; Cheung, K.M.C.; Chu, P.K.; Yeung, K.W.K. Vanadium Dioxide Nanocoating Induces Tumor Cell Death through Mitochondrial Electron Transport Chain Interruption. Glob. Chall. 2019, 3, 1800058. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xing, M.; Zhang, J.; Tian, B. Visible light activated sulfur and iron co-doped TiO2 photocatalyst for the photocatalytic degradation of phenol. Catal. Today 2013, 201, 159–166. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A.; Junk, P.C. High photodegradation efficiency of phenol by mixed-metal-organic frameworks. Inorg. Chem. Front. 2016, 3, 944–951. [Google Scholar] [CrossRef]
- Kosowska, B.; Mozia, S.; Morawski, A.W.; Grzmil, B.; Janus, M.; Kałucki, K. The preparation of TiO2-nitrogen doped by calcination of TiO2·xH2O under ammonia atmosphere for visible light photocatalysis. Sol. Energy Mater. Sol. Cells 2005, 88, 269–280. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafique, S.; Shafiq, I.; Akhter, P.; Belousov, A.S.; Show, P.L.; Park, Y.K.; Hussain, M. Mesoporous LaVO4/MCM-48 nanocomposite with visible-light-driven photocatalytic degradation of phenol in wastewater. Environ. Res. 2023, 218, 114983. [Google Scholar] [CrossRef] [PubMed]
- Atheba, P.; Drogui, P.; Seyhi, B.; Robert, D. Photo-degradation of butyl parahydroxybenzoate by using TiO2-supported catalyst. Water Sci. Technol. 2013, 67, 2141–2147. [Google Scholar] [CrossRef]
- Rosa, D.; Manetta, G.; Di Palma, L. Experimental assessment of the pH effect and ions on the photocatalytic activity of iron-doped titanium dioxide supported on polystyrene pellets: Batch and continuous tests. Chem. Eng. Sci. 2024, 291, 119918. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M. Adsorption Efficiency of Anatase TiO2 Nanoparticles Against Cadmium Ions. J. Inorg. Organomet. Polym. Mater. 2019, 29, 651–658. [Google Scholar] [CrossRef]
- Hashemi, H.; Bahrami, S.; Emadi, Z.; Shariatipor, H.; Nozari, M. Optimization of ammonium adsorption from landfill leachate using montmorillonite/hematite nanocomposite: Response surface method based on central composite design. Desalin. Water Treat. 2021, 232, 39–54. [Google Scholar] [CrossRef]
- Saqib, N.U.; Khan, A.; Alam, I.; Rahim, M. Glass beads immobilized doped TiO2 NPs with enhanced adsorption efficiency for arsenic(III) from aqueous solution. SN Appl. Sci. 2020, 2, 619. [Google Scholar] [CrossRef]
- Dehmani, Y.; Alrashdi, A.A.; Lgaz, H.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.M. Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): Mechanism exploration from both experimental and theoretical studies. Arab. J. Chem. 2020, 13, 5474–5486. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Cabrerizo-Vílchez, M.; Martínez-García, R.; Hidalgo-Álvarez, R. Effect of the particle surface charge density on the colloidal aggregation mechanism. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1996, 53, 4981–4989. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Tombácz, E. Surface charge characterization of metal oxides by potentiometric acid-base titration, revisited theory and experiment. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 414, 302–313. [Google Scholar] [CrossRef]
- Borji, S.H.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Javadi, A.H. Investigation of photocatalytic degradation of phenol by Fe(III)-doped TiO2 and TiO2 nanoparticles. J. Environ. Heal. Sci. Eng. 2014, 12, 101. [Google Scholar] [CrossRef]
- McBryde, W.A.E. Constraints on the determination of stability constants for metal complexes. II. The iron(III) phenolates. Can. J. Chem. 1968, 46, 2385–2392. [Google Scholar] [CrossRef]
- Jabalpurwala, K.E.; Milburn, R.M. Iron(III)-Phenol Complexes. II. Iron (III) and Proton Associations with Some Singly Substituted Phenolate Ions at 25°. J. Am. Chem. Soc. 1966, 88, 3224–3227. [Google Scholar] [CrossRef]
- Dezani, C.; Ribeiro, E.; Goetz, V.; Plantard, G. Continuous flow photoreactor undergoing variable simulated irradiation conditions: Experimentations and modeling. Chem. Eng. J. Adv. 2022, 12, 100422. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Chapter 1. Heterogeneous Photocatalysis: A Promising Advanced Oxidation Process; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Asadipour, A.; Kargar, E. Facile and green synthesis of ZnFe2O4@CMC as a new magnetic nanophotocatalyst for ciprofloxacin degradation from aqueous media. Process Saf. Environ. Prot. 2019, 129, 138–151. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Zhang, G.; Wang, J.; Tang, P.; Ke, T.; Li, W. Heterogeneous activation of persulfate for Rhodamine B degradation with 3D flower sphere-like BiOI/Fe3O4 microspheres under visible light irradiation. Sep. Purif. Technol. 2018, 192, 88–98. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Q.; Yan, F.; Wang, Q. Heterogeneous activation of persulfate by CuMgAl layered double oxide for catalytic degradation of sulfameter. Green Energy Environ. 2022, 7, 105–115. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Zou, D.; Yuan, X.; Xiao, Z.; Deng, Y.; Zhou, Y.; Jiang, L.; Qin, P. Enhanced heterogeneous activation of persulfate by NixCo3−xO4for oxidative degradation of tetracycline and bisphenol A. J. Environ. Chem. Eng. 2020, 8, 104451. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Kobylański, M.P.; Zaleska-Medynska, A. Chapter 2. Fundamentals of metal oxide-based photocatalysis. In Metal Oxide-Based Photocatalysis: Fundamentals and Prospects for Application; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–50. ISBN 9780128116340. [Google Scholar]
- Gao, Y.; Champagne, P.; Blair, D.; He, O.; Song, T. Activated persulfate by iron-based materials used for refractory organics degradation: A review. Water Sci. Technol. 2020, 81, 853–875. [Google Scholar] [CrossRef]
- Liu, N.N.; Song, L.X.; Teng, Y.; Xia, J.; Li, Y.; Wang, W.P. Microspheres of Ferrous Oxalate Dihydrate: Formation, Structure, Physical Properties, and Photocatalytic Activities. J. Phys. Chem. C 2022, 126, 19122–19135. [Google Scholar] [CrossRef]
- Lagos, K.J.; Marinkovic, B.A.; Dosen, A.; Guamán, M.V.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Data on phase and chemical compositions of black sands from “El Ostional” beach situated in Mompiche, Ecuador. Data Br. 2020, 32, 106214. [Google Scholar] [CrossRef]
- Bhavsar, K.S.; Labhane, P.K.; Dhake, R.B.; Sonawane, G.H. Solvothermal synthesis of activated carbon loaded CdS nanoflowers: Boosted photodegradation of dye by adsorption and photocatalysis synergy. Chem. Phys. Lett. 2020, 744, 137202. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef]
- Li, C.; Goetz, V.; Chiron, S. Peroxydisulfate activation process on copper oxide: Cu(III) as the predominant selective intermediate oxidant for phenol and waterborne antibiotics removal. J. Environ. Chem. Eng. 2021, 9, 105145. [Google Scholar] [CrossRef]
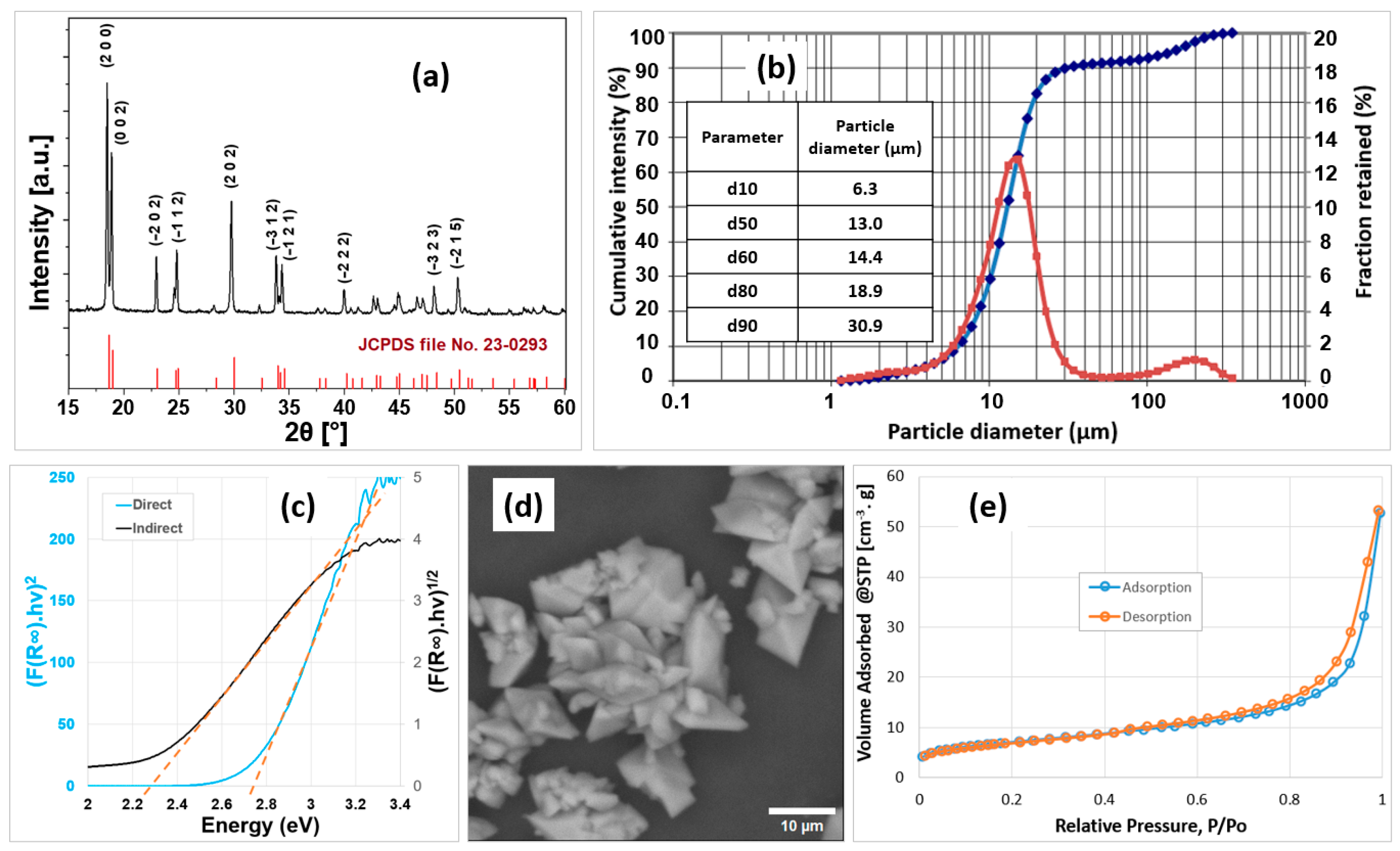
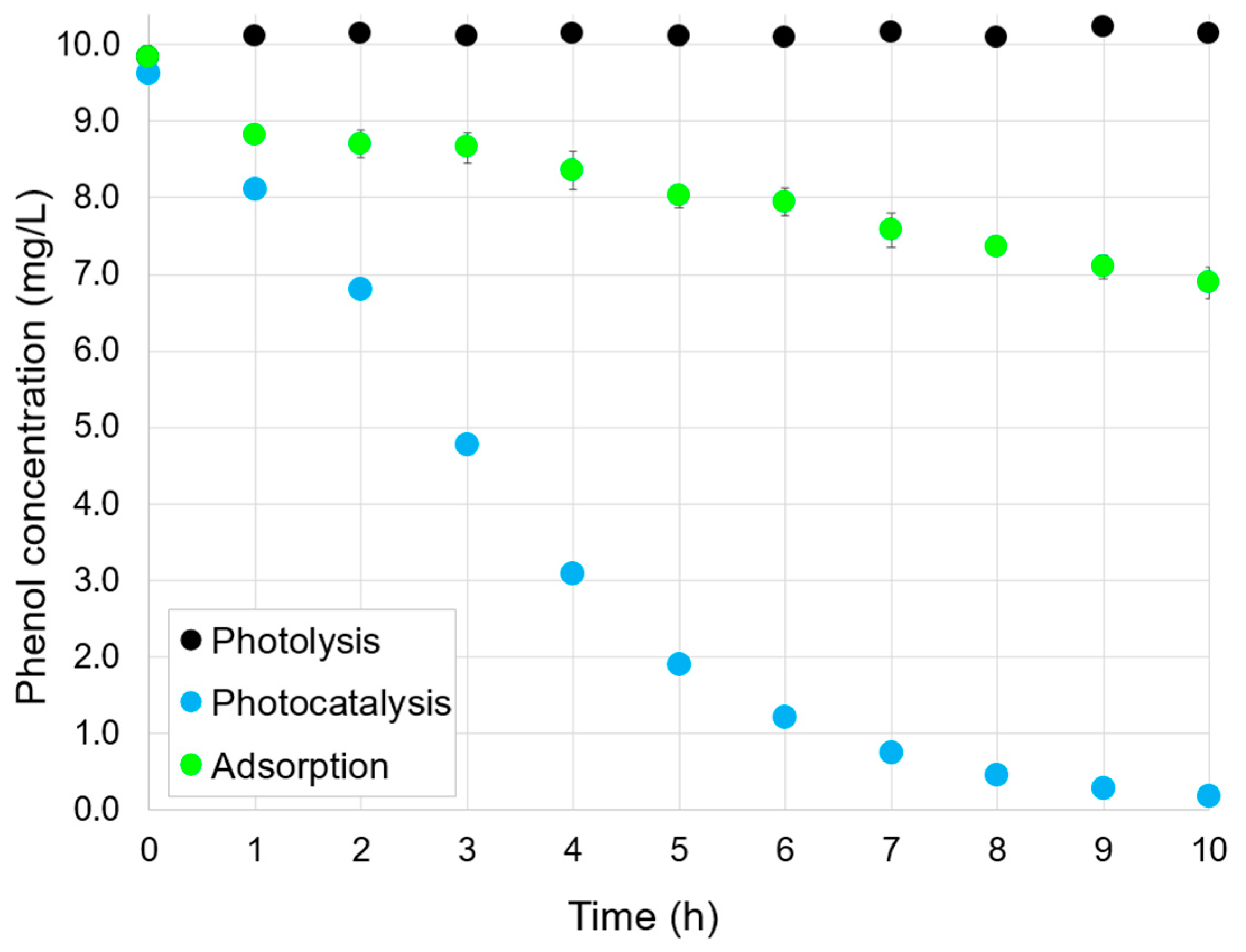
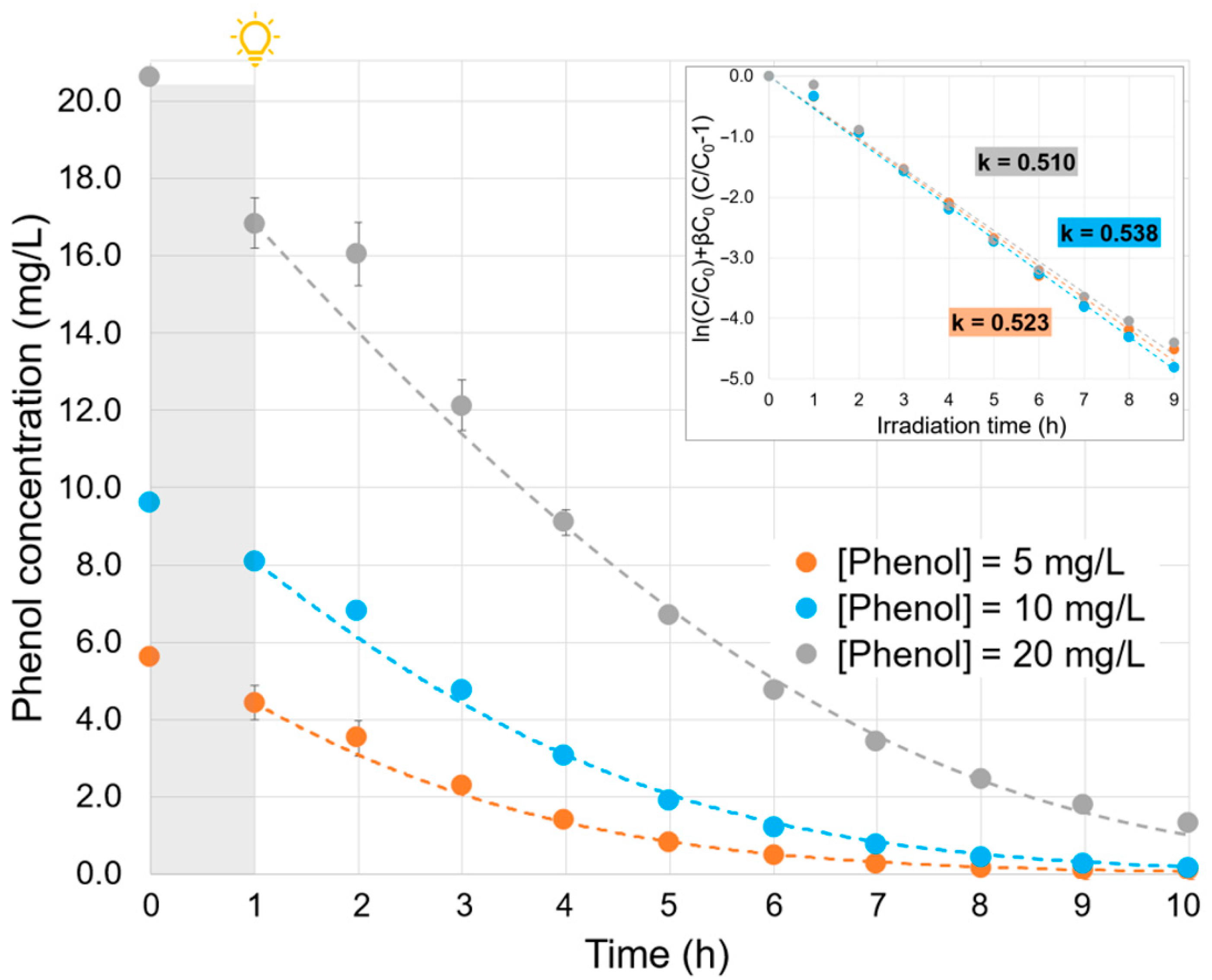
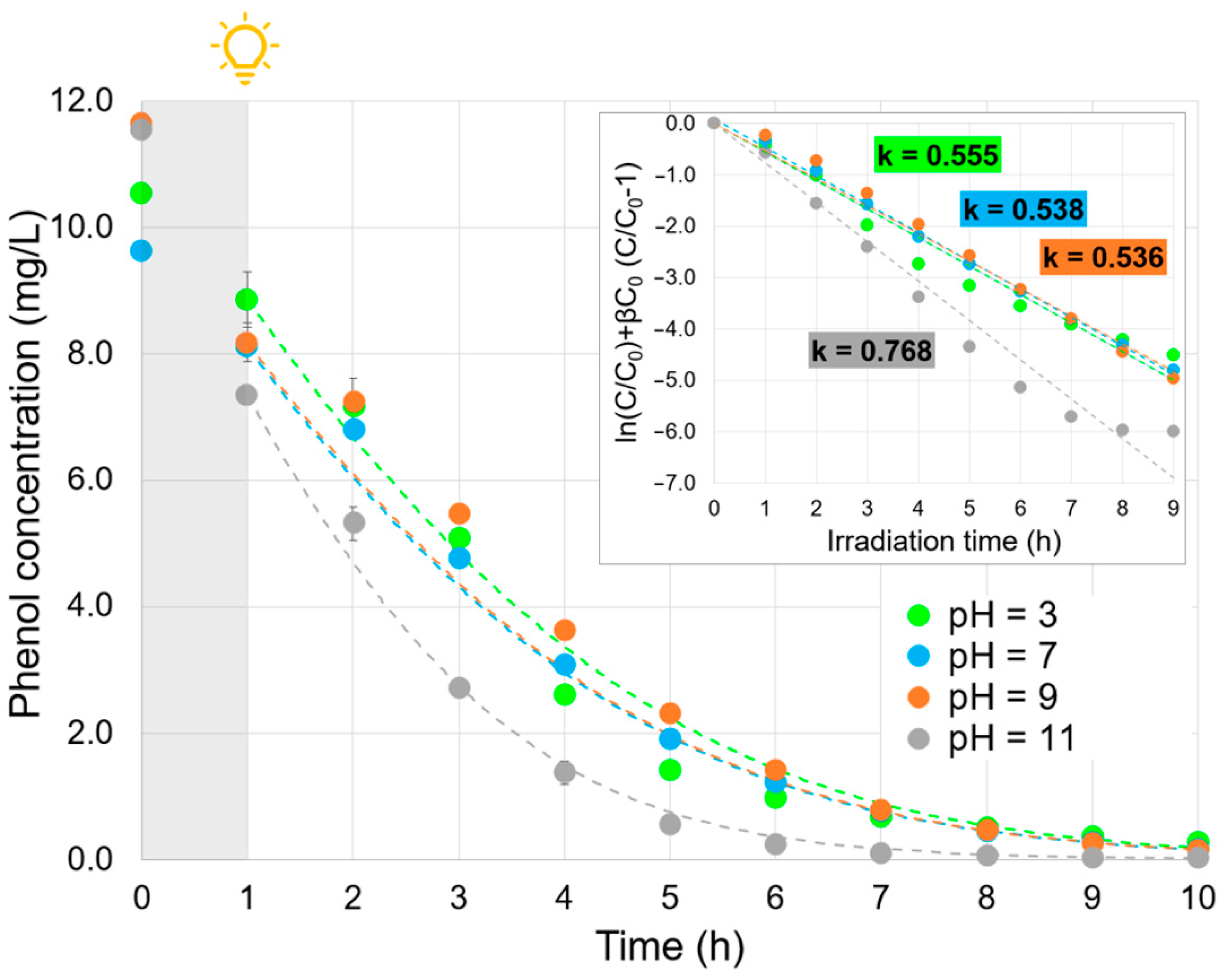
| Parameter | Value |
|---|---|
| Crystalline phase | Monoclinic ferrous oxalate dihydrate (α-FOD) |
| Band gap (eV) | 2.78 (direct transition) 2.28 (indirect transition) |
| d80 (µm) | 18.9 |
| Specific surface area (m2/g) | 25.13 |
| Total pore volume (cm3/g) | 0.082 |
| Average pore radius (nm) | 6.5 |
| Photocatalyst | Dosage (g/L) | Phenol Initial Concentration (mg/L) | Removal (%) | Time (h) | Visible Light Source Power (Intensity)/Type | Reference |
|---|---|---|---|---|---|---|
| Sulfur and iron co-doped TiO2 | 1.0 | 20 | 99.4 | 10 | 1000 W (60 mW/cm2)/tungsten halogen lamp with UV cutoff filters) | [26] |
| Zinc-based MOF ([Cd0.3Zn0.7(oba)(4-bpdh)0.5]n·1.5DMF) 1 | 0.5 | 25 | 78.0 | 2 | 300 W (not reported)/xenon lamp | [27] |
| N-doped TiO2 | 0.2 | 100 | 6.6 | 5 | 100 W (38.5 mW/cm2)/not reported | [28] |
| LaVO4/MCM-48 2 | 3.0 | 20 | 100 | 3 | 500 W (100 mW/cm2)/xenon lamp | [29] |
| α-FOD | 1.0 | 10 | 98.2 | 9 | 18 W (49.3 mW/cm2)/LED lamp | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeas, S.; Guerrero, V.H.; Pontón, P.I.; Goetz, V. Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study. Molecules 2025, 30, 2059. https://doi.org/10.3390/molecules30092059
Galeas S, Guerrero VH, Pontón PI, Goetz V. Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study. Molecules. 2025; 30(9):2059. https://doi.org/10.3390/molecules30092059
Chicago/Turabian StyleGaleas, Salomé, Víctor H. Guerrero, Patricia I. Pontón, and Vincent Goetz. 2025. "Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study" Molecules 30, no. 9: 2059. https://doi.org/10.3390/molecules30092059
APA StyleGaleas, S., Guerrero, V. H., Pontón, P. I., & Goetz, V. (2025). Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study. Molecules, 30(9), 2059. https://doi.org/10.3390/molecules30092059







