Free Radical Scavenging Activity and Inhibition of Enzyme-Catalyzed Oxidation by trans-aryl-Palladium Complexes
Abstract
1. Introduction
2. Results and Discussion
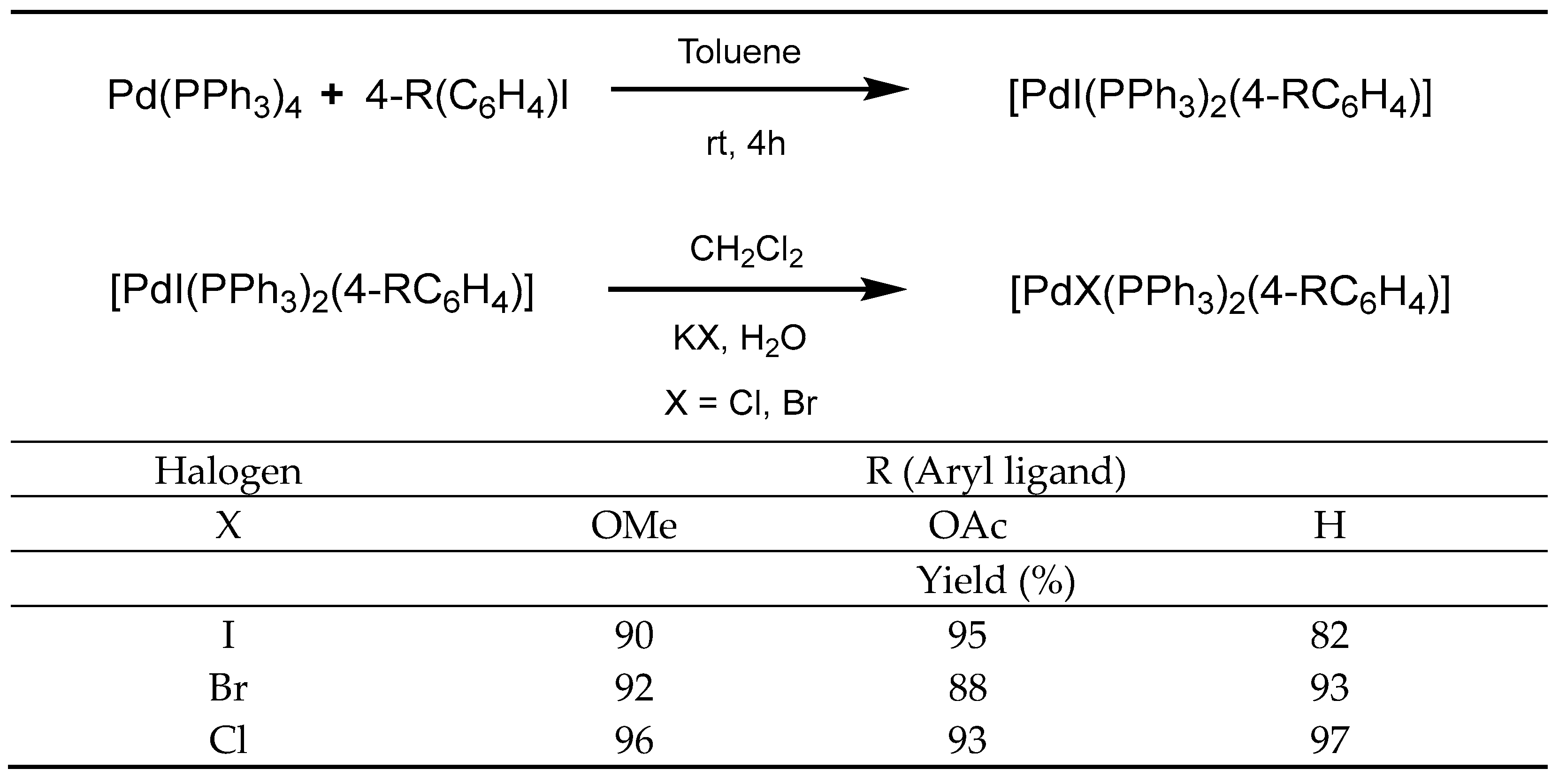
2.1. Synthesis and Characterization of Palladium Complexes
2.2. Mass Spectrometry Analysis
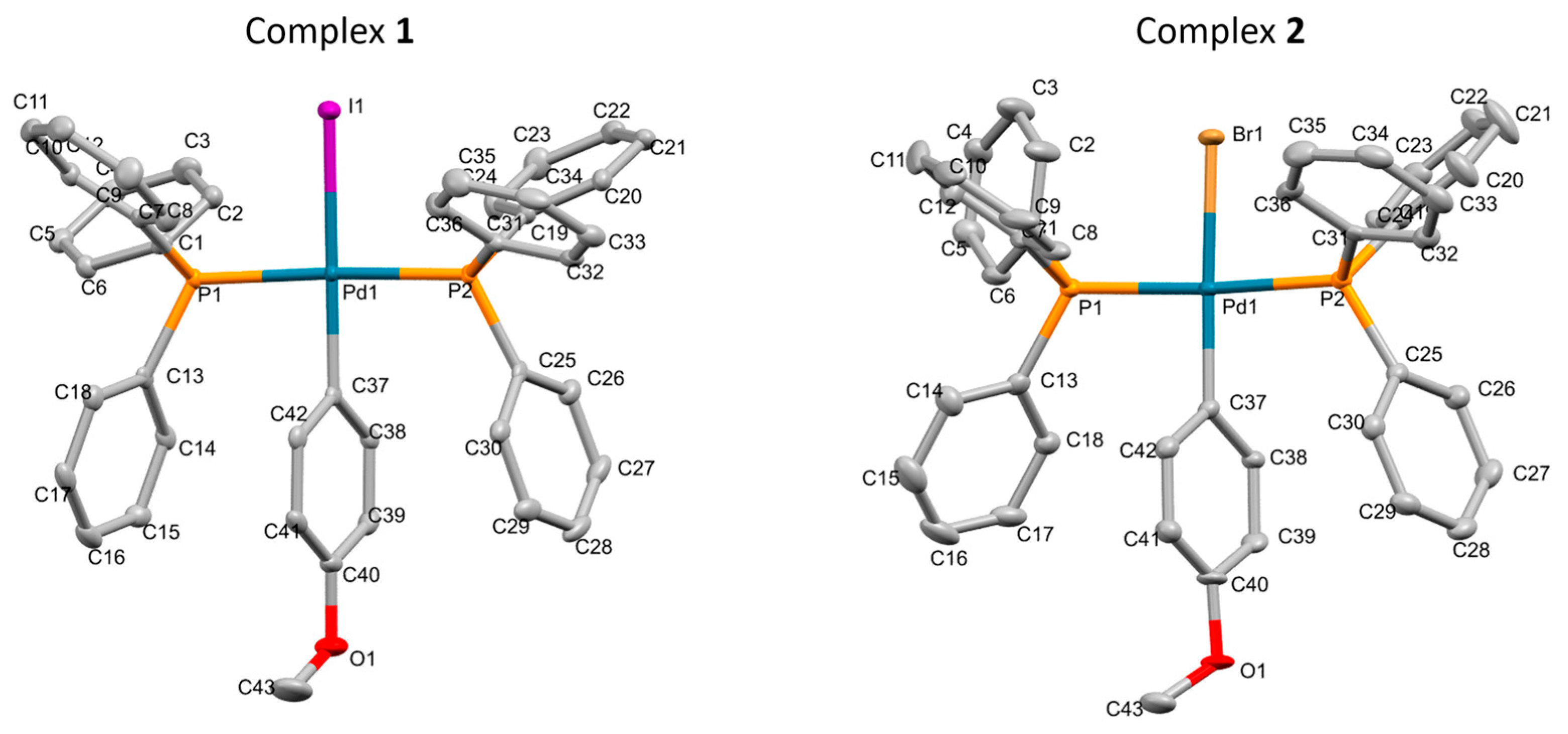
2.3. X-Ray Diffraction Analysis
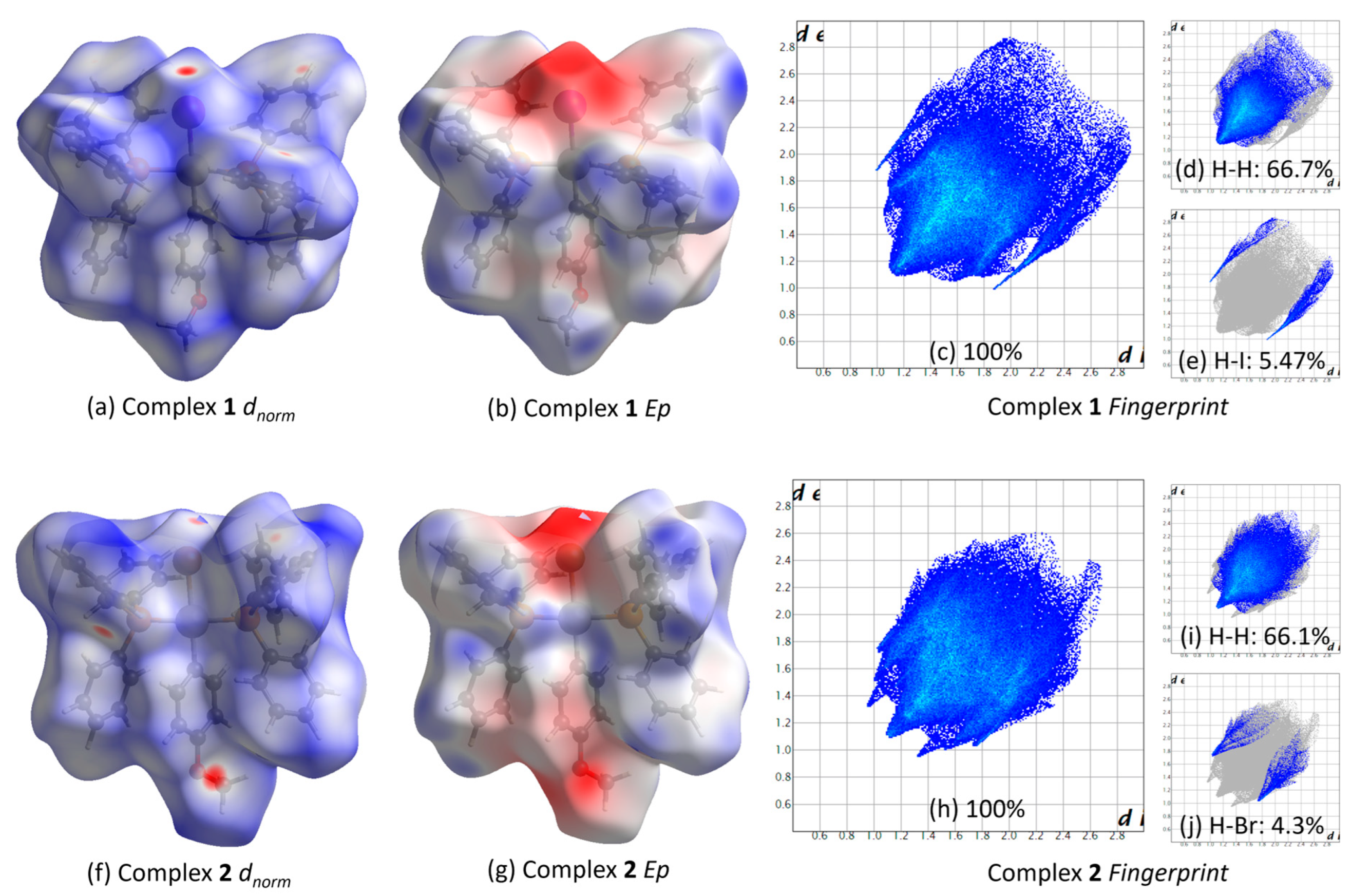
2.4. Hirshfeld Surface Study of Complexes 1 and 2
2.5. Effect of Palladium (II) Complexes on the Free Radical Scavenging Activity
2.6. Antioxidant Efficiencies
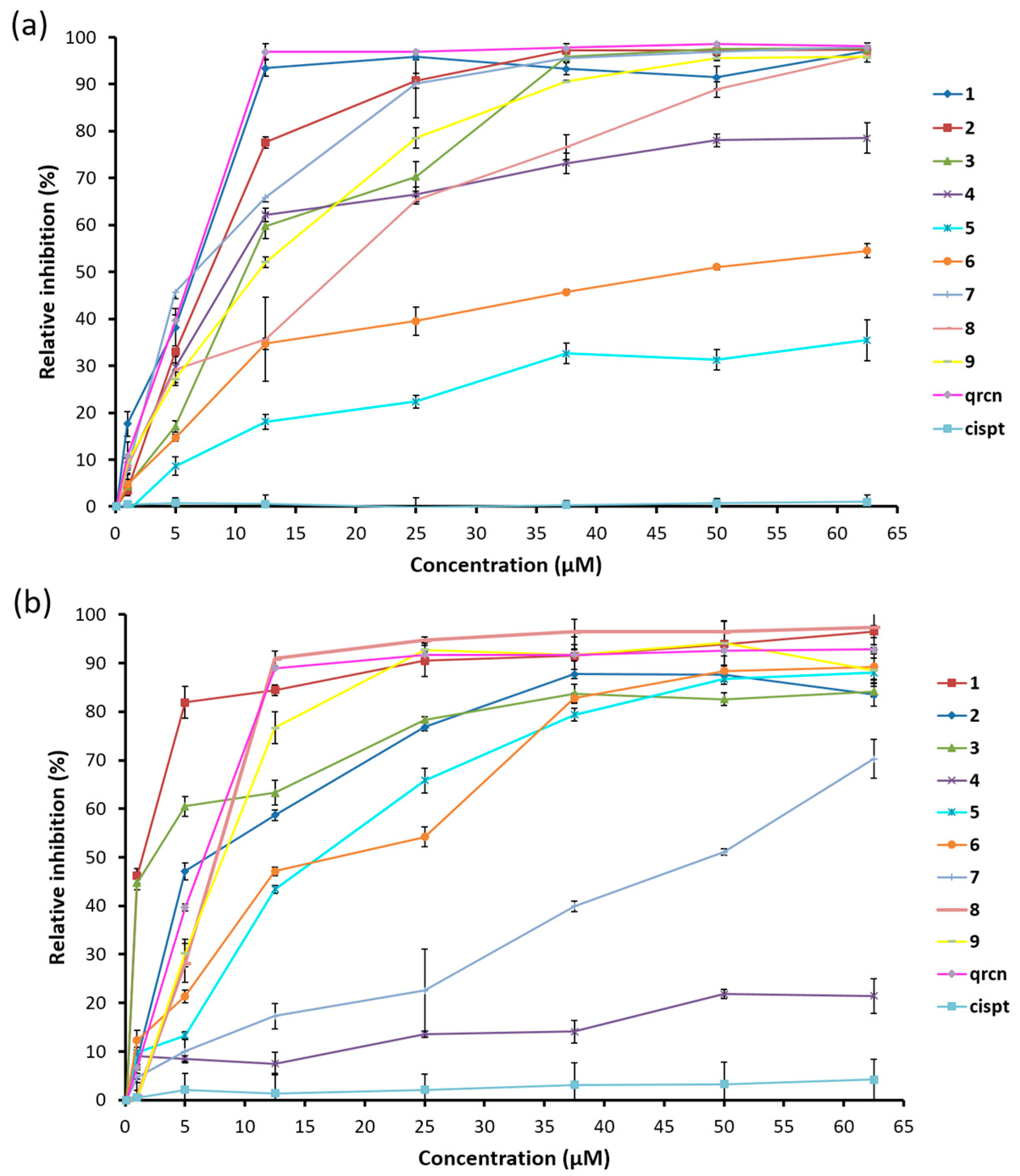
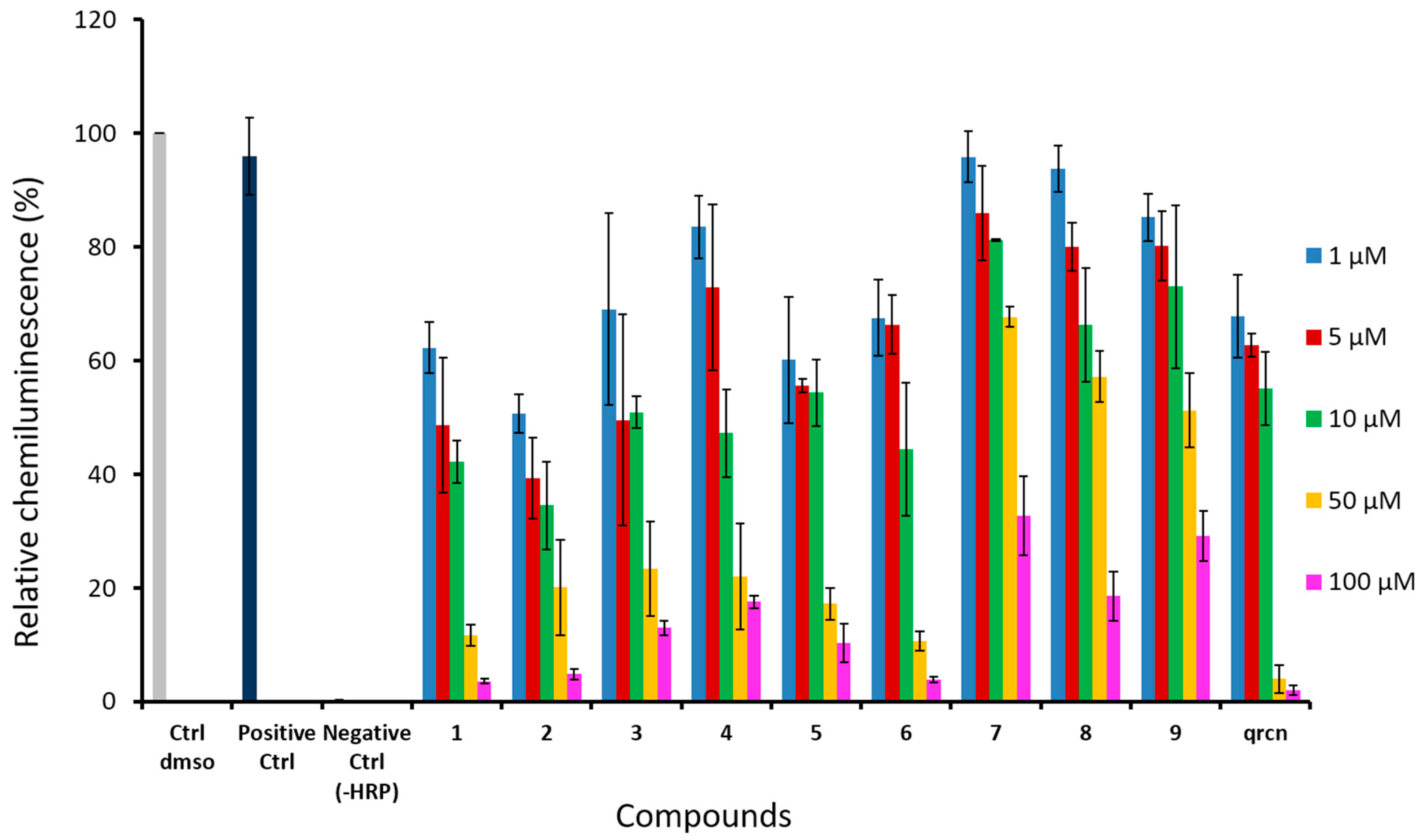
2.7. Enzyme-Catalyzed Oxidation Monitored by Chemiluminescence (CL) Assay
3. Materials and Methods
3.1. Materials and Physical Techniques
3.2. Synthesis of Palladium Complexes
3.2.1. Preparation of Complexes 1, 4, and 7
Trans-Iodo(4-methoxyphenyl)bis(triphenylphosphine)Palladium C43H37OIP2Pd (1)
Trans-Iodo(4-acethoxyphenyl)bis(triphenylphosphine)Palladium C44H37O2IP2Pd (4)
Trans-Iodo(phenyl)bis(triphenylphosphine)Palladium C43H35IP2Pd (7)
3.2.2. Preparation of Complexes 2, 3, 5, 6, 8 and 9
Trans-Bromo(4-methoxyphenyl)bis(triphenylphosphine)Palladium C43H37OBrP2Pd (2)
Trans-Chloro(4-methoxyphenyl) bis(triphenylphosphine)Palladium C43H37OClP2Pd (3)
Trans-Bromo(4-acethoxyphenyl)bis(triphenylphosphine)Palladium C44H37O2BrP2Pd (5)
Trans-Chloro(4-acethoxyphenyl)bis(triphenylphosphine)Palladium C44H37O2ClP2Pd (6)
Trans-Bromo(phenyl)bis(triphenylphosphine)Palladium C42H35BrP2Pd (8)
Trans-Chloro(phenyl)bis(triphenylphosphine)Palladium C42H35ClP2Pd (9)
3.3. X-Ray Crystal Structure Determination
3.4. Free Radicals Scavenging Methods
3.4.1. ABTS Test
3.4.2. DPPH Test
3.5. Antiradical Efficiency
3.6. Chemiluminescence Study of Complexes on Enzyme-Catalyzed Oxidation of L012
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskel. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Bonanno, E.; Sangiorgi, G.; Mauriello, A. Role of Inflammation in Atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer. Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes 2014, 5, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef]
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef]
- Nickers, P.; Kunkler, I.; Scalliet, P. Modem brachytherapy: Current state and future prospects. Eur. J. Cancer. 1997, 33, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Flam, T.; Chauveinc, L.; Servois, V.; Rosenwald, J.-C.; Cosset, J.-M.; Thiounn, N.; Debré, B. La curiethérapie dans le traitement curatif du cancer de la prostate localisé. Progrès Urol. 2000, 10, 3–13. [Google Scholar]
- Colin, P.; Mordon, S.; Nevoux, P.; Marqa, M.F.; Ouzzane, A.; Puech, P.; Bozzini, G.; Leroux, B.; Villers, A.; Betrouni, N. Focal Laser Ablation of Prostate Cancer: Definition, Needs, and Future. Adv. Urol. 2012, 2012, 589160. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.P.M. Platinum and Palladium Polyamine Complexes as Anticancer Agents: The Structural Factor. ISRN Spectroscopy 2013, 2013, 287353. [Google Scholar] [CrossRef]
- Wiltshaw, E. Cisplatin in The Treatment Of Cancer The First Metal Anti-Tumour Drug. Platin. Met. Rev. 1979, 23, 90–98. [Google Scholar] [CrossRef]
- Hambley, T.W. The influence of structure on the activity and toxicity of Pt anti-cancer drugs. Coord. Chem. Rev. 1997, 166, 181–223. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Hernández, W.; Paz, J.; Carrasco, F.; Vaisberg, A.; Spodine, E.; Manzur, J.; Hennig, L.; Sieler, J.; Blaurock, S.; Beyer, L. Synthesis and Characterization of New Palladium(II) Thiosemicarbazone Complexes and Their Cytotoxic Activity against Various Human Tumor Cell Lines. Bioinorg. Chem. Appl. 2013, 2013, 524701. [Google Scholar] [CrossRef]
- Isnard-Bagnis, C.; Moulin, B.; Launay-Vacher, V.; Izzedine, H.; Tostivint, I.; Deray, G. Anticancer drug-induced nephrotoxicity. Nephrol. Ther. 2005, 1, 101–114. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Saeidifar, M.; Mansouri-Torshizi, H. Investigation of the interaction between human serum albumin and antitumor palladium(II) complex containing 1,10-phenanthroline and dithiocarbamate ligands. Nucleosides Nucleotides Nucleic Acids 2015, 34, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Koumousi, E.S.; Zampakou, M.; Raptopoulou, C.P.; Psycharis, V.; Beavers, C.M.; Teat, S.J.; Psomas, G.; Stamatatos, T.C. First Palladium(II) and Platinum(II) Complexes from Employment of 2,6-Diacetylpyridine Dioxime: Synthesis, Structural and Spectroscopic Characterization, and Biological Evaluation. Inorg. Chem. 2012, 51, 7699–7710. [Google Scholar] [CrossRef] [PubMed]
- Samari, F.; Hemmateenejad, B.; Shamsipur, M.; Rashidi, M.; Samouei, H. Affinity of Two Novel Five-Coordinated Anticancer Pt(II) Complexes to Human and Bovine Serum Albumins: A Spectroscopic Approach. Inorg. Chem. 2012, 51, 3454–3464. [Google Scholar] [CrossRef]
- Jovanović, S.; Obrenčević, K.; Bugarčić, Ž.D.; Popović, I.; Žakula, J.; Petrović, B. New bimetallic palladium(II) and platinum(II) complexes: Studies of the nucleophilic substitution reactions, interactions with CT-DNA, bovine serum albumin and cytotoxic activity. Dalton Trans. 2016, 45, 12444–12457. [Google Scholar] [CrossRef]
- Emamia, S.; Ghourchiana, H.; Divsalarb, A. Release of Cyt C from the model membrane due to conformational change induced by anticancer palladium complex. Int. J. Biol. Macromolec. 2011, 48, 243–248. [Google Scholar] [CrossRef]
- Alia, M.A.; Mirzaa, A.H.; Butcherb, R.J.; Tarafderc, M.T.H.; Keat, T.B.; Ali, A.M. Biological activity of palladium (II) and platinum (II) complexes of the acetone Schiff bases of S-methyl- and S-benzyldithiocarbazate and the X-ray crystal structure of the [Pd(asme)2] (asme=anionic form of the acetone Schiff base of S-methyldithiocarbazate) complex. J. Inorg. Biochem. 2002, 92, 141–148. [Google Scholar]
- Das, M.; Livingstone, S.E. Metal chelates as anti-cancer agents. II cytotoxic action of palladium and platinum complexes of 6-mercaptopurine and thioguanine. Br. J. Cancer. 1978, 38, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mohan, R.; Singh, J.K.; Samantaray, M.K.; Shaikh, M.M.; Panda, D.; Ghosh, P. Anticancer and Antimicrobial Metallopharmaceutical Agents Based on Palladium, Gold, and Silver N-Heterocyclic Carbene Complexes. J. Am. Chem. Soc. 2007, 129, 15042–15053. [Google Scholar] [CrossRef]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009, 253, 1384–1397. [Google Scholar] [CrossRef]
- El-Morsy, F.A.; Jean-Claude, B.J.; Butler, I.S.; El-Sayed, S.A.; Mostafa, S.I. Synthesis, characterization and anticancer activity of new zinc(II), molybdate(II), palladium(II), silver(I), rhodium(III), ruthenium(II) and platinum(II) complexes of 5,6-diamino-4-hydroxy-2-mercaptopyrimidine. Inorganica Chim. Acta 2017, 423, 144–155. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, S.; Toole, B.P.; Xu, Y. Cancer may be a pathway to cell survival under persistent hypoxia and elevated ROS: A model for solid-cancer initiation and early development. Int. J. Cancer 2015, 136, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Kinkade, J.M.; Pember, S.O.; Barnes, K.C.; Shapira, R.; Spitznagel, J.K.; Martin, L.E. Differential distribution of distinct forms of myeloperoxidase in different azurophilic granule subpopulations from human neutrophils. Biochem. Biophys. Res. Commun. 1983, 114, 296–303. [Google Scholar] [CrossRef]
- Gaut, J.P.; Yeh, G.C.; Tran, H.D.; Byun, J.; Henderson, J.P.; Richter, G.M.; Brennan, M.-L.; Lusis, A.J.; Belaaouaj, A.; Hotchkiss, R.S.; et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Biochemistry 2001, 98, 11961–11966. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.B.; Ye, Y.; Rosen, H.; Beckman, J.S. Myeloperoxidase and Horseradish Peroxidase Catalyze Tyrosine Nitration in Proteins from Nitrite and Hydrogen Peroxide. Arch. Biochem. Biophys. 1998, 356, 207–213. [Google Scholar] [CrossRef]
- Rosen, H.; Klebanoff, S.J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J. Clin. Invest. 1976, 58, 50–60. [Google Scholar] [CrossRef]
- Nishinaka, Y.; Aramaki, Y.; Yoshida, H.; Masuya, H.; Sugawara, T.; Ichimori, Y. A New Sensitive Chemiluminescence Probe, L-012, for Measuring the Production of Superoxide Anion by Cells. Biochem. Biophys. Res. Commun. 1993, 193, 554–559. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Gloe, T.; Keller, M.; Schoenafinger, K.; Pohl, U. Sensitive Superoxide Detection in Vascular Cells by the New Chemiluminescence Dye L-012. J. Vasc. Res. 1999, 36, 456–464. [Google Scholar] [CrossRef]
- Daiber, A.; August, M.; Baldus, S.; Wendt, M.; Oelze, M.; Sydow, K.; Kleschyov, A.L.; Munzel, T. Measurement of NAD(P) H oxidase-derived superoxide with the luminal analogue L-012. Free Radic. Biol. Med. 2004, 36, 101–111. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts: Innovations in Organic Synthesis; Wiley: Chichester, UK, 1995. [Google Scholar]
- Etsè, K.S.; Boschini, F.; Karegeya, C.; Roex, E.; Zaragoza, G.; Demonceau, A.; Cloots, R.; Mahmoud, A. Exploring organo-palladium(II) complexes as novel organometallic materials for Li-ion batteries. Electrochim. Acta 2020, 337, 135659. [Google Scholar] [CrossRef]
- Roex, E.; Etsè, K.S.; Cloots, R.; Boschini, F.; Mahmoud, A. Improving the electrochemical performances of organo-palladium (II) complex as promising anode material for Li-ion batteries: Effect of double emulsion preparation. J. Power Sources 2021, 496, 229827. [Google Scholar] [CrossRef]
- Flemming, J.P.; Pilon, M.C.; Borbulevitch, O.Y.; Antipin, M.Y.; Grushin, V.V. The trans influence of F, Cl, Br and I ligands in a series of square-planar Pd(II) complexes. Relative affinities of halide anions for the metal centre in trans-[(Ph3P)2Pd(Ph)X]. Inorg. Chim. Acta 1998, 280, 87–98. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Brossmer, C.; Priermeier, T.; Ofele, K. Complexes and mechanisms of metal-catalyzed cc coupling reactions. 2. Oxidative addition of chloroaromatics to pd-0 complexes-synthesis, structure and stability of arylpalladium (ii) chlorides of the phosphorane series. J. Organomet. Chem. 1994, 481, 97–108. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Brossmer, C.; Öfele, K.; Beller, M.; Fischer, H. Zum Mechanismus der Heck-Reaktion: Katalysator-Deaktivierung durch PC-Bindungsbruch. J. Organomet. Chem. 1995, 491, C1–C4. [Google Scholar] [CrossRef]
- Kong, K.-C.; Cheng, C.-H. Facile aryl-aryl exchange between the palladium center and phosphine ligands in palladium (II) complexes. J. Am. Chem. Soc. 1991, 113, 6313–6315. [Google Scholar] [CrossRef]
- Goodson, F.E.; Wallow, T.I.; Novak, B.M. Mechanistic studies on the aryl− aryl interchange reaction of ArPdL2I (L= triarylphosphine) complexes. J. Am. Chem. Soc. 1997, 119, 12441–12453. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayati-Laka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayati-Laka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Etsè, K.S.; Dorosz, J.; Christensen, K.M.; Thomas, J.-Y.; Pop, I.B.; Goffin, E.; Col-Son, T.; Lestage, P.; Danober, L.; Pirotte, B.; et al. Development of thiochroman dioxide analogues of benzothiadiazine dioxides as new positive allosteric modulators of α–amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, ACS Chem. Neuroscince 2021, 12, 2679–2692. [Google Scholar] [CrossRef]
- Fadhel, A.M.; Al-Hamdani, A.A.S. Preparation, characterization, and antioxidant activity of novel metal (Mn (II), Ni (II), Pd (II), Pt (IV)) complexes:Application of Pd complex in Su-zuki-Miyauracross-coupling reaction. Iran. J. Catal. 2024, 14, 142433. [Google Scholar] [CrossRef]
- Ezzatzadeh, E.; Soleimani-Amiri, S.; Hossaini, Z.; Khandan Barani, K. Synthesis and evaluation of the antioxidant activity of new spiro-1,2,4-triazine derivatives applying Ag/Fe3O4/CdO@MWCNT MNCs as efficient organometallic nanocatalysts. Front Chem. 2022, 29, 1001707. [Google Scholar] [CrossRef]
- Bougossa, S.; Mhadhbi, N.; Ahmed, A.B.; Hamdi, M.; Elghniji, K.; Erwann, J.; Hamden, K.; Oueslati, A.; Naïli, H. Design of a new palladium(II) halide complex as a bio-active materi-al: Synthesis, physico-chemical studies, DFT-computations and evaluation of an-ti-inflammatory, antioxidant and anti-gastric damage activities. RSC Adv. 2024, 14, 17413–17433. [Google Scholar] [CrossRef]
- Khan, S.Z.; Rehman, Z.; Butler, I.S.; Bélanger-Gariepy, F. New ternary palladium(II) complexes: Synthesis, characterization, in vitro anticancer and antioxidant activities. Inorg. Chem. Comm. 2019, 105, 140–146. [Google Scholar] [CrossRef]
- Khalil, M.H.; Abdullah, F.O. Synthesis, characterisation, and anticancer and antioxi-dant activities of novel complexes of palladium and an organic Schiff-base ligand. Bull. Chem. Soc. Ethiop. 2024, 38, 605–613. [Google Scholar] [CrossRef]
- Elsayed, S.A.; Badr, H.E.; di Biase, A.; El-Hendawy, A.M. Synthesis, characterization of ruthenium(II), nickel(II), palladium(II), and platinum(II) triphenylphosphine-based com-plexes bearing an ONS-donor chelating agent: Interaction with biomolecules, antioxidant, in vitro cytotoxic, apoptotic activity and cell cycle analysis. J. Inorg. Biochem. 2021, 223, 111549. [Google Scholar] [CrossRef]
- Turan, N.; Buldurun, K.; Bursal, E.; Mahmoudi, G. Pd(II)-Schiff base complexes: Synthesis, characterization, Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions, en-zyme inhibition and antioxidant activities. J. Organomet. Chem. 2022, 970–971, 122370. [Google Scholar] [CrossRef]
- Nimmi, O.S.; George, P. Evaluation of the antioxidant potential of a newly developed polyherbal formulation for antiobesity. Int. J. Pharm. Pharm. Sci. 2012, 4, 505–510. [Google Scholar]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Fauconneau, B.; Waffo-Teguo, P.; Huguet, F.; Barrier, L.; Decendit, A.; Merillon, J.-M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997, 61, 2103–2110. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, J.W.; Lee, B.H.; Chung, K.H.; Chi, D.Y. Syntheses and radical scavenging activities of resveratrol derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent Overview of Po-tent Antioxidant Activity of Coordination Compounds. Antioxidants 2023, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Grushin, V.V. Thermal Stability, Decomposition Paths, and Ph/Ph Exchange Reactions of [(Ph3P)2Pd(Ph)X] (X ) I, Br, Cl, F, and HF2). Organometallics 2000, 19, 1888–1900. [Google Scholar] [CrossRef]
- Tyurin, V.Y.; Moiseeva, A.A.; Shpakovsky, D.B.; Milaeva, E.R. The electrochemical approach to antioxidant activity assay of metal complexes with dipicolylamine ligand, containing 2,6-di-tert-butylphenol groups, based on electrochemical DPPH-test. J. Electroanal. Chem. 2015, 756, 212–221. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Etsè, K.S.; Etsè, K.D.; Nyssen, P.; Mouithys-Mickalad, A. Assessment of anti-inflammatory-like, antioxidant activities and molecular docking of three alkynyl-substituted 3-ylidene-dihydrobenzo[d]isothiazole 1,1-dioxide derivatives. Chem. Biol. Interact. 2021, 344, 109513. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Fitton, P.; Rick, E.A. The addition of aryl halides to tetrakis(triphenylphosphine)palladium(0). J. Organometal. Chem. 1971, 28, 287–291. [Google Scholar] [CrossRef]
- Bruker. APPEX-II; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Beurskens, P.T.; Admiraal, G.; Beurskens, G.; Bosman, W.P.; Garcia-Granda, S.; Gould, R.O.; Smits, J.M.M.; Smykalla, C. DIRDIF92: The DIRDIF Program System, Technical Report of the Crystallography Laboratory; University of Nijimegen: Nijmegen, The Netherlands, 1992. [Google Scholar]
- Sheldrick, G.M. SHELX97 (SHELXS97 and SHELXL97), Programs for Crystal Structure Analysis; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SADABS, Programs for Scaling and Correction of Area Detection Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Floegel, A.; Kim, D.; Chung, S.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta. 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
| Crystal Data | 1 | 2 |
|---|---|---|
| Chemical formula | C43H37OIP2Pd | C43H37OBrP2Pd |
| Mr | 864.96 | 817.97 |
| Crystal system, space group | Monoclinic, Ia | Orthorhombic, Pbca |
| Temperature (K) | 100 | 100 |
| a (Å) | 11.3164 (7) | 11.4818 (11) |
| B (Å) | 13.5866 (8) | 23.717 (3) |
| c (Å) | 23.3413 (16) | 26.133 (3) |
| β (°) | 94.306 (3) | - |
| V (Å3) | 3578.6 (4) | 7116.3 (13) |
| Z | 4 | 8 |
| Radiation type | Mo-Kα radiation | Mo-Kα radiation |
| μ (mm−1) | 1.50 | 1.77 |
| Crystal size (mm) | 0.22 × 0.20 × 0.11 | 0.27 × 0.08 × 0.06 |
| Data collection | ||
| Diffractometer | BRUKER APPEX-II CCD | BRUKER APPEX-II |
| Absorption correction | Multi-scan BRUKER SADABS2012/1 | Multi-scan SADABS2016/2—Bruker AXS area detector scaling and absorption correction |
| Tmin, Tmax | 0.765, 0.825 | 0.693, 0.801 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 64342, 8854, 8583 | 101525, 6743, 5008 |
| Rint | 0.056 | 0.117 |
| (sin θ/λ)max (Å−1) | 0.667 | 0.610 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.022, 0.048, 1.04 | 0.041, 0.107, 1.06 |
| No. of reflections | 8854 | 6743 |
| No. of parameters | 435 | 434 |
| No. of restraints | 2 | - |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.56, −0.30 | 0.64, −1.38 |
| Bond Parameters (Distance (Å) and Angle (°)) | Complex 1 | Complex 2 |
|---|---|---|
| Pd–X | 2.7009 (3) | 2.5120 (5) |
| P1–Pd1 | 2.3343 (9) | 2.3417 (11) |
| P2–Pd1 | 2.3307 (10) | 2.3216 (11) |
| Pd1–C37 | 2.019 (3) | 2.015 (4) |
| C40–O1 | 1.380 (5) | 1.392 (5) |
| C43–O1 | 1.426 (5) | 1.438 (5) |
| C37–Pd1–P2 | 88.75 (9) | 91.33 (11) |
| C37–Pd1–P1 | 88.45 (9) | 91.93 (11) |
| P2–Pd1–P1 | 176.86 (3) | 173.39 (4) |
| C37–Pd1–X | 179.27 (11) | 177.18 (10) |
| P2–Pd1–X | 91.18 (3) | 86.49 (3) |
| P1–Pd1–X | 91.60 (3) | 90.06 (3) |
| C40–O1–C43 | 115.7 (3) | 117.1 (3) |
| Compounds | EC50 (µM) | |
|---|---|---|
| ABTS | DPPH | |
| Qrcn | 5.56 ± 0.97 | 5.79 ± 1.00 |
| 1 | 5.78 ± 0.98 | 1.14 ± 0.90 |
| 2 | 7.01 ± 0.98 | 7.09 ± 0.94 |
| 3 | 11.12 ± 0.94 | 1.90 ± 0.87 |
| 4 | 10.89 ± 0.95 | 1840.77 ± 0.44 |
| 5 | 135.83 ± 0.25 | 15.45 ± 0.96 |
| 6 | 45.81 ± 0.95 | 14.32 ± 0.92 |
| 7 | 6.14 ± 0.94 | 45.08 ± 0.95 |
| 8 | 14.32 ± 0.92 | 6.64 ± 0.91 |
| 9 | 10.35 ± 0.64 | 7.33 ± 0.97 |
| Compounds | EC50 (g Antioxidant/kg DPPH°) | TEC50 (min) | AE (×10−3) | Classification |
|---|---|---|---|---|
| 1 | 49.31 ± 39 | 11 | 1.8437 | Medium |
| 2 | 2898.99 ± 39 | 16 | 0.2155 | Low |
| 3 | 73.49 ± 38 | 63 | 0.2160 | Low |
| 4 | 82,194.89 ± 33 | 78 | 0.0002 | Low |
| 5 | 653.57 ± 20 | 58 | 0.0264 | Low |
| 6 | 573.94 ± 40 | 53 | 0.0328 | Low |
| 7 | 1882.11 ± 37 | 66 | 0.0081 | Low |
| 8 | 260.96 ± 40 | 40 | 0.0955 | Low |
| 9 | 271.97 ± 36 | 36 | 0.1019 | Low |
| Qrcn | 87.98 ± 15 | 61 | 0.1873 | Low |
| Compounds | EC50 (µM) |
|---|---|
| Qrcn | 7.06 ± 2.56 |
| 1 | 3.56 ± 1.87 |
| 2 | 1.48 ± 0.71 |
| 3 | 5.80 ± 2.60 |
| 4 | 11.29 ± 3.30 |
| 5 | 5.28 ± 2.30 |
| 6 | 6.46 ± 2.28 |
| 7 | 66.99 ± 20.11 |
| 8 | 33.34 ± 11.71 |
| 9 | 39.17 ± 17.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etsè, K.S.; Harrad, M.A.; Etsè, K.D.; Zaragoza, G.; Demonceau, A.; Mouithys-Mickalad, A. Free Radical Scavenging Activity and Inhibition of Enzyme-Catalyzed Oxidation by trans-aryl-Palladium Complexes. Molecules 2025, 30, 1122. https://doi.org/10.3390/molecules30051122
Etsè KS, Harrad MA, Etsè KD, Zaragoza G, Demonceau A, Mouithys-Mickalad A. Free Radical Scavenging Activity and Inhibition of Enzyme-Catalyzed Oxidation by trans-aryl-Palladium Complexes. Molecules. 2025; 30(5):1122. https://doi.org/10.3390/molecules30051122
Chicago/Turabian StyleEtsè, Koffi Sénam, Mohamed Anouar Harrad, Kodjo Djidjolé Etsè, Guillermo Zaragoza, Albert Demonceau, and Ange Mouithys-Mickalad. 2025. "Free Radical Scavenging Activity and Inhibition of Enzyme-Catalyzed Oxidation by trans-aryl-Palladium Complexes" Molecules 30, no. 5: 1122. https://doi.org/10.3390/molecules30051122
APA StyleEtsè, K. S., Harrad, M. A., Etsè, K. D., Zaragoza, G., Demonceau, A., & Mouithys-Mickalad, A. (2025). Free Radical Scavenging Activity and Inhibition of Enzyme-Catalyzed Oxidation by trans-aryl-Palladium Complexes. Molecules, 30(5), 1122. https://doi.org/10.3390/molecules30051122







