Analysis of Macroporous Resin Combined Extraction and Purification of Polyphenols from Agrimonia pilosa Ledeb. and Anti-Tumor Effect In Vitro
Abstract
:1. Introduction
2. Results
2.1. Results of Macroporous Resins
2.2. Adsorption Dynamics
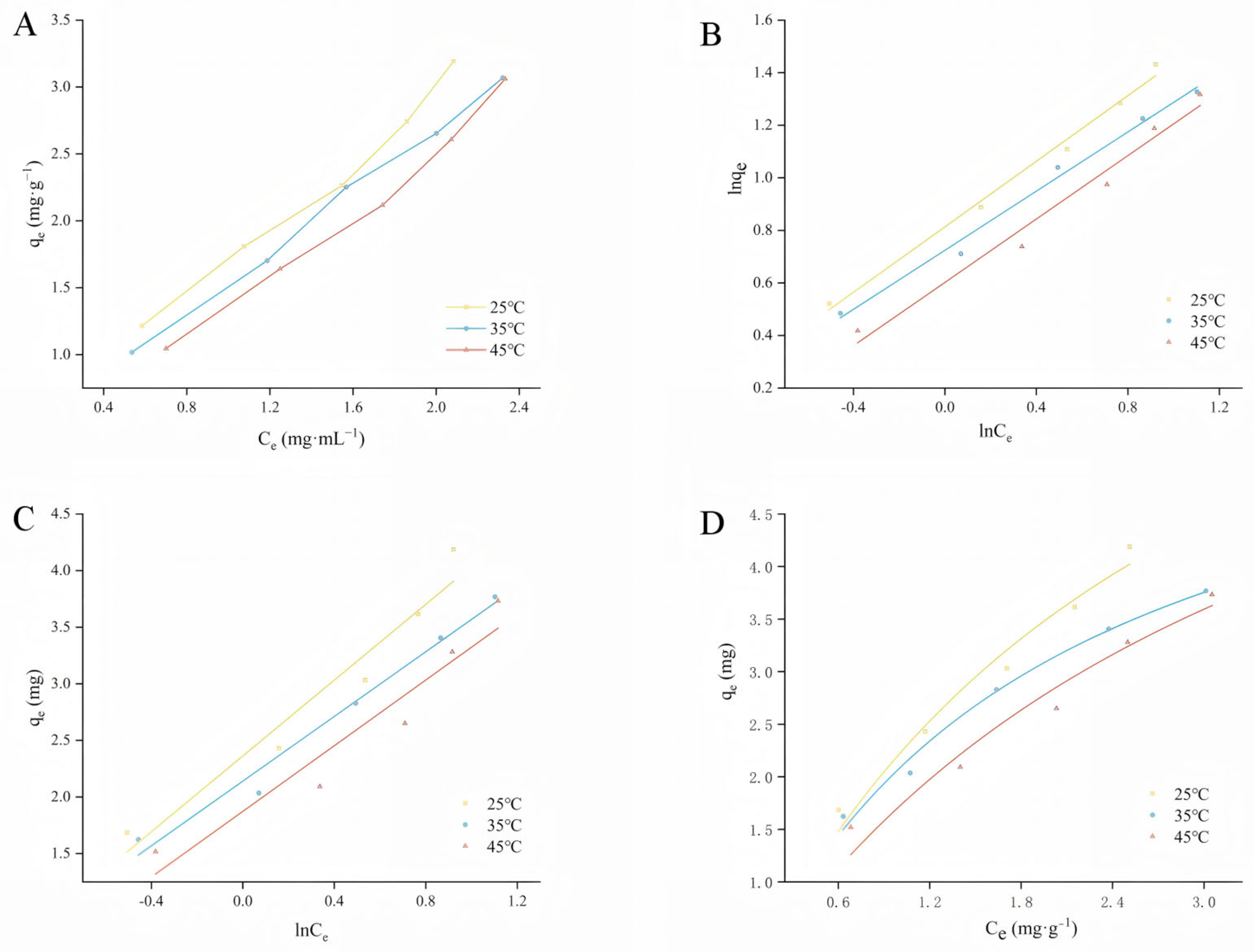
2.3. Adsorption Isotherm
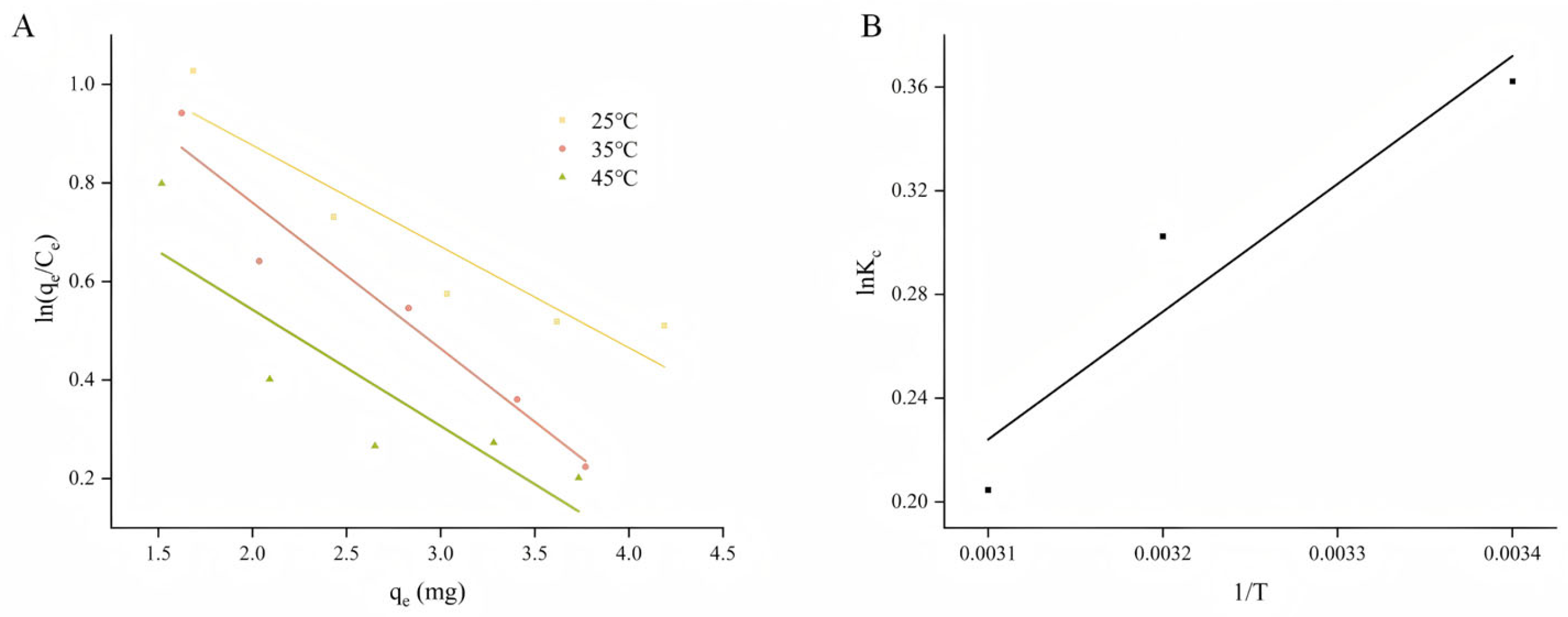
2.4. Adsorption Thermodynamics
2.5. Dynamic Adsorption and Desorption
2.5.1. Dynamic Leakage Curve
2.5.2. Selection of Eluting Agents and Dosage
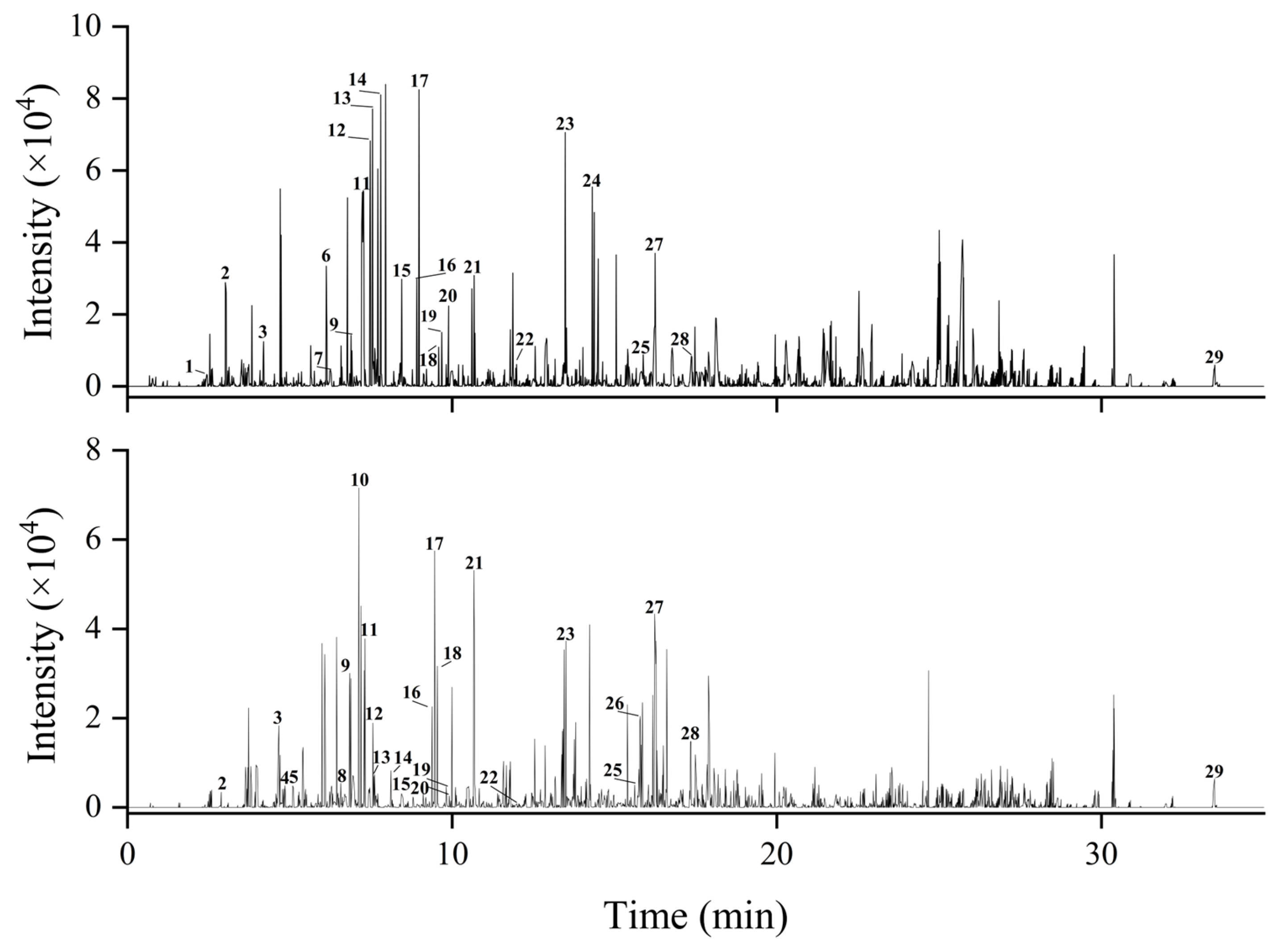
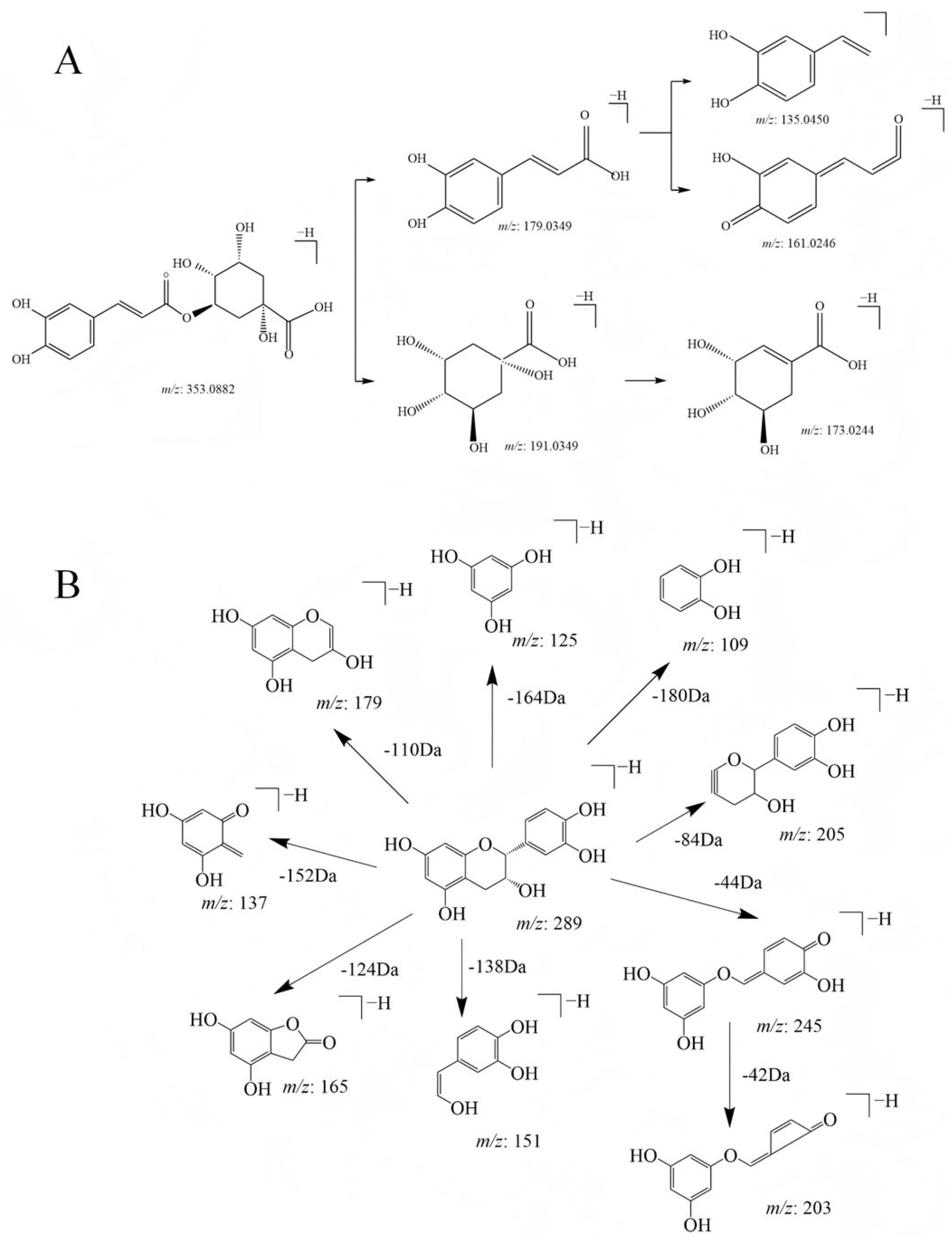
2.6. Characterization of the Extraction Products from the Macroporous Resin of APL
2.7. UHPLC-C-QTRAP-MS/MS-Based Quantitative Analysis of the Macroporous Resin-Extracted Products of APL
2.7.1. Methodological Validation
2.7.2. Selection of Eluting Agents and Dosage
2.8. Antiproliferative Activity of CT26 Tumor Cells and Wound-Healing Assay
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Pretreatment of Macroporous Resins
4.3. Extraction of Crude APL
4.4. Quantification of Total Polyphenols Content
4.5. Resin Screening
4.6. Adsorption Kinetics
- pseudo-first-order kinetic model equation:
- pseudo-second-order kinetic model equation:
- particle diffusion model equation:where qt represents the adsorption capacity of the interval time t (mg·g−1 resin) and qe is the equilibrium adsorption per unit of resin (mg·g−1 resin); k1, k2, and ki correspond to the first-order kinetic constant, the second-order kinetic constant and the particle diffusion kinetic constant, respectively. C is a constant in the particle diffusion model.
4.7. Adsorption Isotherm and Adsorption Thermodynamics
4.8. Dynamic Adsorption and Desorption
4.8.1. Dynamic Leakage Curve
4.8.2. Selection of Eluting Agents and Dosage
4.9. Liquid–Liquid Extraction Test
4.10. Macroporous Resin-Based Qualitative and Quantitative Analysis of APL Extraction Products
4.10.1. Preparation of Standard Solutions
4.10.2. Sample Preparation
4.10.3. Characterization of the Extraction Products from the Macroporous Resin of APL
4.10.4. UHPLC-C-QTRAP-MS/MS-Based Quantitative Analysis of the Macroporous Resin-Extracted Products of APL
4.11. Antiproliferative Activity of CT26 Tumor Cells
4.12. Wound-Healing Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.Q.; Wu, M.H.; Zhou, Y.; Guo, R.Q.; He, N.Y.; Zhu, Y. Exploration of Professor ZHOU Zhongying’s Academic Philosophy on Syndrome Differentiation and Treatment for Digestive System Malignant Tumors. J. Tradit. Chin. Med. 2013, 54, 1186–1188. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.Y.; Zhou, Z.Y. Analysis of Herb Used in Malignant Tumor Treatment Based on Phlegm Syndrome Differentiation by ZHOU Zhongying. J. Tradit. Chin. Med. 2015, 56, 740–744. [Google Scholar] [CrossRef]
- Tao, L.H.P.; Lai, Y.Y.; Cheng, H.B.; Sheng, W.X.; Tan, J.N.; Xu, C.L.; Yu, C.T.; Fan, M.M.; Li, L.; Sheng, Z.J. Effect of Xianlian Jiedu Prescription on Proliferation and Glycolysis of Human Colorectal Cancer HCT-116 Cells and Mechanism. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 72–78. [Google Scholar] [CrossRef]
- Peng, L.R. The Intervention Effect and Mechanism Research of Herba Agrimoniae and Rhizoma Coptidis for Colorectal Inflammatory Cancer Transformation. Master’s Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2022. [Google Scholar]
- Li, J.; Wang, Y.X.; Li, J.S.; Zhang, Y.; Wang, R.R. Screening of Main Effective Components of Compound Agrimonia pilosula Enteritis Capsules and Targets of Enteritis. China Pharm. 2021, 32, 927–932. [Google Scholar]
- Huang, T.; Zhao, C.C.; Xue, M.; Cao, Y.F.; Chen, L.K.; Chen, J.X.; Sun, Y.J.; Zeng, J. Current Progress and Outlook for Agrimonolide: A Promising Bioactive Compound from Agrimonia pilosa Ledeb. Pharmaceuticals 2023, 16, 150. [Google Scholar] [CrossRef]
- Hnit, S.S.T.; Ding, R.; Bi, L.; Xie, C.; Yao, M.; De Souza, P.; Xu, L.; Li, Z.; Dong, Q. Agrimol B present in Agrimonia pilosa Ledeb impedes cell cycle progression of cancer cells through G(0) state arrest. Biomed. Pharmacother. 2021, 141, 111795. [Google Scholar] [CrossRef]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anti-Cancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Lü, P. Inhibitory effects of hyperoside on lung cancer by inducing apoptosis and suppressing inflammatory response via caspase-3 and NF-κB signaling pathway. Biomed. Pharmacother. 2016, 82, 216–225. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Afshari, K.; Haddadi, N.S.; Haj-Mirzaian, A.; Farzaei, M.H.; Rohani, M.M.; Akramian, F.; Naseri, R.; Sureda, A.; Ghanaatian, N.; Abdolghaffari, A.H. Natural flavonoids for the prevention of colon cancer: A comprehensive review of preclinical and clinical studies. J. Cell. Physiol. 2019, 234, 21519–21546. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Igura, K.; Ohta, T.; Kuroda, Y.; Kaji, K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001, 171, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhang, W.; Liu, H.; Jiang, Y.; Li, F.; Wang, D.; Liu, Y.; He, F.; Wu, M.; Ivan Neil Waterhouse, G.; et al. Simultaneous binding of quercetin and catechin to FOXO3 enhances IKKα transcription inhibition and suppression of oxidative stress-induced acute alcoholic liver injury in rats. J. Adv. Res. 2025, 67, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Oriakhi, K.; Orumwensodia, K.O. Combinatorial effect of Gallic acid and Catechin on some biochemical and pro-inflammatory markers in CCl4-mediated hepatic damage in rats. Phytomedicine Plus 2021, 1, 100017. [Google Scholar] [CrossRef]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef]
- Yang, F.Q.; Liu, M.; Li, W.; Che, J.P.; Wang, G.C.; Zheng, J.H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol. Med. Rep. 2015, 11, 1085–1092. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Umeoguaju, F.U.; Akaninwor, J.O.; Essien, E.B.; Amadi, B.A.; Chukeze, E.J.; Nwafor, I.R. Macroporous adsorptive resin-assisted enrichment of polyphenol from Psidium guajava leaves improved its in vitro antioxidant and anti-hemolytic properties. Prep. Biochem. Biotechnol. 2023, 53, 841–848. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Seo, U.M.; Zhao, B.T.; Le, D.D.; Seong, S.H.; Choi, J.S.; Min, B.S.; Woo, M.H. Ellagitannin and flavonoid constituents from Agrimonia pilosa Ledeb. with their protein tyrosine phosphatase and acetylcholinesterase inhibitory activities. Bioorganic Chem. 2017, 72, 293–300. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, X.; Ma, C.; Sun, L.; Feng, Y.; He, Z.; Zhang, H. Purification of polyphenols from Phyllanthus emblica L. pomace using macroporous resins: Antioxidant activity and potential anti-Alzheimer’s effects. J. Food Sci. 2022, 87, 1244–1256. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, J.L.; Li, Z.; Zang, X.Y.; Yang, X.W. Separation and Enrichment of Alkaloids from Coptidis Rhizoma and Euodiae Fructus by Macroporous Resin and Evaluation of the Effect on Bile Reflux Gastritis Rats. Molecules 2022, 27, 724. [Google Scholar] [CrossRef]
- Aljawarneh, R.Y.A.; Che Zain, M.S.; Zakaria, F. Macroporous polymeric resin for the purification of flavonoids from medicinal plants: A review. J. Sep. Sci. 2024, 47, e2400372. [Google Scholar] [CrossRef]
- Pang, J.; Dong, W.; Li, Y.; Xia, X.; Liu, Z.; Hao, H.; Jiang, L.; Liu, Y. Purification of Houttuynia cordata Thunb. Essential Oil Using Macroporous Resin Followed by Microemulsion Encapsulation to Improve Its Safety and Antiviral Activity. Molecules 2017, 22, 293. [Google Scholar] [CrossRef]
- Mi, J.; Zhang, M.; Ren, G.; Zhang, H.; Wang, Y.; Hu, P. Enriched separation of protopanaxatriol ginsenosides, malonyl ginsenosides and protopanaxadiol ginsenosides from Panax ginseng using macroporous resins. J. Food Eng. 2012, 113, 577–588. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, M.; Cao, B.; Che, L.; Su, Y.; Zhou, X.; Li, X.; Chen, C.; Li, G.; Bai, C. Optimization of extraction, separation and purification of baicalin in Scutellaria baicalensis using response surface methodology. Ind. Crops Prod. 2024, 214, 118555. [Google Scholar] [CrossRef]
- Meng, L.; Gui, X.; Yun, Z. Static and dynamic studies of adsorption by four macroporous resins to enrich oridonin from Rabdosia rubescens. Chin. J. Chem. Eng. 2021, 32, 151–158. [Google Scholar] [CrossRef]
- Ahmad, A.; Razali, M.H.; Mamat, M.; Mehamod, F.S.B.; Anuar Mat Amin, K. Adsorption of methyl orange by synthesized and functionalized-CNTs with 3-aminopropyltriethoxysilane loaded TiO2 nanocomposites. Chemosphere 2017, 168, 474–482. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cheng, J.; Zhao, J.; Shi, R.; He, L.; Li, Q.; Chen, Y. Efficient purification of flavonoids from bamboo shoot residues of Phyllostachys edulis by macroporous resin and their hypoglycemic activity. Food Chem. X 2022, 16, 100505. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Huang, X.; Xia, S.; Chen, C.; Nie, Q.; Nie, S. Efficient enrichment of total flavonoids from kale (Brassica oleracea L. var. acephala L.) extracts by NKA-9 resin and antioxidant activities of flavonoids extract in vitro. Food Chem. 2022, 374, 131508. [Google Scholar] [CrossRef]
- Xiong, N.; Yu, R.; Chen, T.; Xue, Y.-P.; Liu, Z.-Q.; Zheng, Y.-G. Separation and purification of l-methionine from E. coli fermentation broth by macroporous resin chromatography. J. Chromatogr. B 2019, 1110–1111, 108–115. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Yuanfeng, W.; Lei, Z.; Jianwei, M.; Shiwang, L.; Jun, H.; Yuru, Y.; Lehe, M. Kinetic and thermodynamic studies of sulforaphane adsorption on macroporous resin. J. Chromatogr. B 2016, 1028, 231–236. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D. Adsorption kinetics, isotherm and thermodynamics studies of flavones from Vaccinium Bracteatum Thunb leaves on NKA-2 resin. Chem. Eng. J. 2014, 254, 579–585. [Google Scholar] [CrossRef]
- Wang, W.; Dong, S.; Zhang, W.; Qi, Z.; Liu, X. Study on adsorption/resolution properties, enrichment and purification of phenolic substances of Inonotus hispidus by macroporous adsorption resin. Ind. Crops Prod. 2024, 216, 118661. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.a.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B 2020, 1138, 121960. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Hernandez, J.; Müller, A.; Jaiswal, R.; Davalos, J.Z.; Kuhnert, N. Energy resolved mass spectrometry of chlorogenic acids and its application to isomer quantification by direct infusion tandem mass spectrometry. Phytochem. Anal. 2018, 29, 406–412. [Google Scholar] [CrossRef]
- Mutungi, M.M.; Muema, F.W.; Kimutai, F.; Xu, Y.-B.; Zhang, H.; Chen, G.-L.; Guo, M.-Q. Antioxidant and Antiproliferative Potentials of Ficus glumosa and Its Bioactive Polyphenol Metabolites. Pharmaceuticals 2021, 14, 266. [Google Scholar] [CrossRef]
- Kagbo-Kue, S.; Ajose, T.; Bakinde, N. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018, 8, 36. [Google Scholar] [CrossRef]
- Ranjbary, A.G.; Bagherzadeh, A.; Sabbaghi, S.S.; Faghihi, A.; Karimi, D.N.; Naji, S.; Kardani, M. Chlorogenic acid induces apoptosis and cell-cycle arrest in colorectal cancer cells. Mol. Biol. Rep. 2023, 50, 9845–9857. [Google Scholar] [CrossRef]
- Huo, L.; Bai, X.; Wang, Y.; Wang, M. Betulinic acid derivative B10 inhibits glioma cell proliferation through suppression of SIRT1, acetylation of FOXO3a and upregulation of Bim/PUMA. Biomed. Pharmacother. 2017, 92, 347–355. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, J.H.; Park, S.; Kweon, D.H.; Kim, S.H.; Ko, S.G. Inhibitory effect of Agrimonia pilosa Ledeb. on inflammation by suppression of iNOS and ROS production. Immunol. Investig. 2010, 39, 159–170. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Deng, T.; Jia, J.; Luo, N.; Li, H. A dual-task method for the simultaneous detoxification and enrichment of stilbene glycoside from Polygonum multiflorum roots extract by macroporous resin. Chem. Eng. J. 2014, 237, 138–145. [Google Scholar] [CrossRef]
- Oliveira, V.d.C.; Rodrigues, S.d.O.; Souto, S.M.T.; da Silva, G.A.; Vilegas, W.; Ferri, B.G.; El-Gazzar, A.M.; Batiha, G.E.-S.; Mahmoud, M.H.; Silva, M.J.D.; et al. Chemical profile and evaluation of the pharmacological activity of the dry extract and fraction of ethyl acetate obtained from the leaves of Mimosa caesalpiniifolia. J. Ethnopharmacol. 2024, 323, 117716. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol Mechanisms against Gastric Cancer and Their Interactions with Gut Microbiota: A Review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef]





| T (K) | ΔS (J·mol−1) | ΔH (KJ·mol−1K) | ||
|---|---|---|---|---|
| ΔG (kJ·mol−1) | ||||
| 298 | 308 | 318 | ||
| −0.90 | −0.77 | −0.54 | −10.84 | −4.09 |
| NO. | R (min) | Identification | Molecular Formula | MW | Measured (m/z) | Characteristic Fragment Ions | Error (ppm) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.47 | 3-Hydroxy-4-methoxybenzoic acid | C8H8O4 | 167.0350 | 167.0362 | 163.0303, 152.0115, 123.0437, 108.0217 | 1.7 | [M-H]− |
| 2 | 2.85 | Protocatechuic acid | C7H6O4 | 153.0193 | 153.0203 | 109.0301, 108.6876, 108.0213, 91.0205, 81.0356 | 6.4 | [M-H]− |
| 3 | 4.4 | Catechin | C15H14O6 | 289.0718 | 289.0724 | 135.0453, 151.0397, 179.0341, 187.0394, 203.0721, 245.0821, 271.0603 | 2.4 | [M-H]− |
| 4 | 5.02 | Caffeic acid | C9H8O4 | 179.0350 | 179.0357 | 135.0439, 134.0360, 117.0358, 89.0417 | 4.1 | [M-H]− |
| 5 | 5.04 | Procyanidin B3 | C30H26O12 | 577.1351 | 577.1346 | 451.1023, 425.0867, 407.0767, 287.0543, 289.0710, 161.0235, 125.0242 | −1 | [M-H]− |
| 6 | 5.72 | (1R,3S,4R,5S)-1,3,4-trihydroxy-5-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxy-cyclohexane-1-carboxylic acid | C16H18O8 | 338.1002 | 337.0929 | 173.0463, 163.0410, 155.0355 | 0.8 | [M-H]− |
| 7 | 6.29 | 4-O-Feruloylquinic acid | C17H20O9 | 368.1107 | 367.1035 | 193.0495, 191.0569, 149.0603, 134.0371, 117.0340 | 0 | [M-H]− |
| 8 | 6.69 | p-Coumaric acid | C9H8O3 | 164.0473 | 163.0401 | 119.0661, 119.0482, 92.9962 | 7.1 | [M-H]− |
| 9 | 6.85 | Gallic acid | C7H6O5 | 169.0143 | 169.0152 | 125.0235, 123.0071, 107.0192, 95.0112, 79.0184 | 5.3 | [M-H]− |
| 10 | 6.97 | Dehydrodicatechin A | C30H24O12 | 575.1195 | 575.1193 | 575.1215, 449.0933, 394.0695, 309.0075, 271.0235, 229.0492, 161.0222, 137.0230 | −0.2 | [M-H]− |
| 11 | 7.3 | Ellagic acid | C14H6O8 | 300.9990 | 300.9991 | 283.9954, 257.0068, 229.0128, 185.0248 | 0.4 | [M-H]− |
| 12 | 7.45 | Rutin | C27H30O16 | 610.1539 | 610.1497 | 447.0893, 413.0954, 300.0516, 300.0263, 271.0226, 151.0040 | 0 | [M-H]− |
| 13 | 7.64 | Hyperoside | C21H20O12 | 463.0882 | 463.0878 | 301.0357, 300.0275, 271.0248, 255.0302, 243.0303, 178.9988, 151.0036 | −0.9 | [M-H]− |
| 14 | 7.85 | Kaempferol | C15H10O6 | 285.0405 | 285.0408 | 217.0491, 198.0295, 175.0426, 151.0041, 133.0297, 132.0214, 121.0280, 107.0153 | 1.1 | [M-H]− |
| 15 | 7.87 | Taxifolin | C15H12O7 | 303.0510 | 303.0516 | 285.04808, 275.0511, 217.0514, 199.0361, 175.0379, 153.0182, 125.022 | 0.9 | [M-H]− |
| 16 | 8.9 | Chlorogenic acid | C16H18O9 | 353.0878 | 353.0882 | 135.0450, 161.0246, 173.0244, 179.0349, 191.0560 | 0.8 | [M-H]− |
| 17 | 8.97 | Quercitrin | C21H20O11 | 447.0933 | 447.0934 | 301.0355, 300.0277, 271.0252, 255.0304, 151.0038 | 0.2 | [M-H]− |
| 18 | 9.67 | Dihydrokaempferol | C15H12O6 | 287.0561 | 287.0567 | 287.0783, 259.0572, 243.0669, 177.0544, 155.0511, 152.0092, 125.0247, 106.0428 | 0.5 | [M-H]− |
| 19 | 9.82 | Isochlorogenic acid B | C25H24O12 | 516.1268 | 515.1195 | 447.1014, 353.0903, 255.0770, 191.0561, 179.0344, 173.0462, 135.0423 | −0.6 | [M-H]− |
| 20 | 9.97 | Dihydrocaffeic acid | C9H10O4 | 182.0579 | 181.0506 | 153.0179, 136.9219, 111.0065, 109.0294, 108.0211, 107.0155 | 6.5 | [M-H]− |
| 21 | 10.62 | Cynaroside | C21H20O10 | 431.0984 | 431.0983 | 229.0503, 227.0346, 255.0299, 285.0403, 284.0323, 431.0974 | 0.2 | [M-H]− |
| 22 | 12.02 | Isochlorogenic acid A | C25H24O12 | 516.1268 | 515.1195 | 173.0495, 353.0903, 173.0457, 179.0344, 191.0561, 135.0423, 179.00540 | −0.6 | [M-H]− |
| 23 | 13.49 | Quercetin | C15H10O7 | 301.0358 | 301.0356 | 283.9909, 257.0407, 219.0657, 178.9984, 151.0032, 121.0292 | −0.6 | [M-H]− |
| 24 | 14.32 | Tiliroside | C30H26O13 | 593.1301 | 593.1306 | 447.0919, 307.0814, 285.0392, 284.0314, 255.0299, 227.0339 | 0.9 | [M-H]− |
| 25 | 15.87 | Apigenin | C15H10O5 | 269.0455 | 269.0463 | 269.0451, 225.0536, 151.0045, 121.0260, 117.0348, 107.0128 | −0.5 | [M-H]− |
| 26 | 16.09 | Kaempferide | C16H12O6 | 299.0561 | 299.0557 | 284.0319, 299.0558 | −1.9 | [M-H]− |
| 27 | 16.29 | Luteolin | C15H10O6 | 285.0405 | 285.0408 | 198.0295, 175.0426, 151.0041, 133.0297 | −1.6 | [M-H]− |
| 28 | 17.35 | Agrimonolide-6-O-glucopyranoside | C24H28O10 | 475.1610 | 475.1603 | 313.1080, 312.8826, 298.0841, 148.0532 | −2 | [M-H]− |
| 29 | 33.31 | Agrimol B | C37H46O12 | 681.2917 | 681.2952 | 653.3028, 447.1237, 285.1277, 116.9352 | 5.2 | [M-H]− |
| Analyte | Linearity | LOD (ng·mL−1) | LOQ (ng·mL−1) | Content (ng·mg−1) | |||
|---|---|---|---|---|---|---|---|
| Calibration Curve | Range (ng·mL−1) | r2 | BEA | ECA | |||
| Catechin (3) | Y = 1849.5x − 3145.2 | 3.37–200.73 | 0.9977 | 1.78 | 3.37 | 66.67 ± 1.33 | 46.00 ± 2.00 |
| Hyperoside (13) | Y = 22,989x − 12,386 | 1.15–199.54 | 0.9980 | 0.80 | 1.15 | 382.56 ± 3.65 | 239.40 ± 2.60 |
| Chlorogenic acid (16) | Y = 6655.7x − 5239 | 1.94–198.5 | 0.9988 | 1.04 | 1.94 | 10.60 ± 0.05 | 3.42 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Z.; Li, H.; Li, T.; Taoli, H.; Pan, L. Analysis of Macroporous Resin Combined Extraction and Purification of Polyphenols from Agrimonia pilosa Ledeb. and Anti-Tumor Effect In Vitro. Molecules 2025, 30, 1478. https://doi.org/10.3390/molecules30071478
Mei Z, Li H, Li T, Taoli H, Pan L. Analysis of Macroporous Resin Combined Extraction and Purification of Polyphenols from Agrimonia pilosa Ledeb. and Anti-Tumor Effect In Vitro. Molecules. 2025; 30(7):1478. https://doi.org/10.3390/molecules30071478
Chicago/Turabian StyleMei, Zhanghao, Huifen Li, Tingting Li, Huiping Taoli, and Linmei Pan. 2025. "Analysis of Macroporous Resin Combined Extraction and Purification of Polyphenols from Agrimonia pilosa Ledeb. and Anti-Tumor Effect In Vitro" Molecules 30, no. 7: 1478. https://doi.org/10.3390/molecules30071478
APA StyleMei, Z., Li, H., Li, T., Taoli, H., & Pan, L. (2025). Analysis of Macroporous Resin Combined Extraction and Purification of Polyphenols from Agrimonia pilosa Ledeb. and Anti-Tumor Effect In Vitro. Molecules, 30(7), 1478. https://doi.org/10.3390/molecules30071478




