Figure 1 presents the general aim of the following research. The main scientific hypothesis was to increase the antibacterial properties of the native fungal chitosan as a result of chemical crosslinking using two amino acids without losing free amino groups and synergistic effects with periclase nanoparticles—highly crystalline magnesium oxide. Fungal chitosan presents several advantages over marine-derived chitosan, making it a superior candidate for hemostatic applications. Its production through controlled fermentation ensures higher purity, eliminating endotoxins and allergens commonly found in shellfish-derived chitosan, thus improving biocompatibility and reducing the risk of immune reactions. Additionally, fungal chitosan exhibits enhanced solubility at physiological pH, facilitating better bioavailability and interaction with biological systems. Its more uniform molecular weight and degree of deacetylation contribute to improved antimicrobial properties and cell adhesion, which are critical for wound healing and infection control. Moreover, fungal chitosan is sustainably sourced from renewable biomass, offering an ethical and consistent alternative to marine-based production. These properties collectively enhance its effectiveness in hemostatic materials by promoting rapid clot formation, reducing inflammation, and supporting tissue regeneration. The addition of periclase (MgO) enhances the properties of chitosan-based hemostatic agents by addressing key limitations associated with pure chitosan. One of the primary benefits is pH modulation, as periclase acts as an alkaline buffer, counteracting the localized acidity that can arise from chitosan degradation. This is particularly important for maintaining a physiological pH in wound environments, reducing the risk of tissue irritation and inflammatory responses. Additionally, periclase contributes to antimicrobial activity, as MgO nanoparticles generate reactive oxygen species (ROS) that disrupt bacterial membranes, enhancing the infection-preventive properties of the hemostatic material.
From a structural perspective, the incorporation of periclase improves the mechanical strength of chitosan-based hydrogels and scaffolds, making them more durable while maintaining flexibility for effective wound coverage. Furthermore, periclase releases bioactive magnesium ions, which play a crucial role in cell proliferation, tissue regeneration, and coagulation processes. Magnesium ions activate platelets and improve fibrin network formation, accelerating clot formation and promoting faster healing. The combined effects of pH regulation, antimicrobial activity, mechanical reinforcement, and bioactivity make periclase an excellent additive for enhancing the performance of chitosan-based hemostatic materials.
2.1. Periclase Characteristics
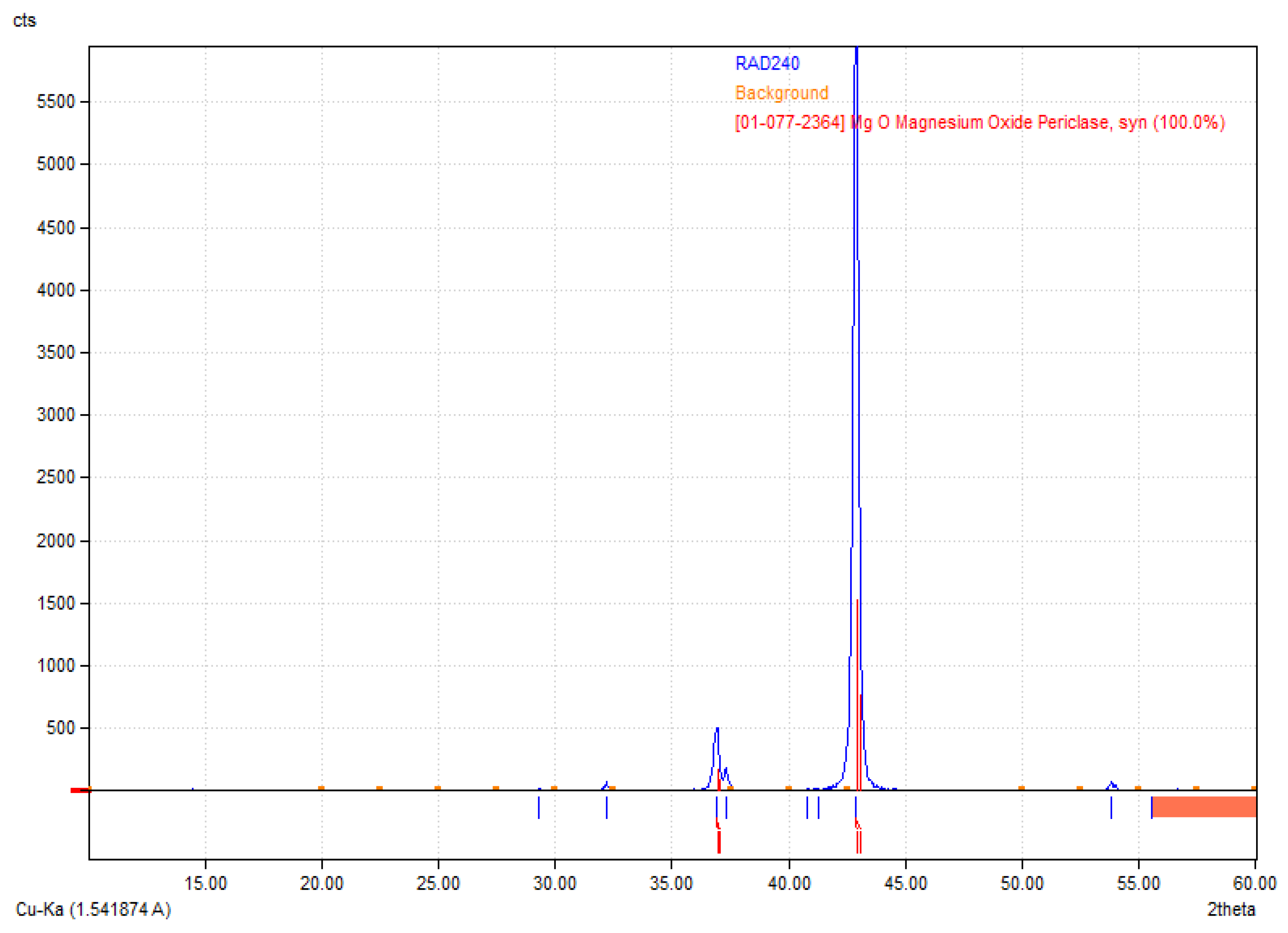
Figure 2 reveals the crystalline structure of the obtained MgO nanoparticles. The X-ray diffraction (XRD) pattern presented corresponds to periclase (MgO), as confirmed by the reference diffraction pattern [01-077-2364] [
35,
36]. The diffractogram exhibits characteristic sharp peaks, indicating a well-crystallized structure. The most intense peak appears around 42.9° 2θ, corresponding to the (200) crystallographic plane of MgO, which is a dominant feature of periclase. Additional peaks observed in the range of 35° to 50° further confirm the presence of cubic MgO. The high intensity of the primary diffraction peak suggests that the sample is highly crystalline, with minimal amorphous content. The absence of additional unidentified peaks indicates phase purity, meaning no significant secondary phases or impurities were detected. The relatively low background signal also supports the conclusion that the material is well-ordered and has minimal structural defects. These results validate the successful synthesis or presence of MgO periclase in the sample, which is relevant for applications where high crystallinity and phase purity are essential, such as in biomaterials.
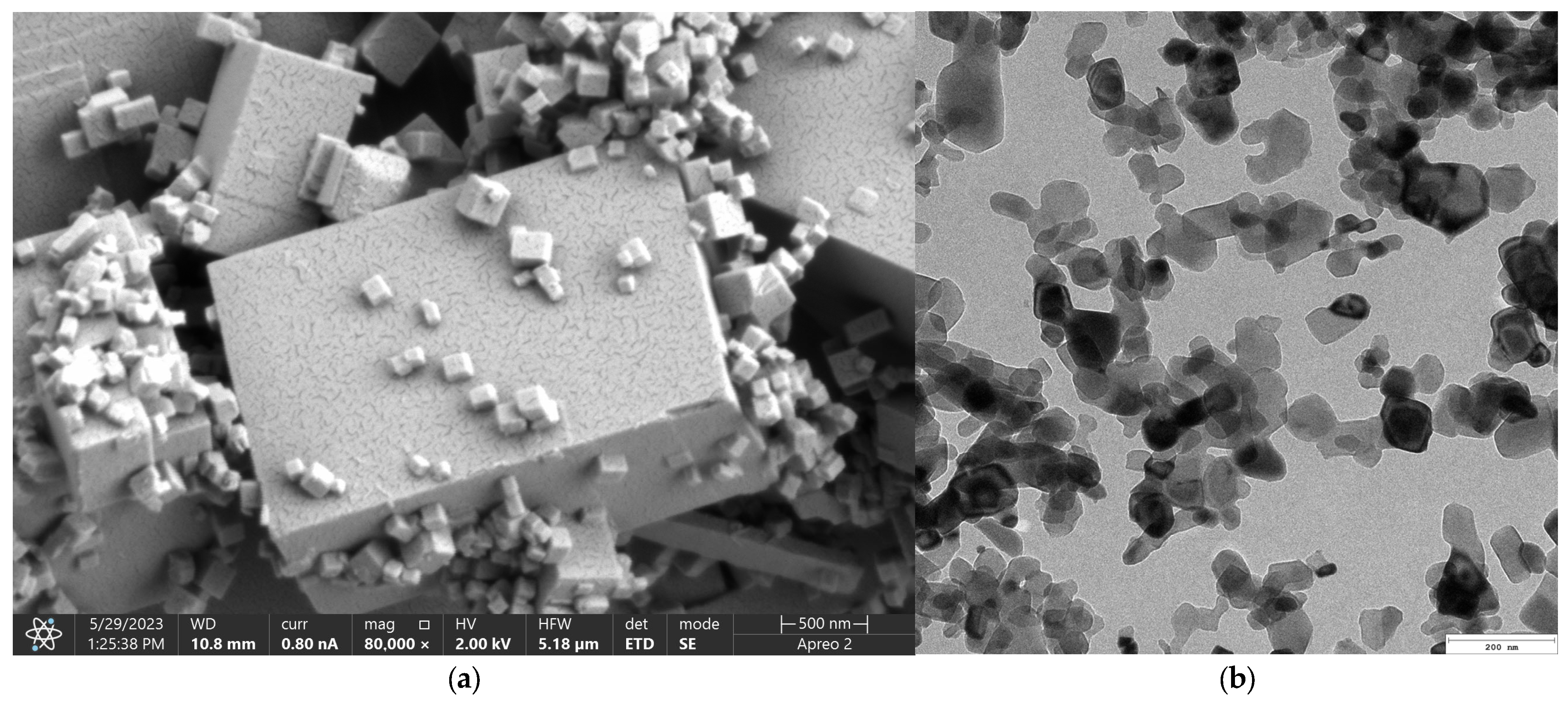
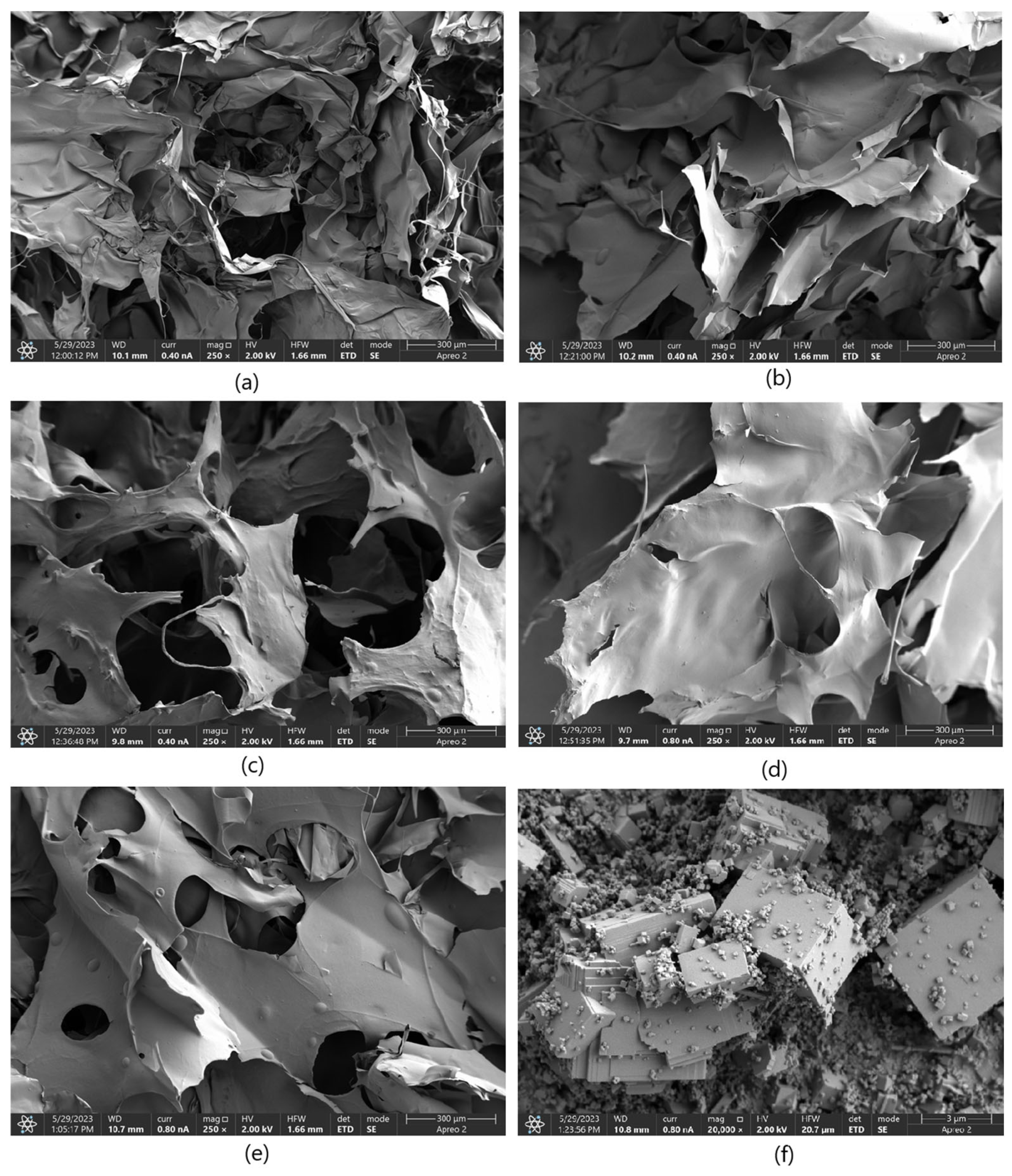
Figure 3 presents microphotographs of the MgO ((a)—SEM and (b)—TEM, respectively). The SEM micrograph of MgO powder (
Figure 3a) reveals a well-defined crystalline morphology, indicative of its high degree of crystallinity. The particles exhibit a cubic structure, consistent with the characteristic morphology of periclase. The surface of the particles appears smooth with sharp edges, confirming the presence of highly ordered crystal planes. Particle sizes are relatively uniform, which agrees with previous reports on MgO periclase synthesis. Additionally, agglomeration is minimal, suggesting a high purity of the synthesized material and an absence of significant structural defects. The high crystallinity observed in the SEM images correlates with the material’s inherent thermal stability and mechanical properties, making it suitable for applications requiring structural integrity and resistance to degradation. The well-defined edges and uniform morphology also indicate a controlled synthesis process, ensuring reproducibility for further applications. The TEM images provide further insight into the microstructural features of MgO periclase. The lattice fringes observed in high-resolution TEM (
Figure 3b) images confirm the highly crystalline nature of the material. Moreover, the TEM analysis highlights the absence of significant defects, dislocations, or amorphous regions, reinforcing the findings from SEM. The uniformity in particle distribution and crystallinity suggests that the synthesis method employed effectively controls nucleation and growth, resulting in a material with excellent structural homogeneity. These structural characteristics of MgO periclase have significant implications for its applications in catalysis, biomedicine, and refractory materials. The high crystallinity ensures optimal thermal and chemical stability, while the well-defined morphology enhances its reactivity in surface-related applications. The absence of amorphous phases also indicates minimal impurities, further confirming the high-quality synthesis of the material.
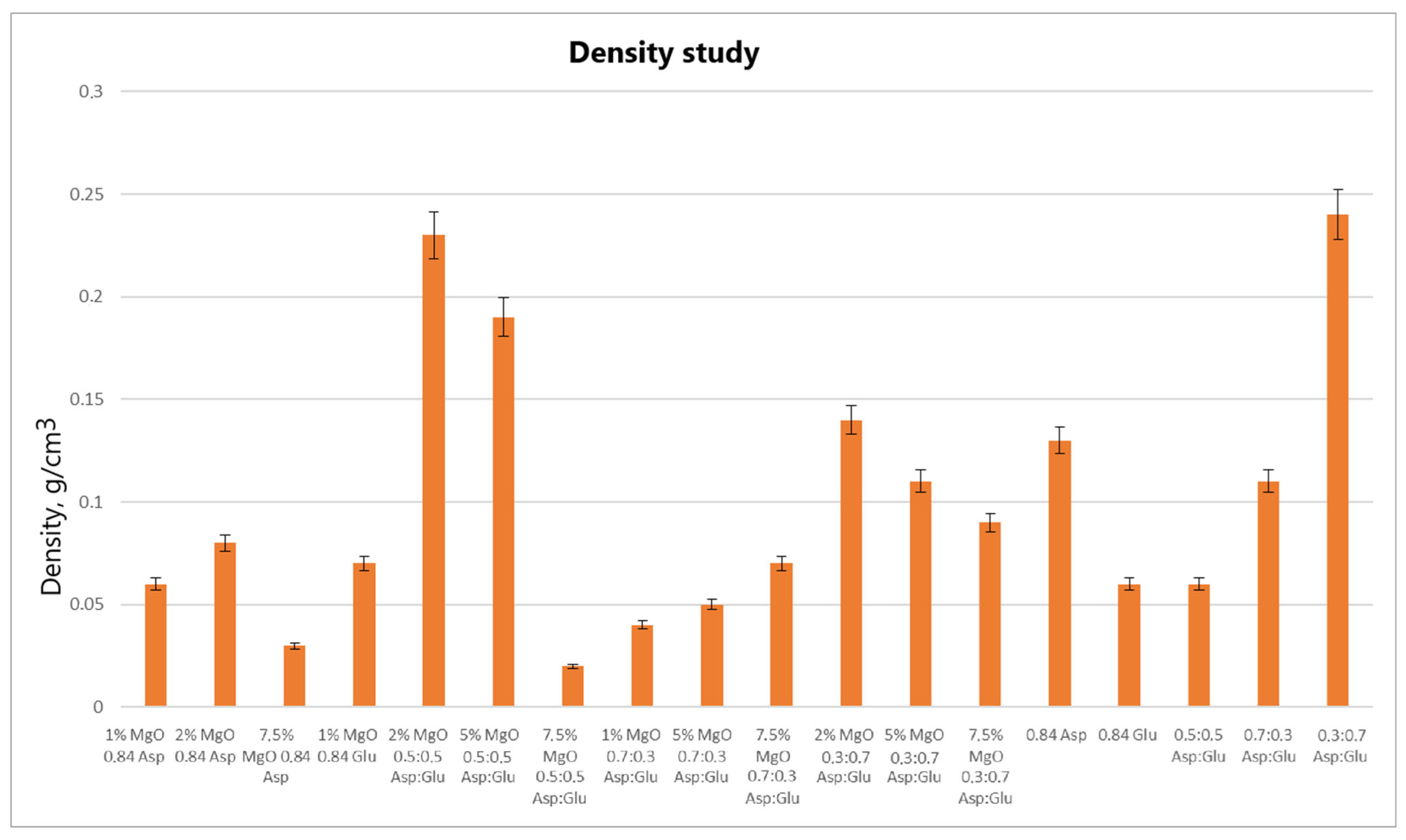
2.2. Weight Swelling Properties
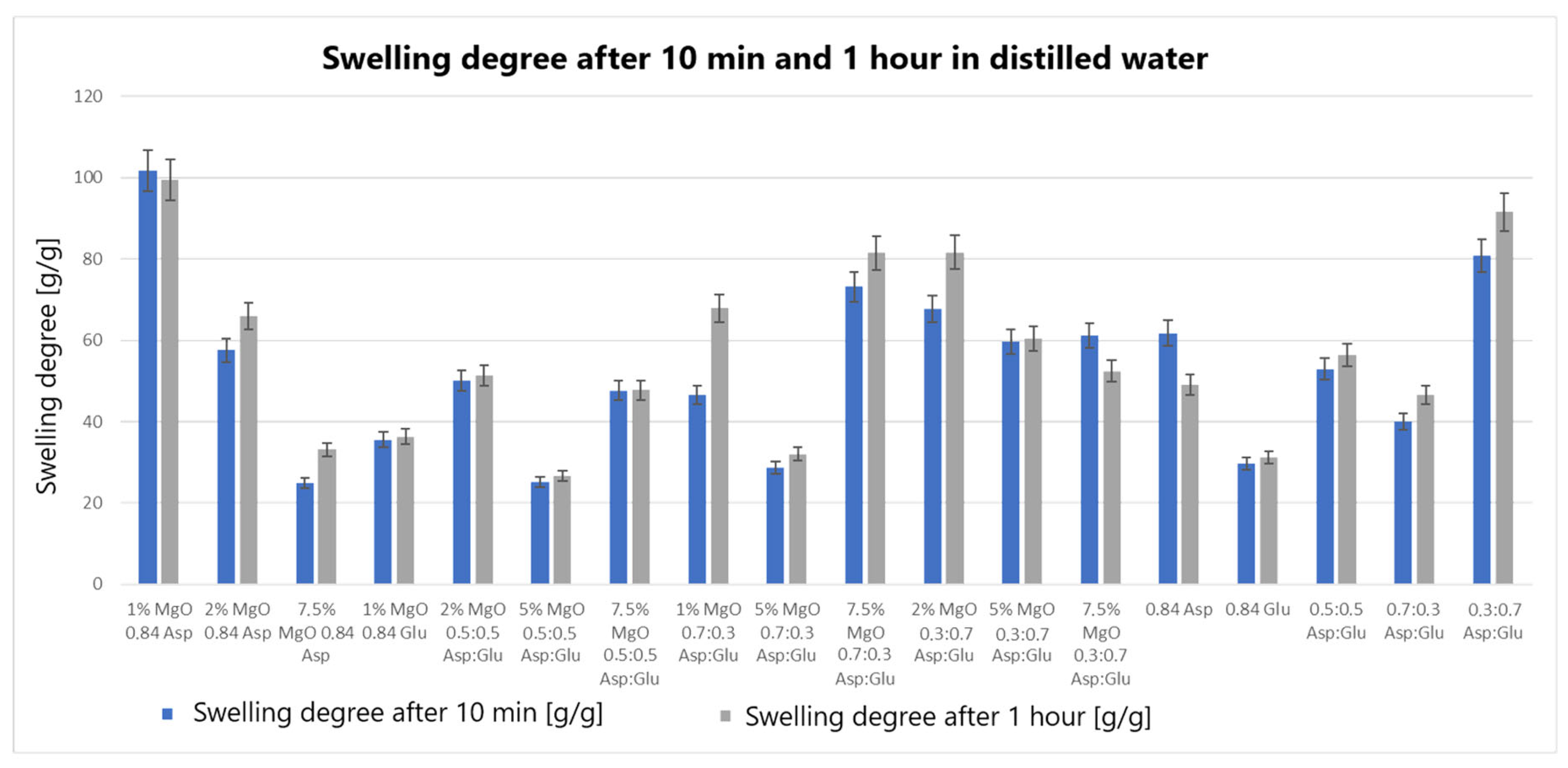
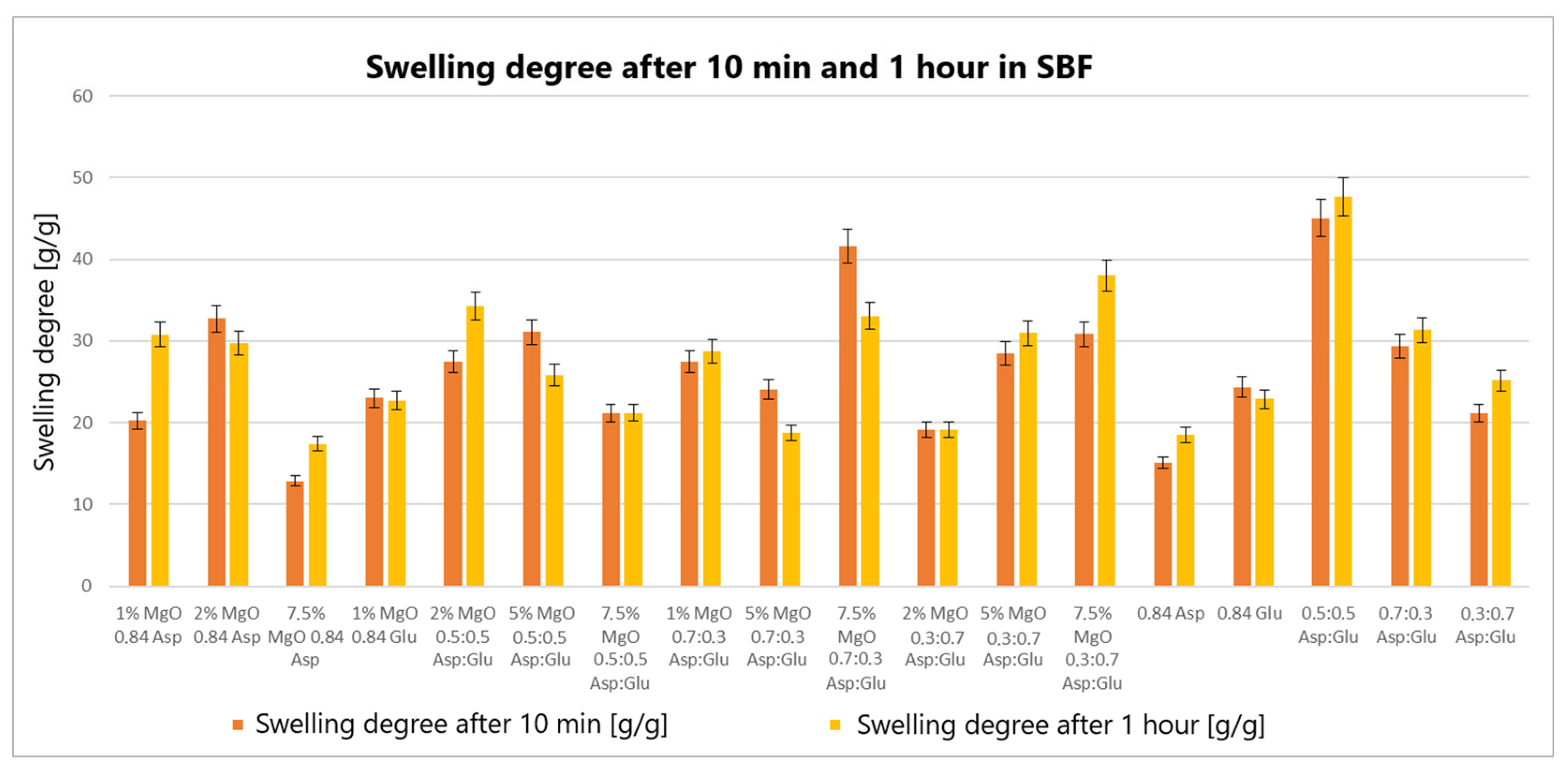
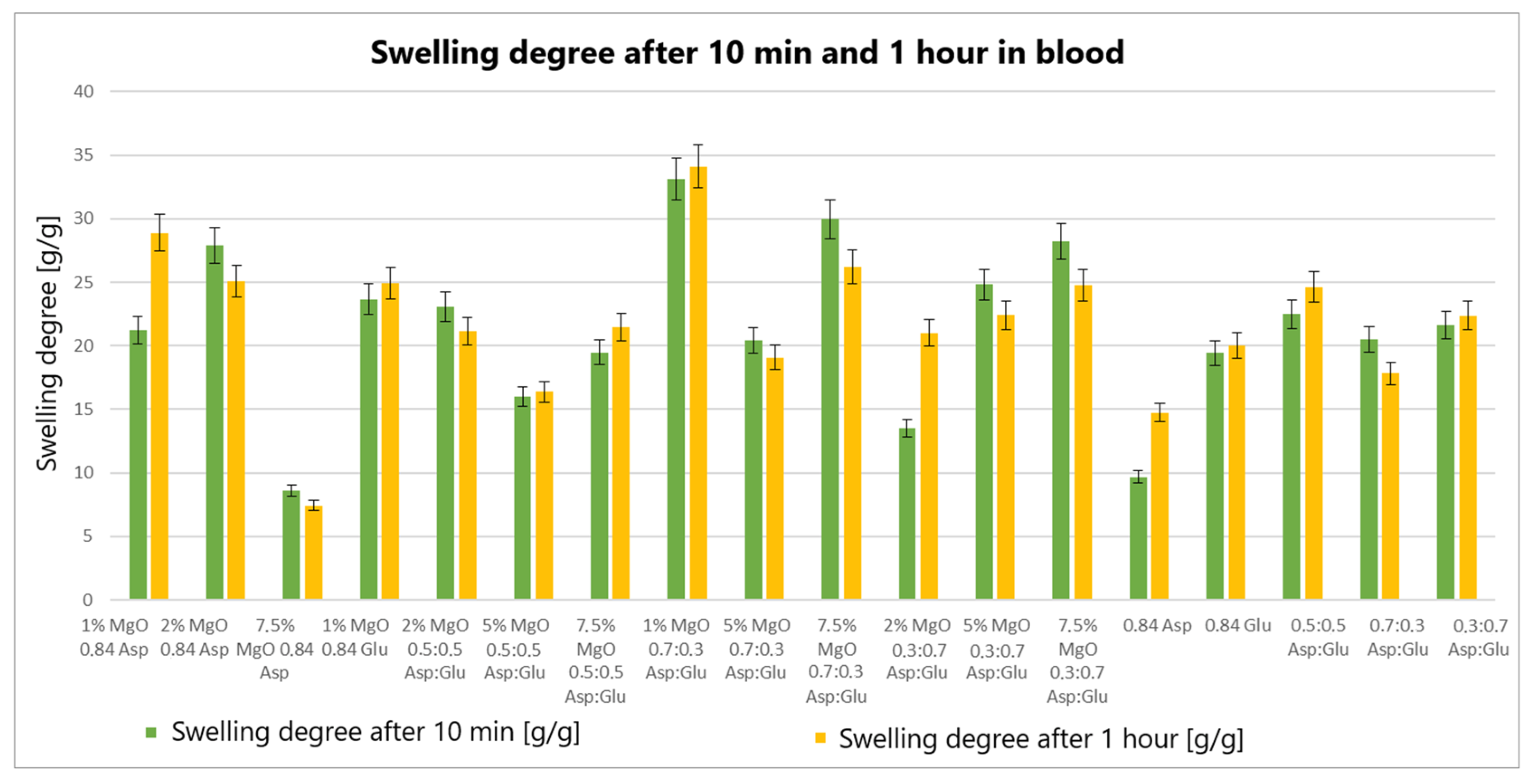
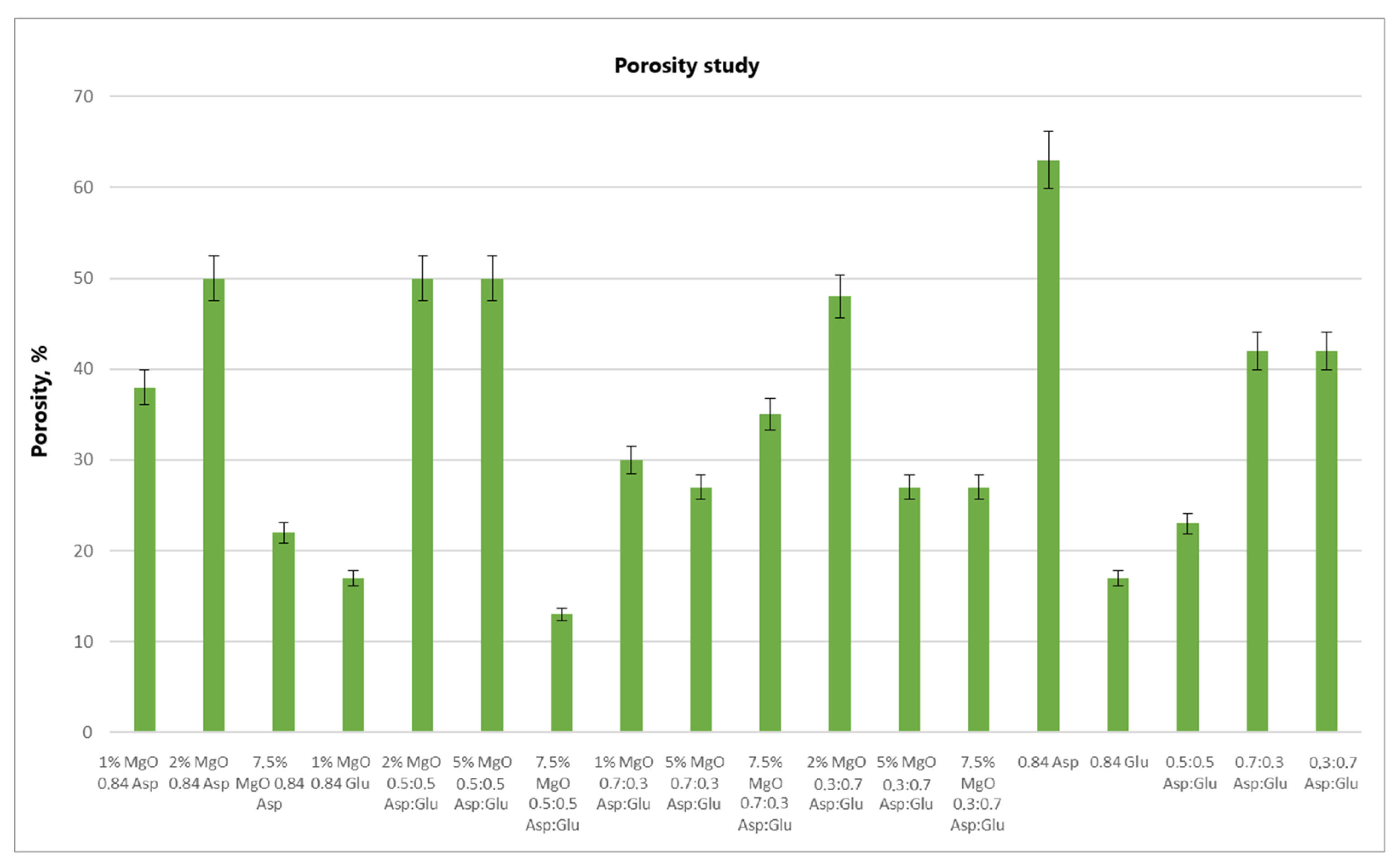
The swelling properties of chitosan-based hydrogels containing periclase (MgO) were compared to those of reference samples in water, simulated body fluid (SBF), and blood. Several formulations demonstrated significantly higher swelling ratios in all media, notably the samples 1% MgO 0.84 Asp, 2% MgO 0.84 Asp, and 7.5% MgO 0.7:0.3 Asp. These samples exhibited enhanced swelling in water (
Figure 4), SBF (
Figure 5), and blood (
Figure 6) compared to the reference hydrogels. Additionally, 1% MgO 0.84 Glu and 1% MgO 0.7:0.3 Asp showed higher swelling ratios in water and blood, while the 5% MgO 0.3:0.7 Asp and 7.5% MgO 0.3:0.7 Asp samples performed better in SBF and blood.
Several formulations exhibited swelling properties comparable to the reference samples. Specifically, in water, the swelling ratios of 2% MgO 0.84 Asp, 2% MgO 0.5:0.5 Asp, and 2% MgO 0.3:0.7 Asp were similar to those of the reference samples. In SBF, the swelling of 7.5% MgO 0.84 Asp and 1% MgO 0.84 Glu was in line with the reference, while in blood, the 5% MgO 0.7:0.3 Asp sample showed similar swelling behavior to the reference. On the other hand, reduced swelling ratios were observed for the 5% MgO 0.5:0.5 Asp, 7.5% MgO 0.5:0.5 Asp, and 5% MgO 0.7:0.3 Asp samples across all media. Additionally, lower swelling ratios were noted in water and SBF for 5% MgO 0.3:0.7 Asp and 7.5% MgO 0.3:0.7 Asp, in water and blood for 7.5% MgO 0.84 Asp, and in blood only for 2% MgO 0.3:0.7 Asp.
The highest swelling properties were observed for the 1% MgO 0.84 Asp sample in water, the 7.5% MgO 0.3:0.7 Asp sample in SBF, and the 1% MgO 0.7:0.3 Asp sample in blood. Conversely, the poorest swelling properties were recorded for 7.5% MgO 0.84 Asp in both SBF and blood, as well as for 5% MgO 0.5:0.5 Asp in water. Over time, certain samples exhibited a decrease in swelling ratios. In water, the 1% MgO 0.84 Asp sample showed a reduction, while in SBF, the 1% MgO 0.84 Glu and 5% MgO 0.5:0.5 Asp samples exhibited decreased swelling. In blood, the 2% MgO 0.5:0.5 Asp and 5% MgO 0.7:0.3 Asp samples experienced similar reductions. Furthermore, in SBF and blood, the 2% MgO 0.84 Asp, 5% MgO 0.7:0.3 Asp, and 7.5% MgO 0.7:0.3 Asp samples showed a noticeable reduction in swelling ratios. A decrease was also observed for 7.5% MgO 0.84 Asp and 7.5% MgO 0.3:0.7 Asp in water and blood. Conversely, some samples exhibited further swelling over time. The 1% MgO 0.84 Glu and 5% MgO 0.5:0.5 Asp samples swelled further in both water and blood, while the 2% MgO 0.5:0.5 Asp, 7.5% MgO 0.5:0.5 Asp, 5% MgO 0.3:0.7 Asp, and 7.5% MgO 0.84 Asp samples continued to swell in water and SBF. In addition, the 1% MgO 0.7:0.3 Asp, 2% MgO 0.3:0.7 Asp, and 7.5% MgO 0.5:0.5 Asp samples showed continuous swelling across all media. Notably, swelling ratios for the 2% MgO 0.84 Asp sample increased further in water, while 1% MgO 0.84 Asp and 7.5% MgO 0.3:0.7 Asp displayed an increase in SBF.
The swelling properties of chitosan-based hydrogels containing periclase varied significantly across different formulations and testing media. The formulations incorporating 1% MgO 0.84 Asp, 7.5% MgO 0.3:0.7 Asp, and 1% MgO 0.7:0.3 Asp displayed the most favorable swelling characteristics, while formulations with higher concentrations of MgO (5% and 7.5%) and certain Asp ratios demonstrated reduced swelling. Temporal changes in swelling behavior were also observed, with some samples swelling further over time and others exhibiting a decrease. These findings suggest that periclase incorporation and the composition of the Asp/Glutamate ratios influence the swelling properties of chitosan-based hemostatic agents, which may have implications for their performance in different physiological environments.
The swelling behavior of chitosan-based hydrogels containing periclase (MgO) is a crucial factor in determining their potential as hemostatic agents. Hemostasis, the process of stopping bleeding, relies on the ability of materials to rapidly absorb blood, promote clot formation, and provide a physical barrier to blood flow. The swelling properties of these hydrogels, particularly in blood, play a significant role in their effectiveness as hemostatic agents. The hydrogels exhibiting high swelling ratios in blood, such as the 1% MgO 0.84 Asp, 7.5% MgO 0.3:0.7 Asp, and 1% MgO 0.7:0.3 Asp samples, demonstrate an ability to absorb large volumes of liquid. This absorption capacity enables the hydrogels to maintain contact with the wound site, providing a larger surface area for interaction with blood components. Swelling in blood helps to quickly form a physical gel matrix that can effectively trap and concentrate blood cells, such as red blood cells and platelets, facilitating the formation of a blood clot. This localized clot formation is crucial for sealing the wound and preventing further blood loss.
The incorporation of MgO in the hydrogels may further enhance their hemostatic properties. Magnesium ions released from MgO can influence the coagulation cascade, potentially accelerating clot formation. Additionally, the periclase content can help neutralize acidic conditions at the wound site caused by blood acidification, promoting a more favorable environment for coagulation. The ability of these hydrogels to swell and create a gel-like matrix while interacting with blood components aligns with the mechanism of action required for an effective hemostatic agent. Furthermore, the continued swelling observed in certain formulations over time suggests that these hydrogels can adapt to the dynamic nature of wound healing. As the hydrogel swells, it may conform to the wound’s shape, filling gaps and providing additional structural support to the tissue. This sustained interaction with blood and the wound site can enhance the retention of clotting factors and blood cells, ensuring that the hemostatic effect persists as long as necessary. In contrast, hydrogels with lower swelling ratios in blood, such as those with 5% MgO 0.5:0.5 Asp and 7.5% MgO 0.5:0.5 Asp, may not provide the same level of fluid absorption or surface area for clot formation. These formulations may be less effective in rapidly stopping bleeding, as their reduced swelling capacity limits their ability to interact with the wound site and absorb blood effectively.
2.3. Chemical Structure Analysis
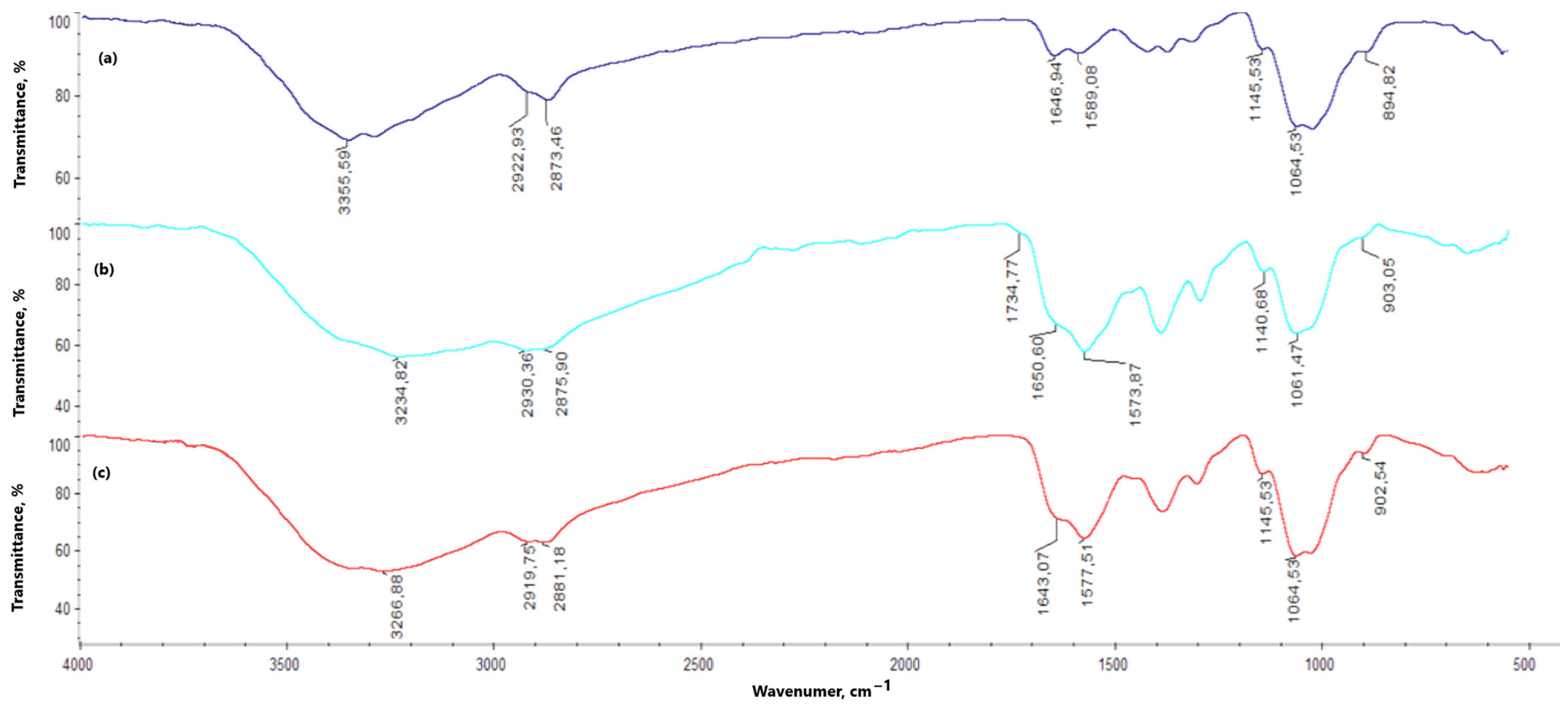
In the chitosan FT-IR spectrum (
Figure 7), a noticeable band appears at 3355.59 cm
−1, corresponding to the free hydroxy groups present in the chitosan molecule. This band is also observed in the spectra of the hydrogels, both with and without periclase (3100–3300 cm
−1); however, compared to the original chitosan band, it is broader and of lower intensity. The bands related to amino groups appear at 1589.08 and 1145.53 cm
−1, with a marked increase in their intensity for the hydrogel samples (both with and without periclase). The band at 1646.94 cm
−1 corresponds to N-acetylglucosamine. For all hydrogels (with and without periclase) cross-linked with L-aspartic acid, this band shows reduced intensity—1% MgO 0.84 Asp, 2% MgO 0.5:0.5 Asp, 5% MgO 0.5:0.5 Asp, 7.5% MgO 0.5:0.5 Asp, 1% MgO 0.7:0.3 Asp, 5% MgO 0.7:0.3 Asp, 7.5% MgO 0.7:0.3 Asp, 5% MgO 0.3:0.7 Asp, 7.5% MgO 0.3:0.7 Asp—or is not observed in the spectrum—2% MgO 0.84 Asp, 7.5% MgO 0.84 Asp. A different result is observed with L-glutamic acid as the primary cross-linking agent, where an increase in band intensity is noted relative to the reference sample and chitosan—1% MgO 0.84 Glu. When analyzing the hydrogels in terms of periclase concentration, a decrease in the intensity of this band is also observed. Bonds between -CH- and -CH
2- groups correspond to values of 2922.93 and 2873.46 cm
−1, while β-glycosidic bonds between chitosan mer units appear at 1064.53 cm
−1 (1060–1070 cm
−1). The band at 894.82 cm
−1 is attributed to glucopyranose rings. In the spectra of hydrogels, with and without periclase, these bands show no shifts. However, bands corresponding to the bonds between -CH- and -CH
2- groups (2800 to 3000 cm
−1) and β-glycosidic bonds (1060–1070 cm
−1) do exhibit shifts. In some samples without periclase (0.5:0.5 Asp, 0.7:0.3 Asp, and 0.3:0.7 Asp), bands are also noted at 1734.77, 1725.77, and 1727.93 cm
−1, corresponding to ester bonds formed between hydroxy groups in chitosan and carboxylic groups from amino acids. Below are the spectra of chitosan and samples with and without periclase.
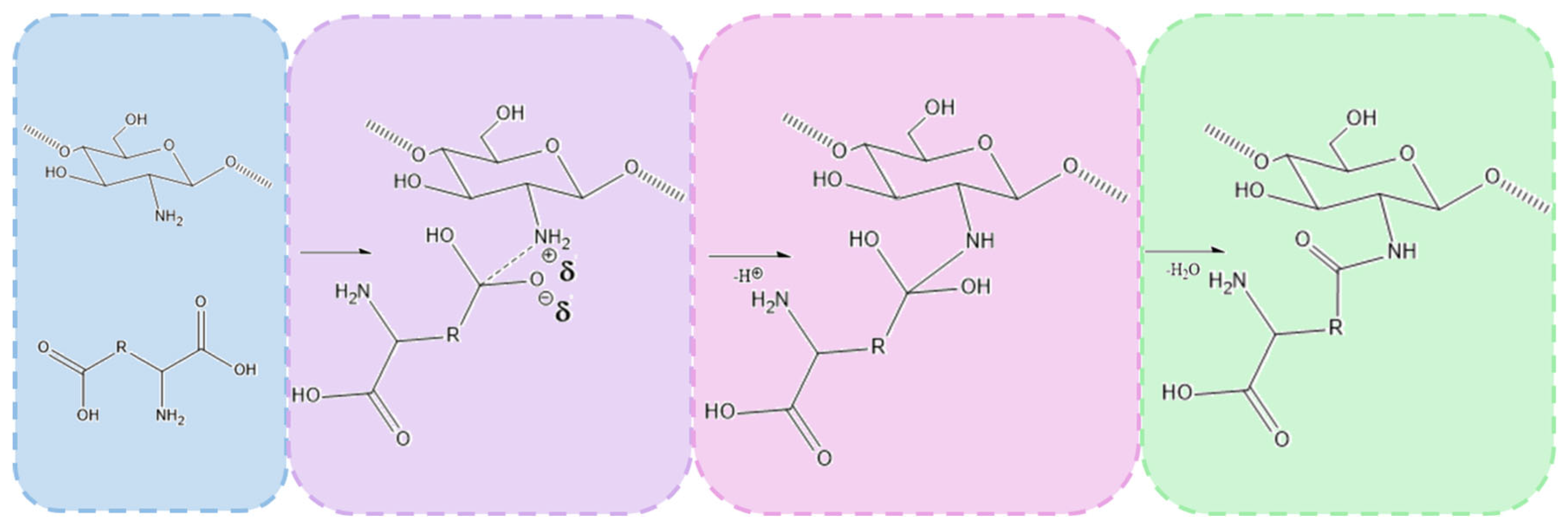
The chemical crosslinking mechanism between chitosan and amino acids containing two carboxylic groups, such as aspartic acid and glutamic acid, involves the formation of amide bonds through a condensation reaction between the amine groups (-NH2) of chitosan and the carboxylic groups (-COOH) of the amino acids. Under microwave-assisted conditions, this process is significantly enhanced due to rapid heating and increased molecular mobility, which facilitates efficient bond formation.
The reaction begins with the thermal activation of the carboxylic groups, leading to their conversion into more reactive intermediates, such as anhydrides or acyl species. This activation promotes a nucleophilic attack by the primary amine groups of chitosan, resulting in the formation of covalent amide (-CONH-) bonds that link the amino acids to the chitosan backbone. Given that both aspartic acid and glutamic acid contain two carboxylic groups, they can react with multiple chitosan chains, creating a crosslinked polymer network. This crosslinking process enhances the structural integrity and stability of the hydrogel.
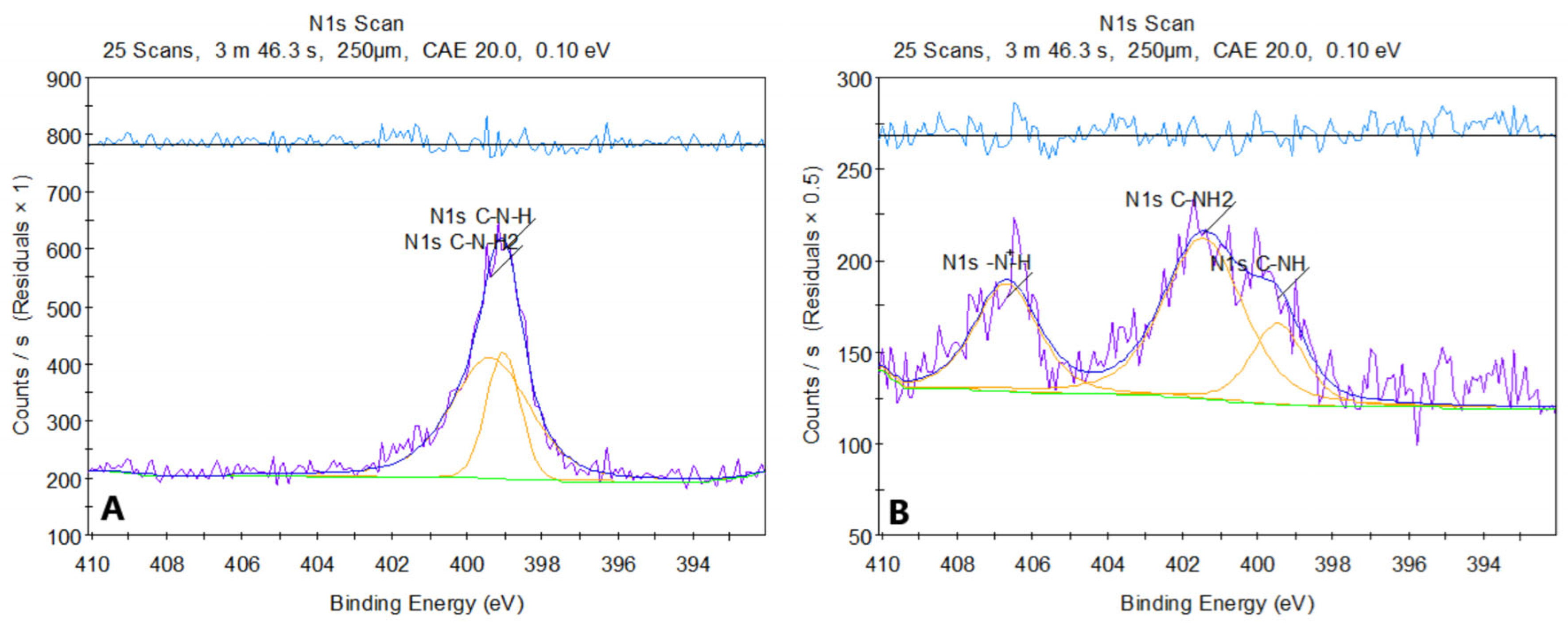
Figure 8 presents the XPS spectrum of pure chitosan (a) and sample 2. The XPS spectrum of chitosan before modification shows two peaks in the N 1s region, located around ~399 eV. The first corresponds to the amine group C-NH
2 (~399 eV), and the second to the amide group C-NH (~398.8–399.5 eV). Both signals are characteristic of the structures present in chitosan, where nitrogen exists in the form of amine and amide groups, without significant protonation or other strong chemical modifications.
After modification of chitosan with asparagine acid, the N 1s spectrum shows a shift in the peaks towards higher binding energies. The peak around ~400 eV (C-NH) has shifted compared to the original spectrum, which may suggest the formation of amide bonds (-CONH-) between chitosan and aspartic acid. Additionally, a new peak around ~401 eV (R-NH3+) appeared, indicating protonation of amine groups due to the reaction in an acidic environment. The new feature in the ~406–407 eV range is particularly noteworthy and may arise from protonated amides (CONH2+), suggesting that some of the amide or amine groups underwent modification, likely due to interactions with dicarboxylic acid.
The changes in the spectrum after modification indicate the introduction of new functional groups, such as amides, and protonation of amine groups. The analysis of this spectrum suggests that the chemical modification of chitosan with aspartic acid leads to the formation of new amide bonds and changes in the chemical environment of nitrogen.
The successful formation of amide bonds is confirmed by both FT-IR and XPS analyses. FT-IR spectra reveal the appearance of characteristic amide I (C=O stretching at ~1640–1660 cm
−1) and amide II (N-H bending at ~1550 cm
−1) bands. XPS analysis further supports the crosslinking mechanism by detecting a new N 1s peak (~399–401 eV), corresponding to amide nitrogen, along with shifts in the O 1s and C 1s spectra, indicative of newly formed amide linkages. Overall, microwave-assisted crosslinking between chitosan and dicarboxylic amino acids results in the formation of a stable, covalently bonded network that enhances the mechanical properties, hemostatic potential, and antibacterial activity of the hydrogel. Proposed mechanism is given in
Figure 9.
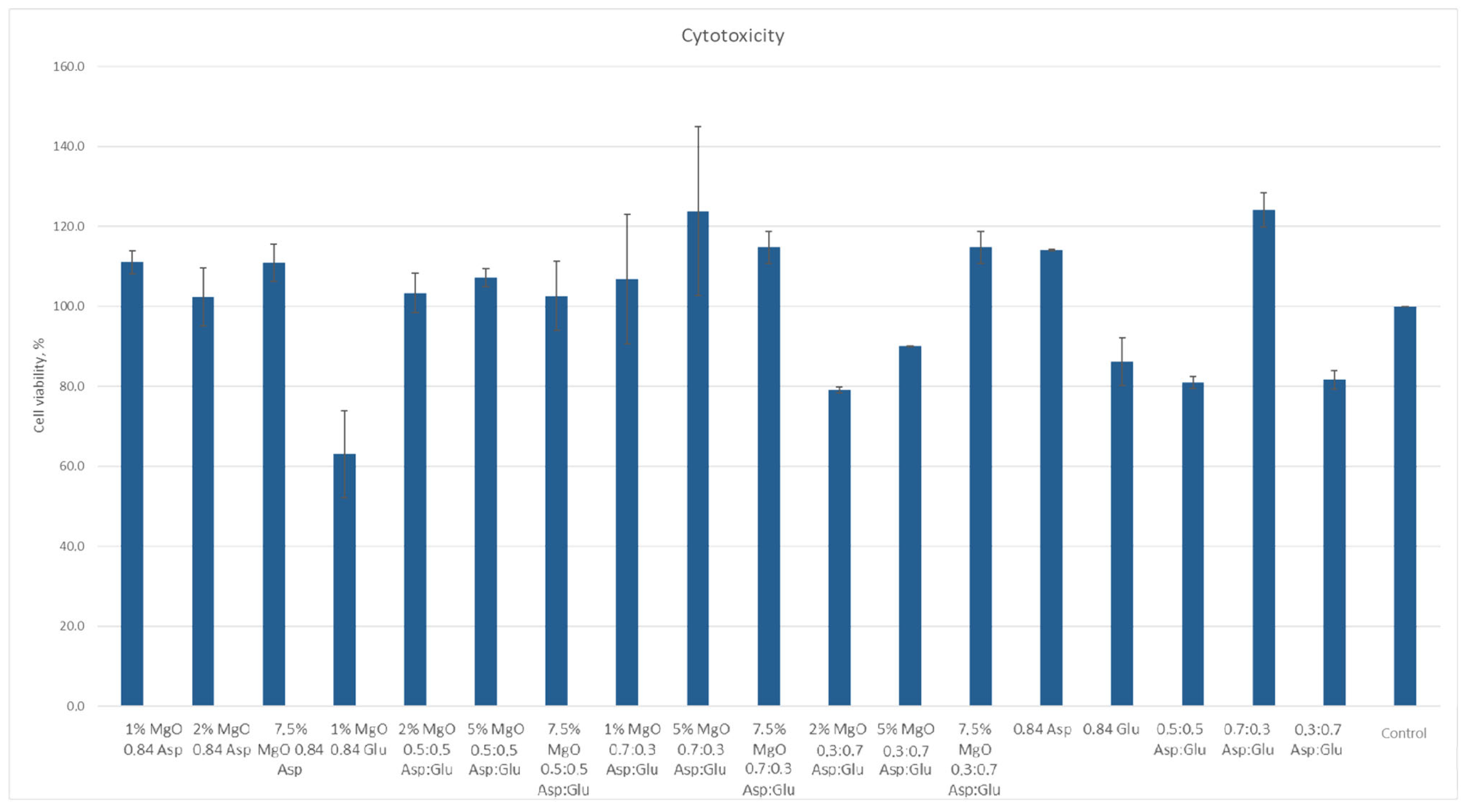
2.7. Cytototxicity Study
According to ISO 10933 [
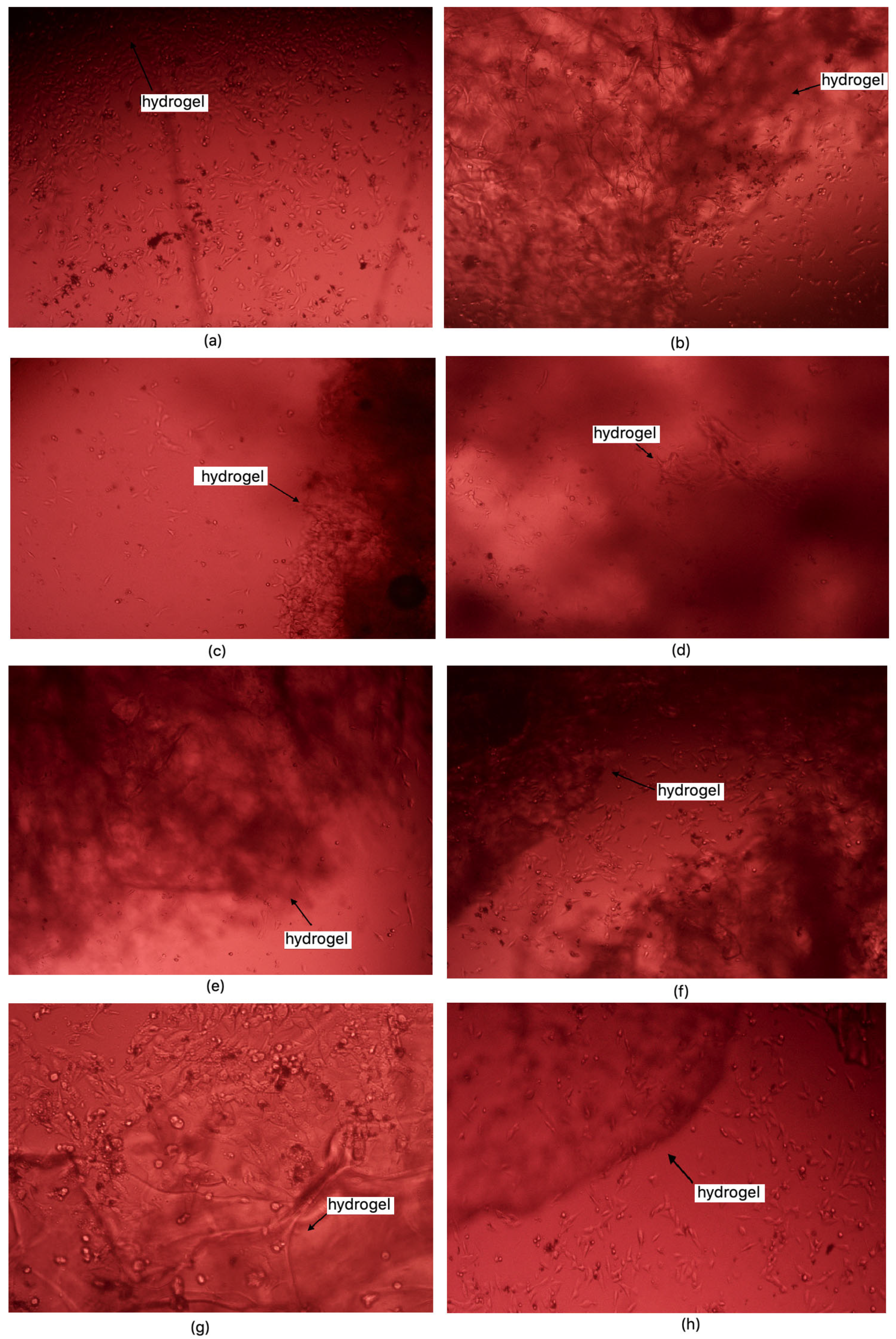
37] standards for medical devices, a material is considered non-cytotoxic if cell viability is at least 70% relative to the control sample. Among the samples studied, all except 1% MgO 0.84 Glu were non-cytotoxic. The sample with the lowest cytotoxicity was 5% MgO 0.7:0.3 Asp, while the highest cytotoxicity (excluding 1% MgO 0.84 Glu) was observed in 2% MgO 0.3:0.7 Asp. Increased cell proliferation relative to the control was observed in samples of 1% MgO 0.84 Asp, 2% MgO 0.84 Asp, 7.5% MgO 0.84 Glu, 2% MgO 0.5:0.5 Asp, 5% MgO 0.5:0.5 Asp, 7.5% MgO 0.5:0.5 Asp, 1% MgO 0.7:0.3 Asp, 5% MgO 0.7:0.3 Asp, 7.5% MgO 0.7:0.3 Asp, and 7.5% MgO 0.3:0.7 Asp. Greater cell survival in extracts of biomaterials containing periclase, compared to samples without it, was observed for 2% MgO 0.5:0.5 Asp, 5% MgO 0.5:0.5 Asp, 7.5% MgO 0.5:0.5 Asp, 5% MgO 0.3:0.7 Asp, and 7.5% MgO 0.3:0.7 Asp. Microscopic images show increased proliferation of L929 mouse fibroblasts on most hydrogel structures (except 1% MgO 0.84 Glu). A greater number of cells over time, comparing 24 to 48 h after the start of incubation, is also evident. Most cells display normal morphology, with an elongated shape and flat adherence to the surface of the plate. Dead cells, appearing dark and forming aggregates, are also visible in the microscopic images (
Figure 16 and
Figure 17).
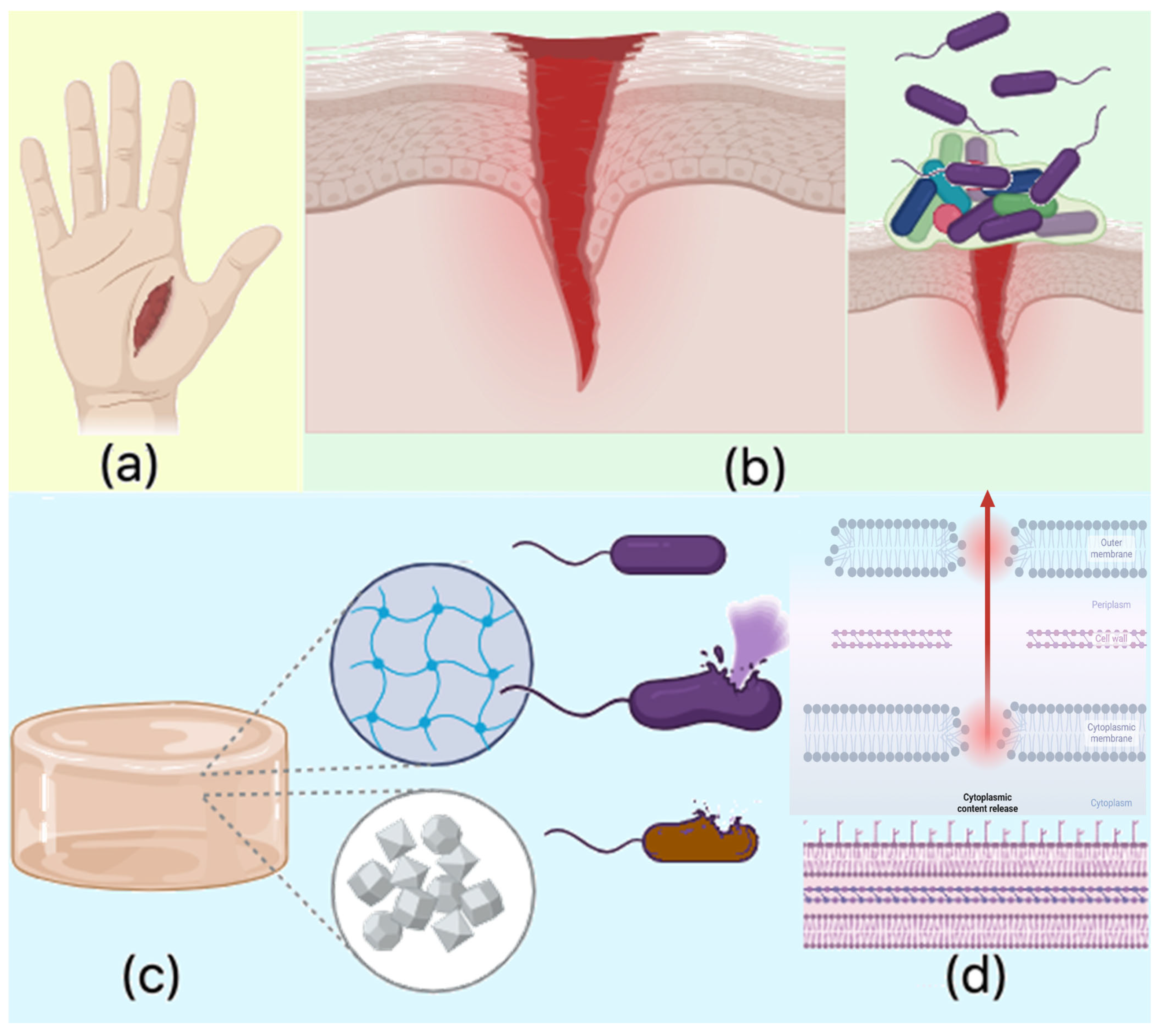
Figure 18 reveals possible future application of newly developed biomaterials. Both FT-IR and XPS analyses provided clear evidence of the successful crosslinking of chitosan with dicarboxylic amino acids—namely aspartic acid, glutamic acid, and their combination—resulting in the formation of amide bonds between the amino groups of chitosan and the carboxyl groups of the amino acids. This modification was crucial in enhancing the hemostatic properties of the material while maintaining the biological activity of chitosan. The introduction of amino acids as crosslinkers presents significant advantages over conventional chemical crosslinkers such as glutaraldehyde or genipin, which tend to drastically reduce the availability of amino groups due to excessive crosslinking. Chitosan’s hemostatic efficiency is largely attributed to its cationic nature, which stems from its free amino groups. However, when crosslinked with traditional agents, these groups are significantly diminished, leading to reduced biological activity. In contrast, the incorporation of amino acids as crosslinking agents helps to preserve the essential amino functionalities within the hydrogel network. Although amide bonds form between the amino groups of chitosan and the carboxyl groups of aspartic and glutamic acids, the final hydrogel structure retains free amino groups from the amino acids themselves. This is a crucial aspect, as these amino groups contribute to the hemostatic potential by maintaining interactions with negatively charged components of blood, promoting platelet adhesion and activation. Furthermore, the protonation of the amino groups in the amino acids at different pH values significantly broadens the biomedical applicability of the developed hydrogels. Unlike the amino groups in chitosan, which predominantly exist in a protonated NH
3+ state at acidic pH, the amino groups in the incorporated amino acids display pH-dependent protonation behavior. This property enhances the material’s functionality in varying physiological environments, ensuring effective hemostatic action across a broader range of conditions. Another advantage of this approach is the biocompatibility of the employed crosslinking agents. While glutaraldehyde and genipin are known to induce cytotoxicity due to the formation of highly reactive aldehyde or secondary crosslinking structures, the use of naturally occurring amino acids minimizes potential adverse biological effects. This makes the amino acid-crosslinked chitosan hydrogels more suitable for clinical applications, where minimizing immune responses and cytotoxicity is of paramount importance. Overall, the results indicate that chemical crosslinking with dicarboxylic amino acids is an effective strategy for enhancing the structural and hemostatic properties of chitosan-based hydrogels while preserving their biological activity.
2.8. Antibacterial Properties Study
The performed study demonstrated a notable inhibitory effect on the growth of both
Staphylococcus aureus and
Escherichia coli. Colony counting for
S. aureus was not conducted due to the density and merging of colonies. However, results for the higher dilution of samples were recorded and summarized in
Table 6,
Table 7,
Table 8 and
Table 9. Among the various hydrogel formulations, the sample 1% MgO 0.84 Glu exhibited the strongest antibacterial properties against both bacterial strains. However, given its cytotoxicity, the second-best antibacterial hydrogels were identified as 7.5% MgO 0.7:0.3 Asp for
E. coli and 5% MgO 0.3:0.7 Asp for
S. aureus. Nevertheless, all the hydrogels demonstrated inhibitory effects on the growth and proliferation of both bacterial strains, with a clear reduction in colony count observed 2 h and 24 h post-infection of the biomaterials.
The observed antibacterial properties align with existing knowledge regarding chitosan’s antimicrobial activity, which is primarily attributed to the protonable NH2 groups present on its molecular structure. These cationic groups facilitate electrostatic interactions with the negatively charged bacterial cell membranes, leading to membrane disruption and subsequent leakage of intracellular contents. Chitosan’s antimicrobial effect is multifaceted: chitosan of lower molecular mass can penetrate bacterial cells and interact with negatively charged genetic material, such as DNA, while chitosan with higher molecular mass tends to interact with the cell wall and membrane components, blocking both passive and active nutrient transport. Furthermore, chitosan can chelate crucial ions like Mg2+ and Ca2+, disrupting essential cellular processes.
In this study, the antibacterial activity of the chitosan-based hydrogels was further enhanced by the incorporation of periclase nanoparticles (MgO). The release of magnesium ions (Mg
2+) from MgO contributes to membrane destabilization, impeding bacterial metabolic functions and ultimately leading to cell death. Additionally, the magnesium ions may induce oxidative stress by generating reactive oxygen species (ROS), which are harmful to bacterial cells. This oxidative damage further disrupts bacterial cell functioning, contributing to the hydrogels’ antibacterial properties. The crystalline nature of the MgO nanoparticles may also interfere with biofilm formation, a critical factor in bacterial resistance, by preventing the attachment and accumulation of bacterial cells at the wound site. Additionally, the hydrogel’s ability to swell in biological fluids such as simulated body fluid (SBF) and blood increases its surface area, enhancing its interaction with bacterial cells. This physical property, combined with the chemical antibacterial effects of chitosan and MgO, prevents bacterial adhesion and proliferation, significantly reducing the risk of infection at the wound site. The combination of chitosan and MgO in the hydrogel matrix results in a potent antibacterial effect against both
S. aureus and
E. coli, with enhanced activity observed against
E. coli. The antibacterial properties are attributed to several mechanisms, including the disruption of bacterial cell membranes by chitosan, ion chelation, and the generation of oxidative stress by magnesium ions. Moreover, the crystalline nature of MgO nanoparticles contributes to the inhibition of biofilm formation. These properties make the chitosan-based MgO hydrogels highly effective in preventing bacterial infection, establishing them as promising candidates for use as hemostatic agents in wound care applications (
Figure 19).
The number of colony-forming units (CFU) of
Escherichia coli is summarized in
Table 6.
After accounting for the dilutions and the volume of bacterial suspension taken for swabbing.
The number of colony-forming units (CFU) of
Staphylococcus aureus is summarized in
Table 8.