Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of LiCl in RAW 264.7 Cells
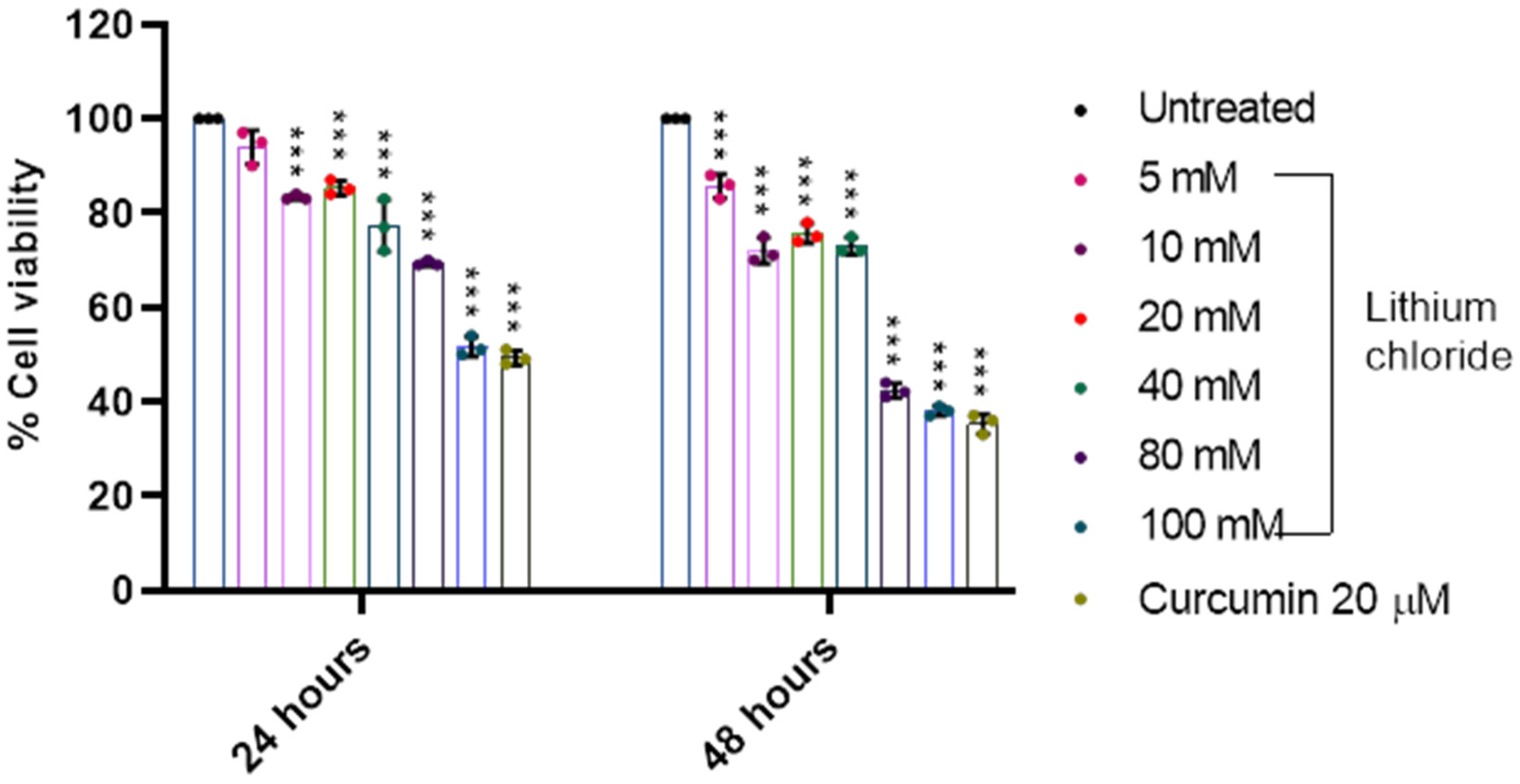
2.2. Effect of LiCl on the Cell Viability of A549 Lung Adenocarcinoma Cells
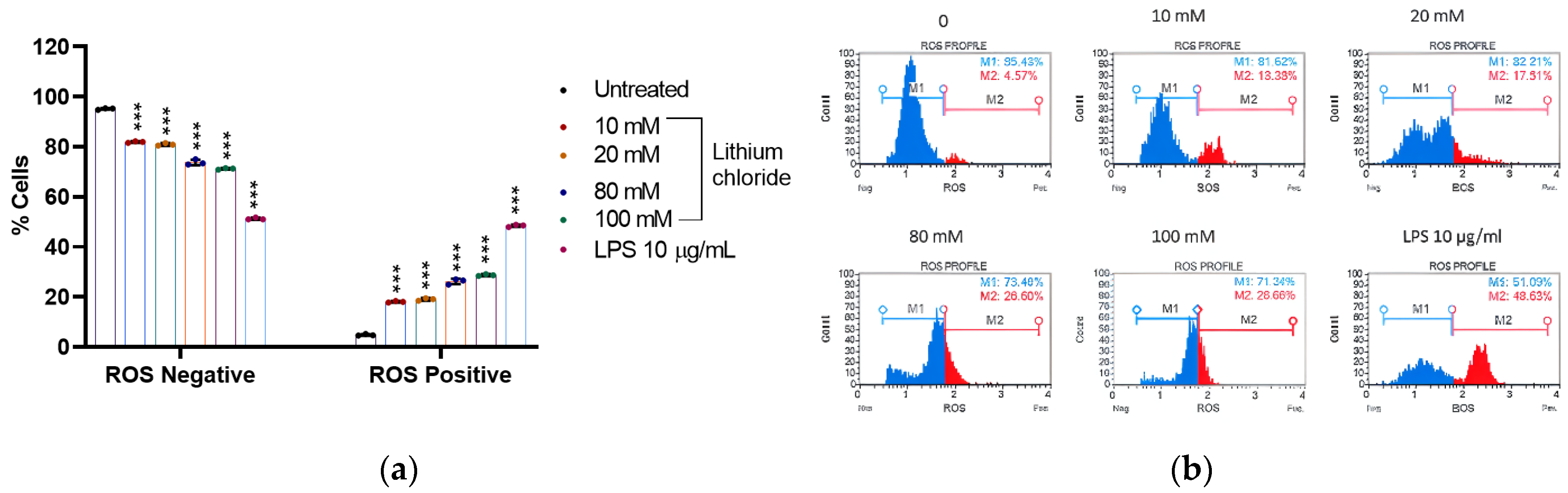
2.3. Effect of Lithium on the Production of Reactive Oxygen Species in A549 Cells
2.4. Effect of Lithium on the Production of Nitric Oxide in A549 Cells
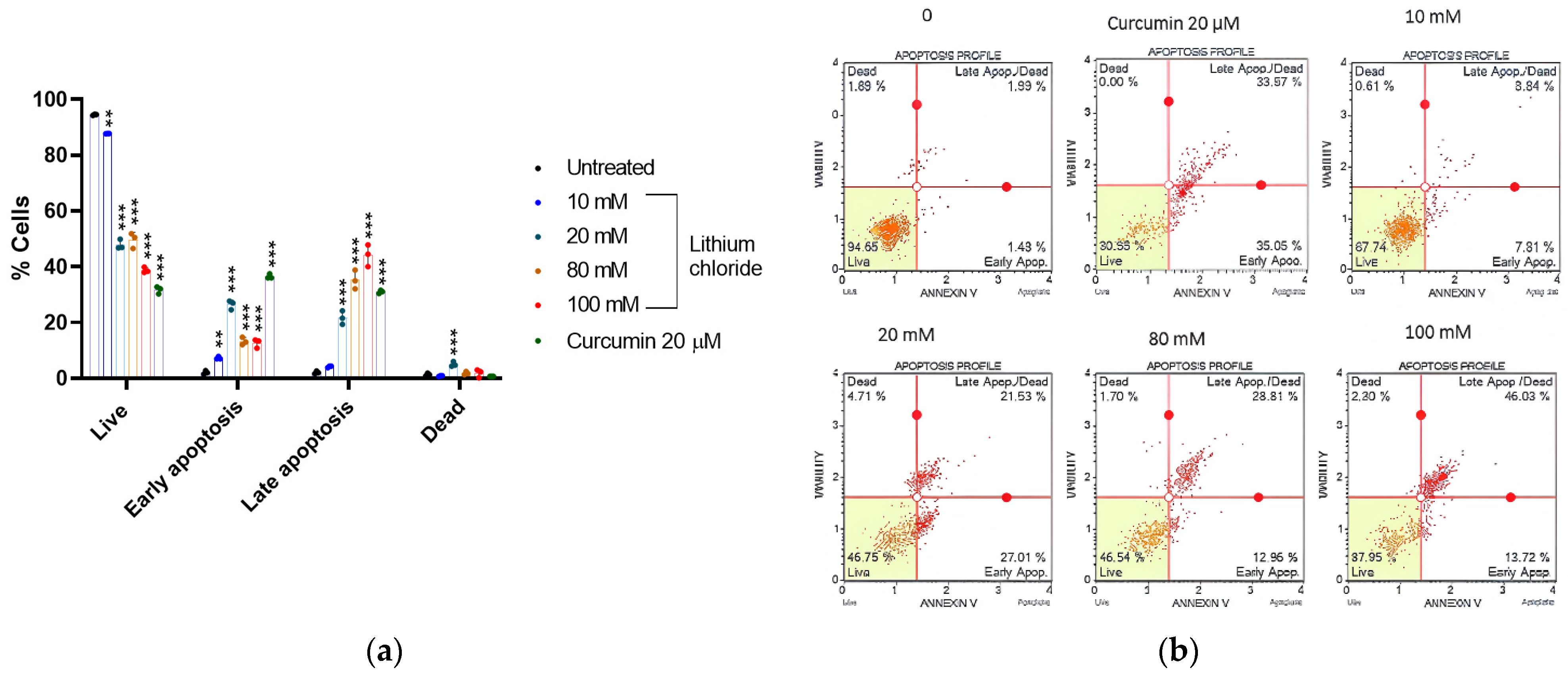
2.5. Mode of Cell Death Induced by LiCl in A549 Cells
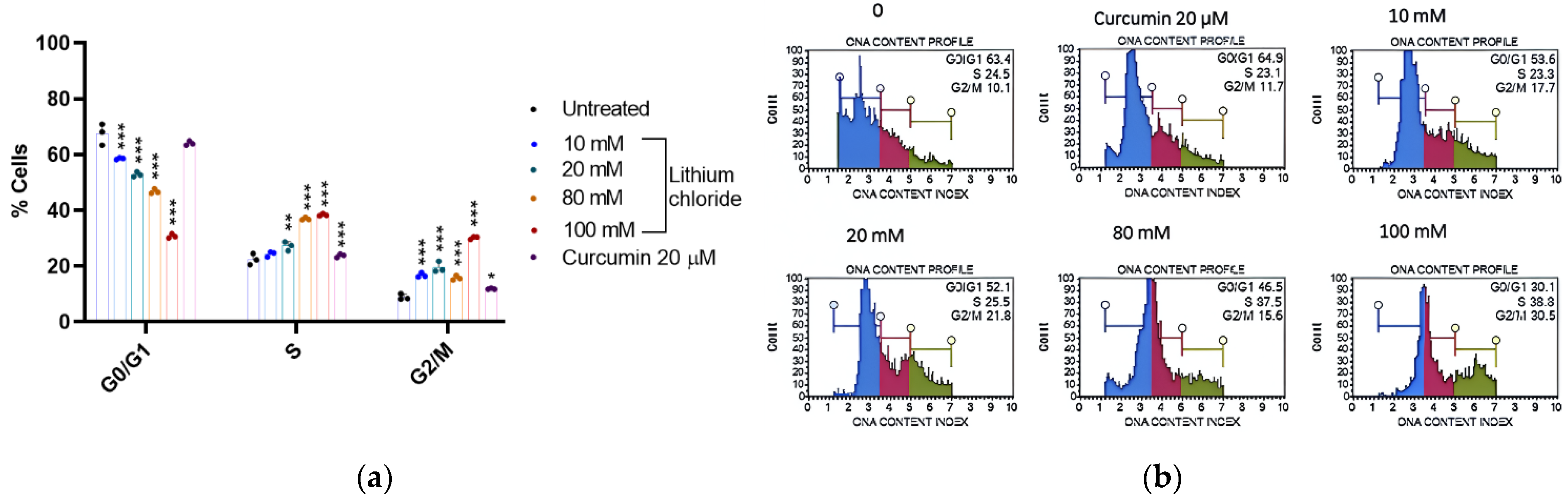
2.6. Cell Cycle Arrest Potential of Lithium Chloride
3. Discussion
4. Materials and Methods
4.1. Cell Line and Culture
4.2. Cell Viability Assay
4.3. Oxidative Stress Assay
4.4. DAF2-DA Nitric Oxide Measurement Assay
4.5. Annexin-V and (PI) Apoptosis Detection Assay
4.6. Cell Cycle Arrest Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LiCl | Lithium chloride |
| FDA | Food and Drug Administration |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| DNA | Deoxyribonucleic acid |
| LPS | Lipopolysaccharide |
Appendix A
| 24 h | 48 h | |
|---|---|---|
| 0 | 100.000 ± 0.000 | 100.000 ± 0.000 |
| 5 mM | 93.667 ± 5.033 | 94.333 ± 1.1555 |
| 10 mM | 98.667 ± 0.577 | 84.333 ± 0.577 |
| 20 mM | 88.333 ± 1.155 | 76.667 ± 1.155 |
| 40 mM | 73.333 ± 2.887 | 63.333 ± 2.517 |
| 80 mM | 66.667 ± 1.528 | 52.667 ± 5.508 |
| 100 mM | 57.000 ± 2.000 | 44.000 ± 1.732 |
| Curcumin 20 µM | 93.333 ± 3.055 | 78.667 ± 1.528 |
| 24 h | 48 h | |
|---|---|---|
| 0 | 100.000 ± 0.000 | 100.000 ± 0.000 |
| 5 mM | 94.000 ± 3.606 | 85.667 ± 2.516 |
| 10 mM | 83.333 ± 0.577 | 72.000 ± 2.646 |
| 20 mM | 85.333 ± 1.528 | 75.667 ± 2.082 |
| 40 mM | 77.333 ± 5.508 | 73.000 ± 1.732 |
| 80 mM | 69.333 ± 0.577 | 42.333 ± 1.528 |
| 100 mM | 51.667 ± 2.082 | 38.000 ± 1.000 |
| Curcumin 20 µM | 49.333 ± 1.528 | 35.333 ± 2.082 |
| ROS Negative | ROS Positive | |
|---|---|---|
| 0 | 95.170 ± 0.352 | 4.830 ± 0.352 |
| 10 mM | 81.933 ± 0.326 | 18.067 ± 0.326 |
| 20 mM | 80.957 ± 0.531 | 19.043 ± 0.531 |
| 80 mM | 73.800 ± 1.087 | 26.200 ± 1.087 |
| 100 mM | 71.243 ± 0.326 | 28.727 ± 0.365 |
| LPS 10 μg/mL | 51.303 ± 0.396 | 48.470 ± 0.452 |
| % Live Negative | % Nitric Oxide + Live | % Nitric Oxide + Dead | % Total Nitric Oxide | |
|---|---|---|---|---|
| 0 | 93.977 ± 1.698 | 1.943 ± 0.580 | 4.080 ± 1.237 | 6.023 ± 1.698 |
| 10 Mm | 82.840 ± 2.705 | 0.610 ± 0.185 | 16.540 ± 2.875 | 17.150 ± 2.713 |
| 20 mM | 58.980 ± 5.172 | 1.220 ± 0.085 | 39.680 ± 5.130 | 40.900 ± 5.209 |
| 80 mM | 54.353 ± 1.382 | 7.343 ± 5.699 | 38.287 ± 4.317 | 45.543 ± 1.297 |
| 100 mM | 34.367 ± 3.305 | 1.073 ± 0.459 | 64.563 ± 3.760 | 65.970 ± 3.119 |
| LPS 10 μg/mL | 48.250 ± 3.123 | 9.497 ± 0.482 | 42.257 ± 2.668 | 51.753 ± 3.128 |
| % Live | % Early Apoptotic | % Late Apoptotic | % Dead | |
|---|---|---|---|---|
| 0 | 94.490 ± 0.204 | 2.067 ± 0.634 | 2.073 ± 0.471 | 1.370 ± 0.506 |
| 10 mM | 87.723 ± 0.029 | 7.257 ± 0.479 | 4.190 ± 0.303 | 0.833 ± 0.194 |
| 20 mM | 47.867 ± 1.781 | 26.433 ± 1.652 | 21.677 ± 2.423 | 5.027 ± 0.898 |
| 80 mM | 49.613 ± 2.747 | 13.270 ± 1.352 | 35.280 ± 3.381 | 1.843 ± 0.569 |
| 100 mM | 38.733 ± 1.021 | 12.633 ± 1.613 | 44.130 ± 3.928 | 1.840 ± 1.456 |
| Curcumin 20 µM | 31.717 ± 1.293 | 36.527 ± 0.826 | 31.027 ± 0.544 | 0.727 ± 0.006 |
| G0/G1 | S | G2/M | |
|---|---|---|---|
| 0 | 67.467 ± 3.790 | 22.400 ± 2.007 | 8.900 ± 1.044 |
| 10 mM | 58.533 ± 0.306 | 24.333 ± 0.907 | 16.767 ± 0.814 |
| 20 mM | 52.867 ± 0.862 | 27.200 ± 1.609 | 19.533 ± 1.973 |
| 80 mM | 46.800 ± 0.889 | 36.933 ± 0.493 | 15.767 ± 0.862 |
| 100 Mm | 30.767 ± 0.764 | 38.367 ± 0.379 | 30.167 ± 0.493 |
| Curcumin 20 µM | 64.033 ± 0.777 | 23.767 ± 0.611 | 11.833 ± 0.231 |
References
- Pikin, O.V.; Ryabov, A.B.; Glushko, V.A.; Kolbanov, K.I.; Amiraliev, A.M.; Vursol, D.A.; Bagrov, V.A.; Barmin, V. Surgery for non-small cell lung carcinoma after previous chemoradiotherapy alone. Khirurgiia 2016, 11, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.; Baum, S.L.; Dransfield, M.T.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; et al. Lung cancer screening, version 1.2015: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2015, 13, 23–34. [Google Scholar] [CrossRef]
- D’Souza, R.; Rajji, T.K.; Mulsant, B.H.; Pollock, B.G. Use of lithium in the treatment of bipolar disorder in late-life. Curr. Psychiatry Rep. 2011, 13, 488–492. [Google Scholar] [CrossRef]
- Kovacsics, C.E.; Gottesman, T.D.; Gould, T.D. Lithium’s antisuicidal efficacy: Elucidation of neurobiological targets using endophenotype strategies. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 175–198. [Google Scholar] [CrossRef]
- Post, R.M. Treatment of bipolar depression: Evolving recommendations. Psychiatr. Clin. North Am. 2016, 39, 11–33. [Google Scholar] [CrossRef]
- Matsebatlela, T.M.; Gallicchio, V.; Becker, R.P. Lithium modulates cancer cell growth, apoptosis, gene expression, and cytokine production in HL-60 promyelocytic leukaemia cells and their drug-resistant sub-clones. Biol. Trace Elem. Res. 2012, 149, 323–330. [Google Scholar] [CrossRef]
- Novetsky, A.P.; Thompson, D.M.; Zighelboim, I.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Goodfellow, P.J. Lithium chloride and inhibition of glycogen synthase kinase 3β as a potential therapy for serous ovarian cancer. Int. J. Gynecol. Cancer 2012, 23, 361–366. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Liu, X.; Liu, J.; Lu, X.; Tao, K.; Wang, G.; Wang, J. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3β/NF-κB signalling pathway. Oxidative Med. Cell. Longev. 2014, 2014, 241864. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Tang, Y.; He, W.; Pan, H.; Jiang, W.; Wang, L.; Deng, W. Lithium chloride promotes neuronal differentiation of rat neural stem cells and enhances neural regeneration in Parkinson’s disease model. Cytotechnology 2017, 69, 277–287. [Google Scholar] [CrossRef]
- Cimarosti, H.; Rodnight, R.; Tavares, A.; Paiva, R.; Valentim, L.; Rocha, E.; Salbego, C. An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci. Lett. 2001, 315, 33–36. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Song, S.; Zhang, H. Cancer therapeutic strategies based on metal ions. Chem. Sci. 2021, 12, 12234–12247. [Google Scholar] [CrossRef]
- Makola, R.T.; Mbazima, V.G.; Mokgotho, M.P.; Gallicchio, V.S.; Matsebatlela, T.M. The effect of lithium on inflammation-associated genes in lipopolysaccharide-activated RAW 264.7 macrophages. Int. J. Inflamm. 2020, 2020, 8340195. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding apoptosis and apoptotic path-ways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Matson, J.P.; Cook, J.G. Cell cycle proliferation decisions: The impact of single-cell analyses. FEBS J. 2017, 284, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Bhavya, K.; Ramprasad, P.; Kalia, M.; Pal, D. Anti-cancer drug molecules targeting cancer cell cycle and proliferation. Adv. Protein Chem. Struct. Biol. 2023, 135, 343–395. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Zhu, K.; Liu, W.; Luo, Y.; Yuan, X.; Wang, J.; Cheng, T.; Zhang, X. Lithium chloride with immunomodulatory function for regulating titanium nanoparticle-stimulated inflammatory response and accelerating osteogenesis through suppression of MAPK signalling pathway. Int. J. Nanomed. 2019, 14, 7475–7488. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, S.; Xiao, L.; Han, P.; Xie, K.; Ivanovski, S.; Xiao, Y.; Zhou, Y. LiCl-induced immunomodulatory periodontal regeneration via the activation of the Wnt/β-catenin signaling pathway. J. Periodontal Res. 2022, 57, 835–848. [Google Scholar] [CrossRef]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, X.; Zhang, R.; Wang, K.; Wang, Y.; Hua, Z.C. Lithium enhances TRAIL-induced apoptosis in human lung carcinoma A549 cells. Biometals 2013, 26, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.-S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef] [PubMed]
- ArulJothi, K.N.; Kumaran, K.; Senthil, S.; Nidhu, A.B.; Munaff, N.; Janitri, V.B.; Kirubakaran, R.; Singh, S.K.; Gupt, G.; Dua, K.; et al. Implications of reactive oxygen species in lung cancer and exploiting it for therapeutic interventions. Med. Oncol. 2022, 40, 43. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Li, H.; Zhao, X.; Zhang, H. LiCl induces apoptosis via CHOP/NOXA/Mcl-1 axis in human choroidal melanoma cells. Cancer Cell Int. 2021, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–915. [Google Scholar] [CrossRef]
- Li, C.Q.; Pang, B.; Kiziltepe, T.; Trudel, L.J.; Engelward, B.P.; Dedon, P.C.; Wogan, G.N. Threshold effects of nitric oxide-induced toxicity and cellular responses in wild-type and p53-null human lymphoblastoid cells. Chem. Res. Toxicol. 2006, 19, 399–406. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Wang, B.; Li, P.; Liu, P. LiCl treatment induces programmed cell death of Schwannoma cells through AKT- and MTOR-mediated necroptosis-sis. Neurochem. Res. 2017, 42, 2363–2371. [Google Scholar] [CrossRef]
- Sabancı, P.A.; Ergüven, M.; Yazıhan, N.; Aktaş, E.; Aras, Y.; Civelek, E.; Aydoseli, A.; Imer, M.; Gürtekin, M.; Bilir, A. Sorafenib and lithium chloride combination treatment shows promising synergistic effects in human glioblastoma multiforme cells in vitro but midkine is not implicated. Neurol. Res. 2014, 36, 189–197. [Google Scholar] [CrossRef]
- Razmi, M.; Rabbani-Chadegani, A.; Hashemi-Niasari, F.; Ghadam, P. Lithium chloride attenuates mitomycin C&-induced necrotic cell death in MDA-MB-231 breast cancer cells via HMGB1 and Bax signalling. J. Trace Elem. Med. Biol. 2018, 48, 87–96. [Google Scholar] [CrossRef]
- Li, L.; Song, H.; Zhong, L.; Yang, R.; Yang, X.Q.; Jiang, K.I.; Liu, B.Z. Lithium chloride promotes apoptosis in human leukemia NB4 cells by inhibiting glycogen synthase kinase-3 beta. Int. J. Med. Sci. 2015, 12, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [CrossRef]
- Hart, D.A. Lithium Ions as Modulators of Complex Biological Processes: The Conundrum of Multiple Targets, Responsiveness and Non-Responsiveness, and the Potential to Prevent or Correct Dysregulation of Systems during Aging and in Disease. Biomolecules 2024, 14, 905. [Google Scholar] [CrossRef]
- Chen, W.; Wu, R.; Wang, X.; Li, Y.; Hao, T. Effect of lithium on cell cycle progression of pig airway epithelial cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 318–321. [Google Scholar] [CrossRef]
- Han, S.; Meng, L.; Jiang, Y.; Cheng, W.; Tie, X.; Xia, J.; Wu, A. Lithium enhances the antitumor effect of temozolomide against TP53 wild-type glioblastoma cells via NFAT1/FasL signalling. Br. J. Cancer 2017, 116, 1302–1311. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, C.L.; Wen, J.F.; Wang, Y.J.; Hu, Y.B.; Ren, H.Z. Lithium inhibits proliferation of human esophageal cancer cell line Eca-109 by inducing a G2/M cell cycle arrest. World J. Gastroenterol. 2008, 14, 3982–3989. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tang, L.; Tan, Y.; Xiao, J.; Wei, K.; Zhang, X.; Ma, Y.; Tong, S.; Chen, J.; Zhou, N.; et al. Lithium carbonate revitalizes tumour-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 2024, 25, 552–561. [Google Scholar] [CrossRef]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, B.; Zhan, M.; Hua, Z.C. Lithium in cancer therapy: Friend or foe? Cancers 2023, 15, 1095. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramushu, P.; Mangoakoane, D.D.; Makola, R.T.; Matsebatlela, T.M. Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells. Molecules 2025, 30, 1797. https://doi.org/10.3390/molecules30081797
Ramushu P, Mangoakoane DD, Makola RT, Matsebatlela TM. Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells. Molecules. 2025; 30(8):1797. https://doi.org/10.3390/molecules30081797
Chicago/Turabian StyleRamushu, Pearl, Dikgale D. Mangoakoane, Raymond T. Makola, and Thabe M. Matsebatlela. 2025. "Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells" Molecules 30, no. 8: 1797. https://doi.org/10.3390/molecules30081797
APA StyleRamushu, P., Mangoakoane, D. D., Makola, R. T., & Matsebatlela, T. M. (2025). Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells. Molecules, 30(8), 1797. https://doi.org/10.3390/molecules30081797







