A Facile One-Pot Preparation and Catalytic Application of Tunable Silica-Coated Aqueous Gold Nanoparticles
Abstract
:1. Introduction
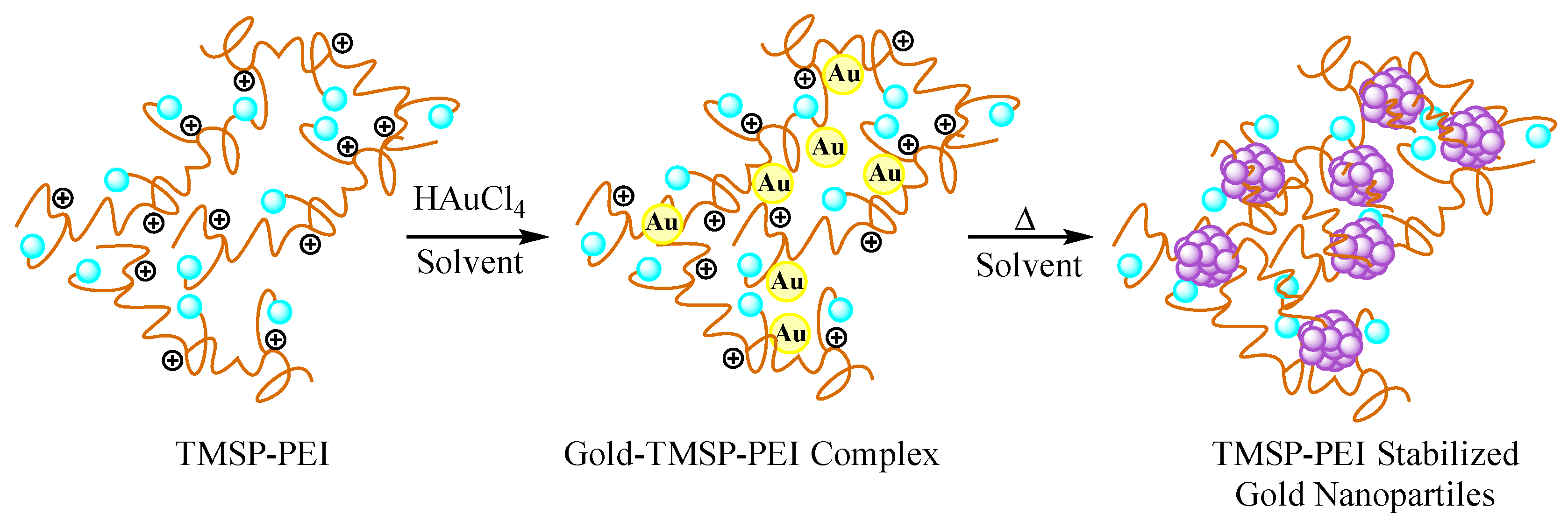
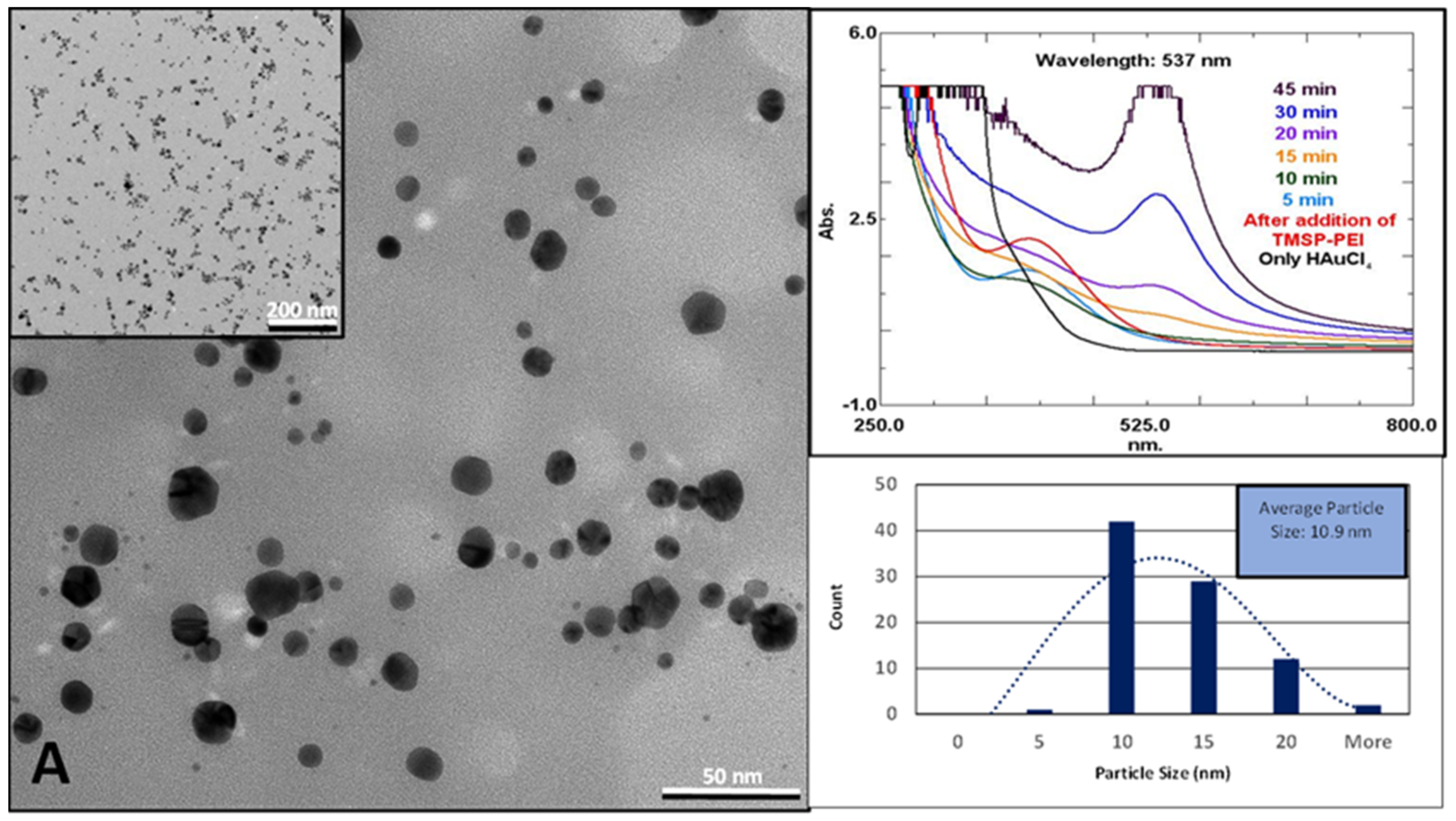
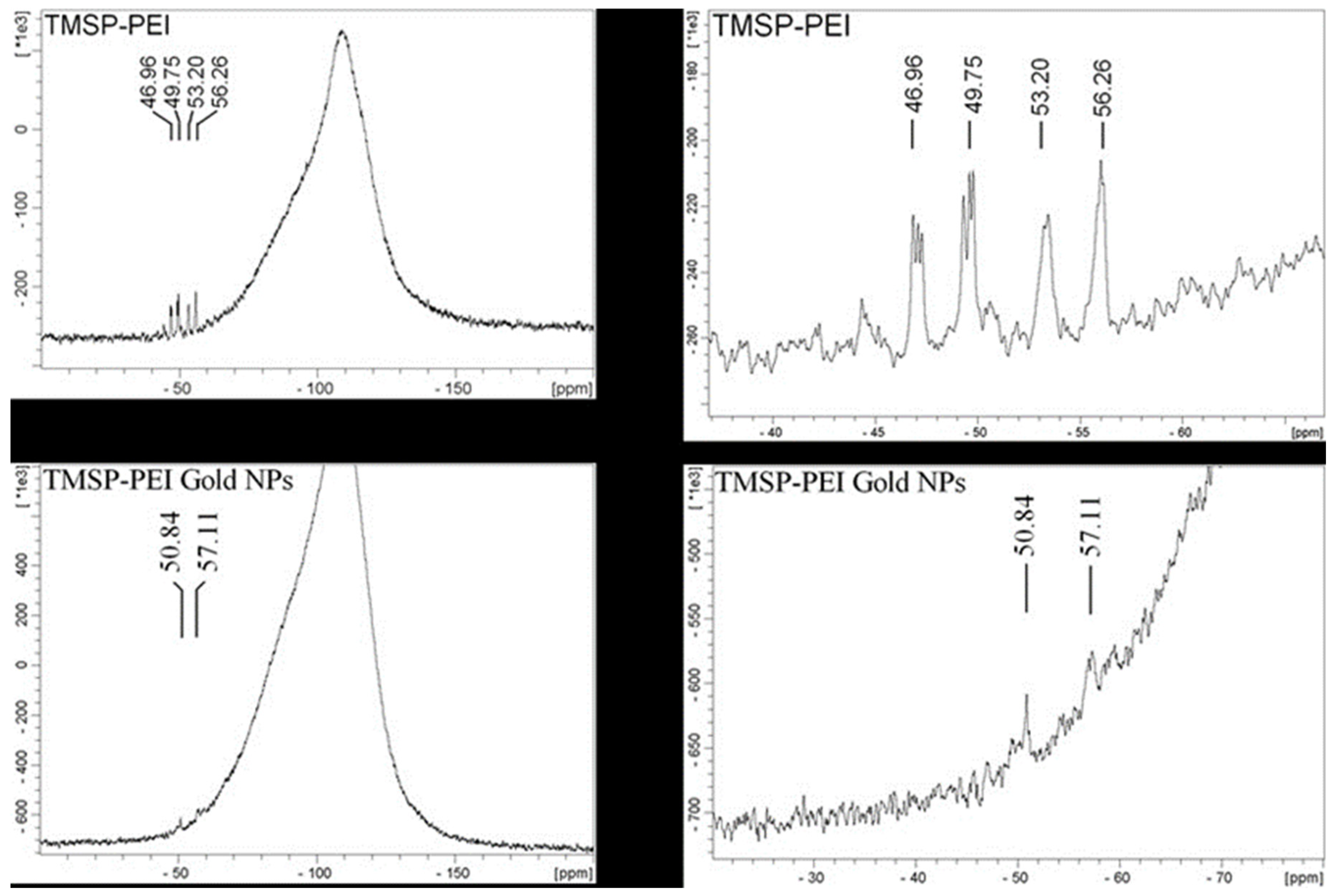
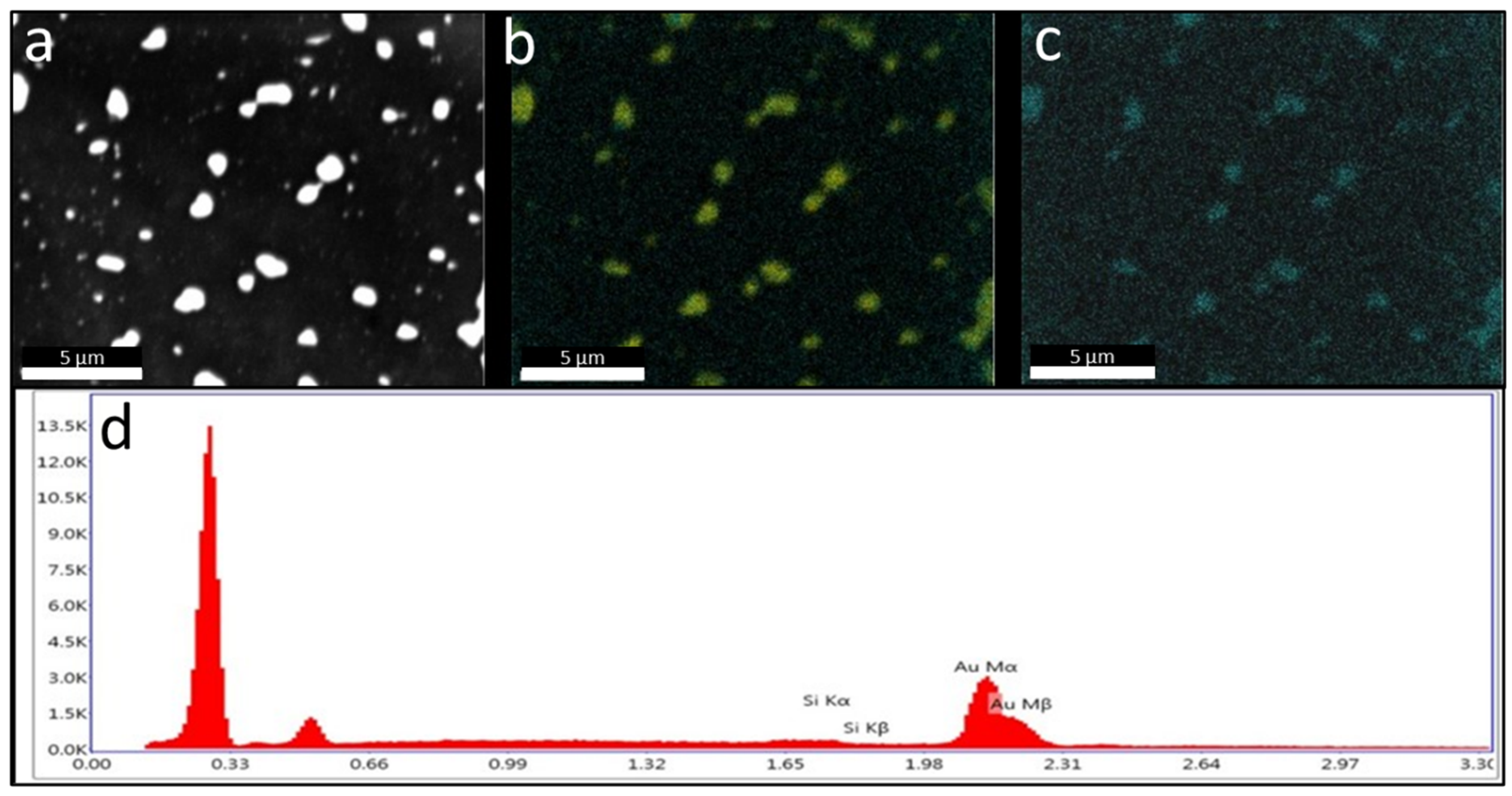
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Silica-Coated Gold Nanoparticles
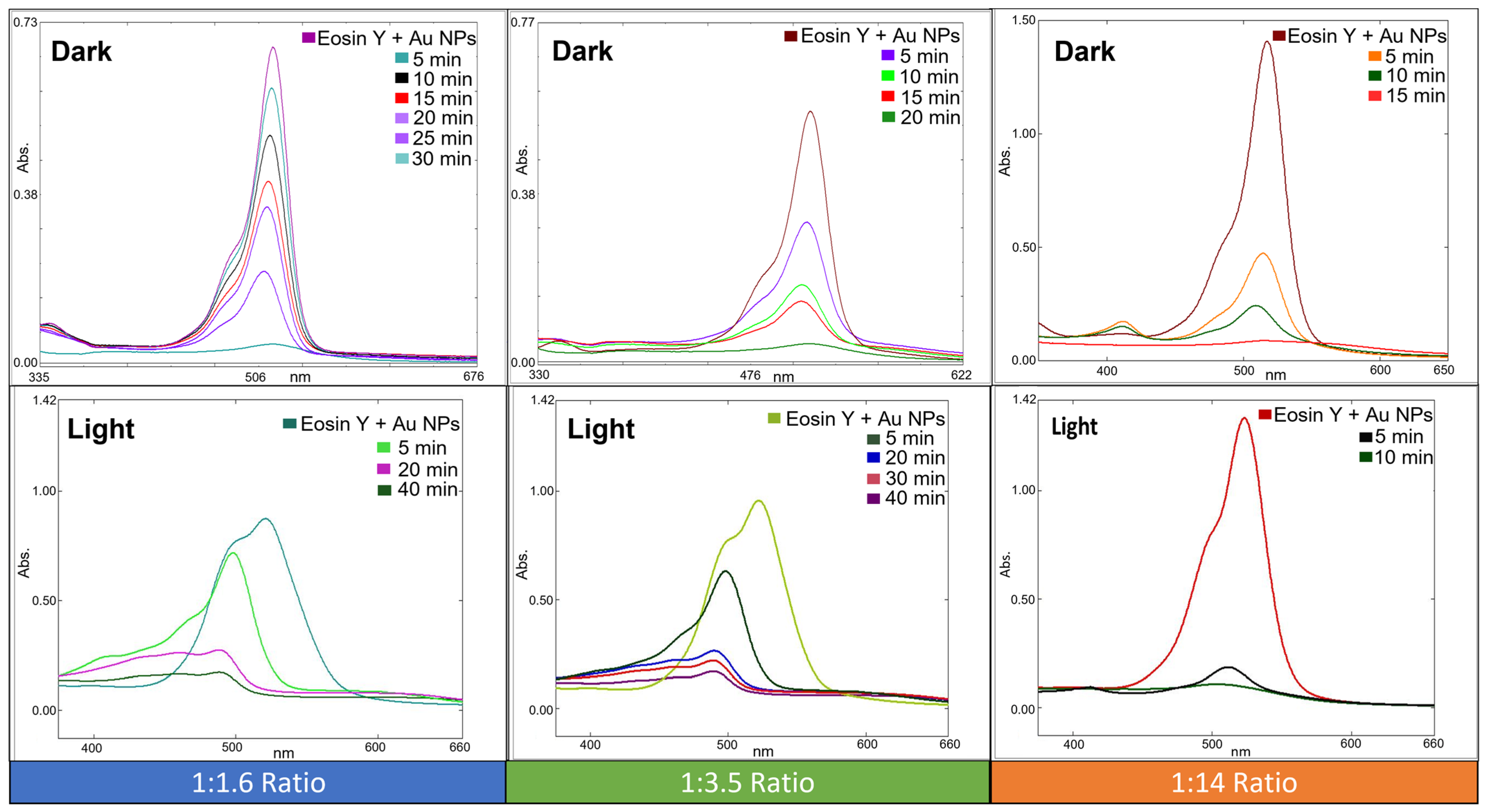
3.3. Experimental Procedure for the Catalytic Reduction of Eosin Y
3.3.1. General Experimental Setup for the Investigation of the Catalytic Reduction of Eosin Y in the Dark
3.3.2. General Experimental Setup for the Investigation of the Catalytic Reduction of Eosin Y in the Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| Au NPs | Gold nanoparticles |
| PEI | Polyethylenimime |
| TMSP-PEI | Trimethoxysilylpropyl-(polyethylenimime) |
| Eosin Y | Tetrabormofluorescein |
| IPA | Isopropanol |
References
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Mochrie, S.G.J.; Lurio, L.B.; Rühm, A.; Lennox, R.B. Polymer-Stabilized Gold Nanoparticles and Their Incorporation into Polymer Matrices. J. Am. Chem. Soc. 2001, 123, 10411–10412. [Google Scholar] [CrossRef]
- Sindram, J.; Krüsmann, M.; Otten, M.; Pauly, T.; Nagel-Steger, L.; Karg, M. Versatile Route toward Hydrophobically Polymer-Grafted Gold Nanoparticles from Aqueous Dispersions. J. Phys. Chem. B 2021, 125, 8225–8237. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Lee, S.-H.; Lee, S.; Choi, S.-H.; Hawker, C.J.; Kim, B.-S. Highly Stable Au Nanoparticles with Double Hydrophilic Block Copolymer Templates: Correlation between Structure and Stability. Polym. Chem. 2017, 8, 4528–4537. [Google Scholar] [CrossRef]
- Bhol, P.; Mohanty, M.; Mohanty, P.S. Polymer-Matrix Stabilized Metal Nanoparticles: Synthesis, Characterizations and Insight into Molecular Interactions between Metal Ions, Atoms and Polymer Moieties. J. Mol. Liq. 2021, 325, 115135. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Lennox, R.B. Polymer-Stabilized Gold Nanoparticles with High Grafting Densities. Langmuir 2004, 20, 2867–2873. [Google Scholar] [CrossRef]
- Pérignon, N.; Mingotaud, A.-F.; Marty, J.-D.; Rico-Lattes, I.; Mingotaud, C. Formation and Stabilization in Water of Metal Nanoparticles by a Hyperbranched Polymer Chemically Analogous to PAMAM Dendrimers. Chem. Mater. 2004, 16, 4856–4858. [Google Scholar] [CrossRef]
- Zhang, F.; Lees, E.; Amin, F.; Rivera Gil, P.; Yang, F.; Mulvaney, P.; Parak, W.J. Polymer-Coated Nanoparticles: A Universal Tool for Biolabelling Experiments. Small 2011, 7, 3113–3127. [Google Scholar] [CrossRef]
- Virca, C.N.; Winter, H.M.; Goforth, A.M.; Mackiewicz, M.R.; McCormick, T.M. Photocatalytic Water Reduction Using a Polymer Coated Carbon Quantum Dot Sensitizer and a Nickel Nanoparticle Catalyst. Nanotechnology 2017, 28, 195402. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A ‘green’ Chitosan–Silver Nanoparticle Composite as a Heterogeneous as Well as Micro-Heterogeneous Catalyst. Nanotechnology 2007, 19, 015603. [Google Scholar] [CrossRef]
- Kelsch, A.; Tomcin, S.; Rausch, K.; Barz, M.; Mailänder, V.; Schmidt, M.; Landfester, K.; Zentel, R. HPMA Copolymers as Surfactants in the Preparation of Biocompatible Nanoparticles for Biomedical Application. Biomacromolecules 2012, 13, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-J.; Du, J.-Z.; Sun, T.-M.; Zhang, P.-Z.; Wang, J. Gold Nanoparticles Capped with Polyethyleneimine for Enhanced siRNA Delivery. Small 2010, 6, 239–246. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. Controlled Synthesis of Polyethylenimine Coated Gold Nanoparticles: Application in Glutathione Sensing and Nucleotide Delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ling, M.; Jia, M.; Huang, P.; Li, C.; Yan, B. Facile Synthesis and Characterization of Polyethylenimine-Coated Fe3O4 Superparamagnetic Nanoparticles for Cancer Cell Separation. Mol. Med. Rep. 2014, 9, 1080–1084. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zu, Y.; Fu, Y.; Li, N.; Guo, N.; Liu, R.; Zhang, Y. Synthesis of Polyethylenimine (PEI) Functionalized Silver Nanoparticles by a Hydrothermal Method and Their Antibacterial Activity Study. Mater. Sci. Eng. C 2014, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Muñoz, M.; Giron-Gonzalez, M.D.; Salto-Gonzalez, R.; Jodar-Reyes, A.B.; De Jesus, S.E.; Lopez-Jaramillo, F.J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Polyethyleneimine-Coated Gold Nanoparticles: Straightforward Preparation of Efficient DNA Delivery Nanocarriers. Chem. Asian J. 2016, 11, 3365–3375. [Google Scholar] [CrossRef]
- Ratanajanchai, M.; Soodvilai, S.; Pimpha, N.; Sunintaboon, P. Polyethylenimine-Immobilized Core–Shell Nanoparticles: Synthesis, Characterization, and Biocompatibility Test. Mater. Sci. Eng. C 2014, 34, 377–383. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Chen, C.-C.; Jao, M.-W. Effects of Polymer Micelles of Alkylated Polyethylenimines on Generation of Gold Nanoparticles. J. Phys. Chem. B 2005, 109, 9445–9450. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Liang, W.-J.; Wang, F.-Y. Hyperbranch-Polyethyleniminated Functional Polymers. I. Synthesis, Characterization of Novel ABA Type of Dumbbell-like Polyethyleniminated Polyoxypropylenediamines, and Their Complexing Properties with Copper(II) Ions in Aqueous Solution. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1360–1370. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. One-Step Synthesis and Characterization of Polyelectrolyte-Protected Gold Nanoparticles through a Thermal Process. Polymer 2004, 45, 2181–2184. [Google Scholar] [CrossRef]
- Wang, S.; Yan, J.; Chen, L. Formation of Gold Nanoparticles and Self-Assembly into Dimer and Trimer Aggregates. Mater. Lett. 2005, 59, 1383–1386. [Google Scholar] [CrossRef]
- Note, C.; Kosmella, S.; Koetz, J. Poly(Ethyleneimine) as Reducing and Stabilizing Agent for the Formation of Gold Nanoparticles in w/o Microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 150–156. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Cole, S.R.; Kitchens, C.L. Synthesis and Enhanced Colloidal Stability of Cationic Gold Nanoparticles Using Polyethyleneimine and Carbon Dioxide. ACS Sustain. Chem. Eng. 2013, 1, 826–832. [Google Scholar] [CrossRef]
- Kim, K.; Lee, H.B.; Lee, J.W.; Park, H.K.; Shin, K.S. Self-Assembly of Poly(Ethylenimine)-Capped Au Nanoparticles at a Toluene−Water Interface for Efficient Surface-Enhanced Raman Scattering. Langmuir 2008, 24, 7178–7183. [Google Scholar] [CrossRef]
- Pugh, T.L.; Heller, W. Coagulation and Stabilization of Colloidal Solutions with Polyelectrolytes. J. Polym. Sci. 1960, 47, 219–227. [Google Scholar] [CrossRef]
- Radziuk, D.; Skirtach, A.; Sukhorukov, G.; Shchukin, D.; Möhwald, H. Stabilization of Silver Nanoparticles by Polyelectrolytes and Poly(Ethylene Glycol). Macromol. Rapid Commun. 2007, 28, 848–855. [Google Scholar] [CrossRef]
- Wuelfing, W.P.; Gross, S.M.; Miles, D.T.; Murray, R.W. Nanometer Gold Clusters Protected by Surface-Bound Monolayers of Thiolated Poly(Ethylene Glycol) Polymer Electrolyte. J. Am. Chem. Soc. 1998, 120, 12696–12697. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsu, C.-H.; Kuo, P.-L. Effects of Alkylated Polyethylenimines on the Formation of Gold Nanoplates. Langmuir 2007, 23, 6801–6806. [Google Scholar] [CrossRef]
- Köth, A.; Koetz, J.; Appelhans, D.; Voit, B. “Sweet” Gold Nanoparticles with Oligosaccharide-Modified Poly(Ethyleneimine). Colloid. Polym. Sci. 2008, 286, 1317–1327. [Google Scholar] [CrossRef]
- Köth, A.; Tiersch, B.; Appelhans, D.; Gradzielski, M.; Cölfen, H.; Koetz, J. Synthesis of Core-Shell Gold Nanoparticles with Maltose-Modified Poly(Ethyleneimine). J. Dispers. Sci. Technol. 2012, 33, 52–60. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, L.; Peng, C.; Li, D.; Li, J.; Wen, S.; Shen, M.; Zhang, G.; Shi, X. Synthesis and Characterization of PEGylated Polyethylenimine-Entrapped Gold Nanoparticles for Blood Pool and Tumor CT Imaging. ACS Appl. Mater. Interfaces 2014, 6, 17190–17199. [Google Scholar] [CrossRef]
- Chauhan, B.P.S.; Matam, S.; Johnson, Q.R.; Patel, A.; Moran, K.; Onyechi, B. Generation of Zerovalent Metal Core Nanoparticles Using N-(2-Aminoethyl)-3-Aminosilanetriol. J. Vis. Exp. 2016, 108, e53507. [Google Scholar] [CrossRef]
- Fazzini, S.; Nanni, D.; Ballarin, B.; Cassani, M.C.; Giorgetti, M.; Maccato, C.; Trapananti, A.; Aquilanti, G.; Ahmed, S.I. Straightforward Synthesis of Gold Nanoparticles Supported on Commercial Silica-Polyethyleneimine Beads. J. Phys. Chem. C 2012, 116, 25434–25443. [Google Scholar] [CrossRef]
- Chauhan, B.; Sardar, R.; Latif, U.; Chauhan, M.; L’Amoreaux, W. Nanoengineering of Metallic Solutions through Silicone Constructs. Acta Chim. Slov. 2005, 52, 361–370. [Google Scholar]
- Yao, T.; Sun, Z.; Li, Y.; Pan, Z.; Wei, H.; Xie, Y.; Nomura, M.; Niwa, Y.; Yan, W.; Wu, Z.; et al. Insights into Initial Kinetic Nucleation of Gold Nanocrystals. J. Am. Chem. Soc. 2010, 132, 7696–7701. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.; Webster, F.; Kiemle, D.; Bryce, D. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 81–108. ISBN 978-0-470-61637-6. [Google Scholar]
- Kisner, A.; Lenk, S.; Mayer, D.; Mourzina, Y.; Offenhäusser, A. Determination of the Stability Constant of the Intermediate Complex during the Synthesis of Au Nanoparticles Using Aurous Halide. J. Phys. Chem. C 2009, 113, 20143–20147. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Mahmoud; Tabor, C.E.; El-Sayed, M.A.; Ding, Y.; Wang, Z.L. A New Catalytically Active Colloidal Platinum Nanocatalyst: The Multiarmed Nanostar Single Crystal. J. Am. Chem. Soc. 2008, 130, 4590–4591. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Weng, G. Nanocatalysis Production of Photoactive Radicals. Catal. Commun. 2013, 38, 63–66. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Yoshida, T. Spectroelectrochemical Studies on Redox Reactions of Eosin Y and Its Polymerization with Zn2+ Ions. J. Electroanal. Chem. 2011, 662, 384–395. [Google Scholar] [CrossRef]
- Bannerjee, N.R.; Negi, A.S. Polarograms of Eosin (2,4,5,7-Tetrabromo-(R)-Fluorescein) in Aqueous Buffers. Electrochim. Acta 1973, 18, 335–342. [Google Scholar] [CrossRef]
- Issa, I.M.; Issa, R.M.; Ghoneim, M.M.; Temerk, Y.M. Polarography of Eosin and Erythrosin in Solutions of Varying pH at the DME. Electrochim. Acta 1973, 18, 265–270. [Google Scholar] [CrossRef]
- Goux, A.; Pauporté, T.; Lincot, D.; Dunsch, L. In Situ ESR and UV/Vis Spectroelectrochemical Study of Eosin Y Upon Reduction with and without Zn(II) Ions. ChemPhysChem 2007, 8, 926–931. [Google Scholar] [CrossRef]
- Langer, N.; Kedem, O. Effect of Ligands and Their Removal on the Au Nanoparticle-Catalyzed Reduction of 4-Nitrophenol. J. Phys. Chem. C 2022, 126, 13705–13713. [Google Scholar] [CrossRef]
- Ansar, S.M.; Kitchens, C.L. Impact of Gold Nanoparticle Stabilizing Ligands on the Colloidal Catalytic Reduction of 4-Nitrophenol. ACS Catal. 2016, 6, 5553–5560. [Google Scholar] [CrossRef]




| Solvent (25 mL), Reaction Time: 6 h | |||
|---|---|---|---|
| Approximate Gold Chloride-to-TMSP-PEI Ratio | IPA (70 °C) | Methanol (55 °C) | Water (60 °C) |
| Molar Amounts | Molar Amounts | Molar Amounts | |
| [Gram Amounts] | [Gram Amounts] | [Gram Amounts] | |
| 1:1.6 | 0.1 mmol:0.16 mmol | 0.1 mmol:0.16 mmol | 0.1 mmol:0.16 mmol |
| [0.0393 g:0.0824 g] | [0.0393 g:0.0824 g] | [0.0393 g:0.0824 g] | |
| 1:3.5 | 0.1 mmol:0.35 mmol | 0.1 mmol:0.35 mmol | 0.1 mmol:0.35 mmol |
| [0.0393 g:0.180 g] | [0.0393 g:0.180 g] | [0.0393 g:0.1800 g] | |
| 1:14 | 0.1 mmol:1.4 mmol | 0.1 mmol:1.4 mmol | 0.1 mmol:1.4 mmol |
| [0.0393 g:0.721 g] | [0.0393 g:0.721 g] | [0.0393 g:0.721 g] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, E.; Moran, K.; Johnson, Q.R.; Lakhal, A.; Chauhan, B.P.S. A Facile One-Pot Preparation and Catalytic Application of Tunable Silica-Coated Aqueous Gold Nanoparticles. Molecules 2025, 30, 1355. https://doi.org/10.3390/molecules30061355
Cook E, Moran K, Johnson QR, Lakhal A, Chauhan BPS. A Facile One-Pot Preparation and Catalytic Application of Tunable Silica-Coated Aqueous Gold Nanoparticles. Molecules. 2025; 30(6):1355. https://doi.org/10.3390/molecules30061355
Chicago/Turabian StyleCook, Elijah, Kelly Moran, Qiaxian R. Johnson, Asmaa Lakhal, and Bhanu P. S. Chauhan. 2025. "A Facile One-Pot Preparation and Catalytic Application of Tunable Silica-Coated Aqueous Gold Nanoparticles" Molecules 30, no. 6: 1355. https://doi.org/10.3390/molecules30061355
APA StyleCook, E., Moran, K., Johnson, Q. R., Lakhal, A., & Chauhan, B. P. S. (2025). A Facile One-Pot Preparation and Catalytic Application of Tunable Silica-Coated Aqueous Gold Nanoparticles. Molecules, 30(6), 1355. https://doi.org/10.3390/molecules30061355







