Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines
Abstract
1. Introduction
2. C-C Bond Formation
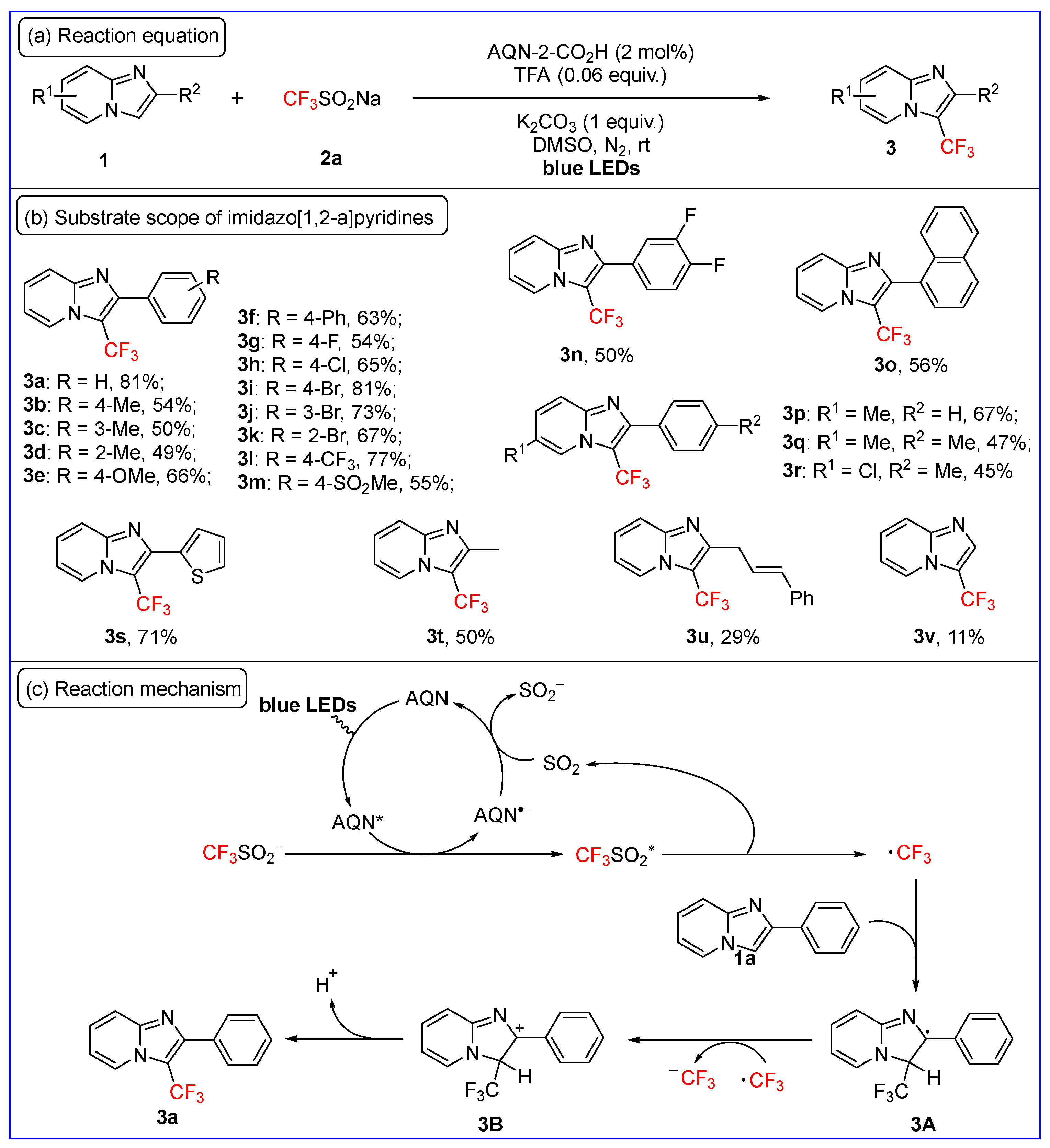
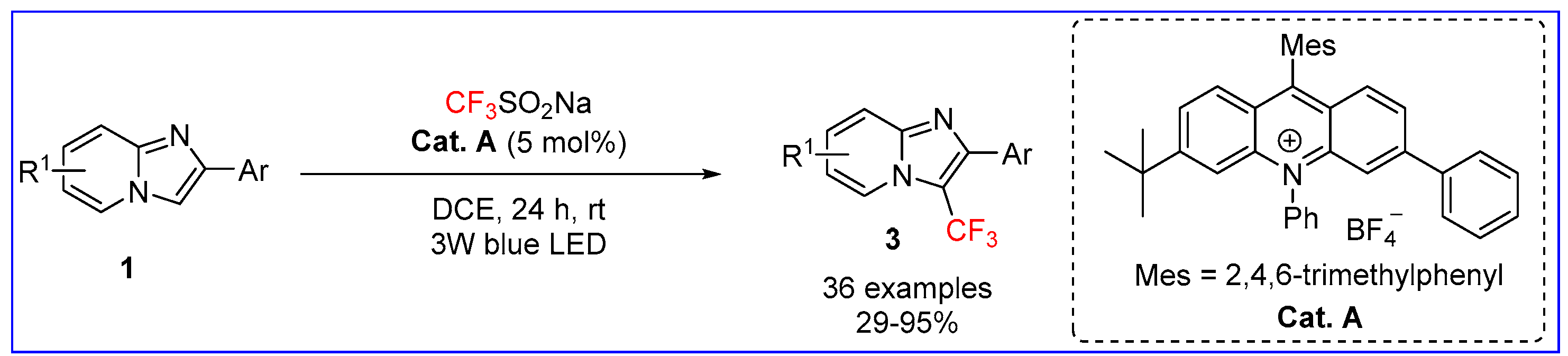
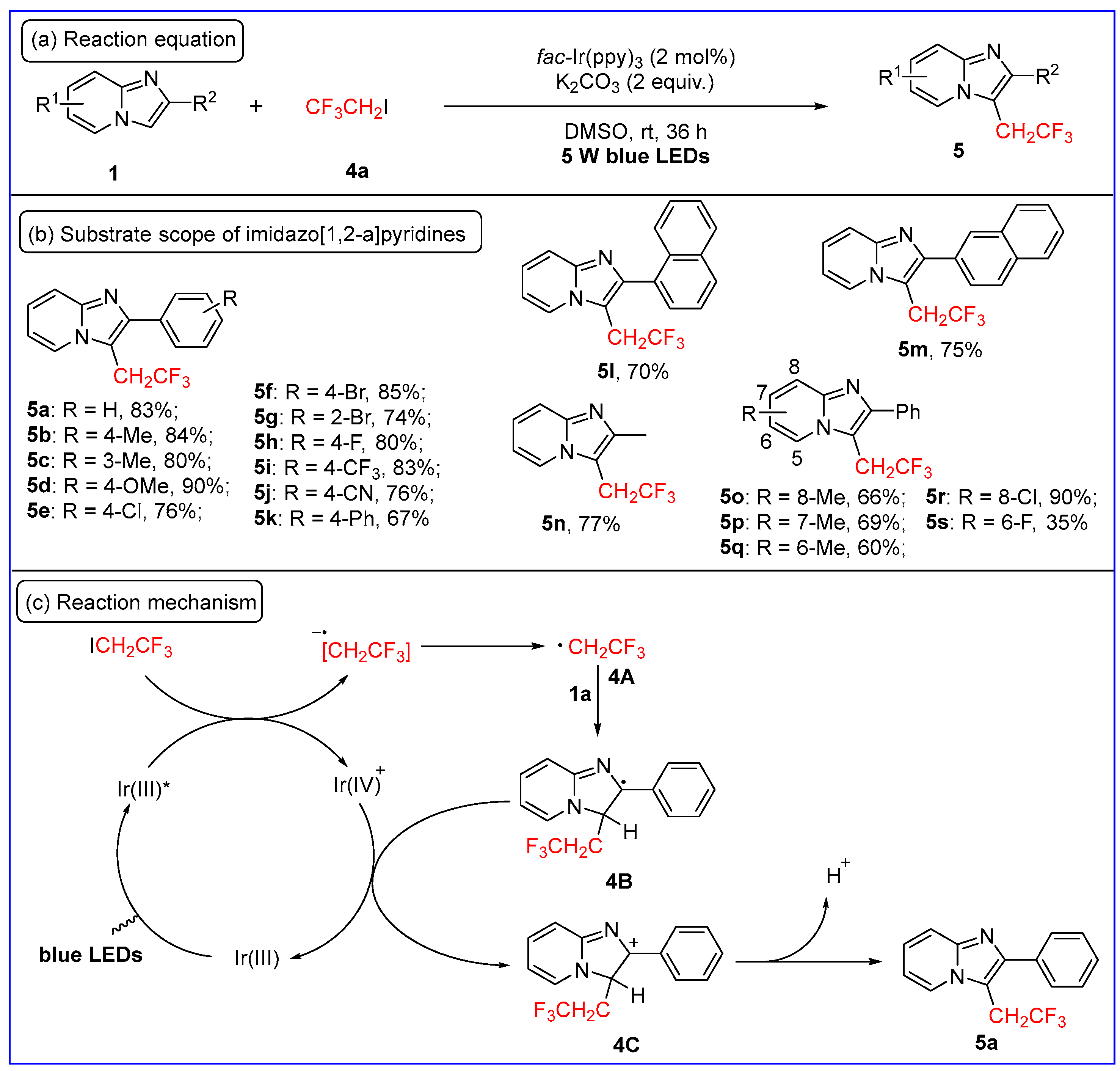
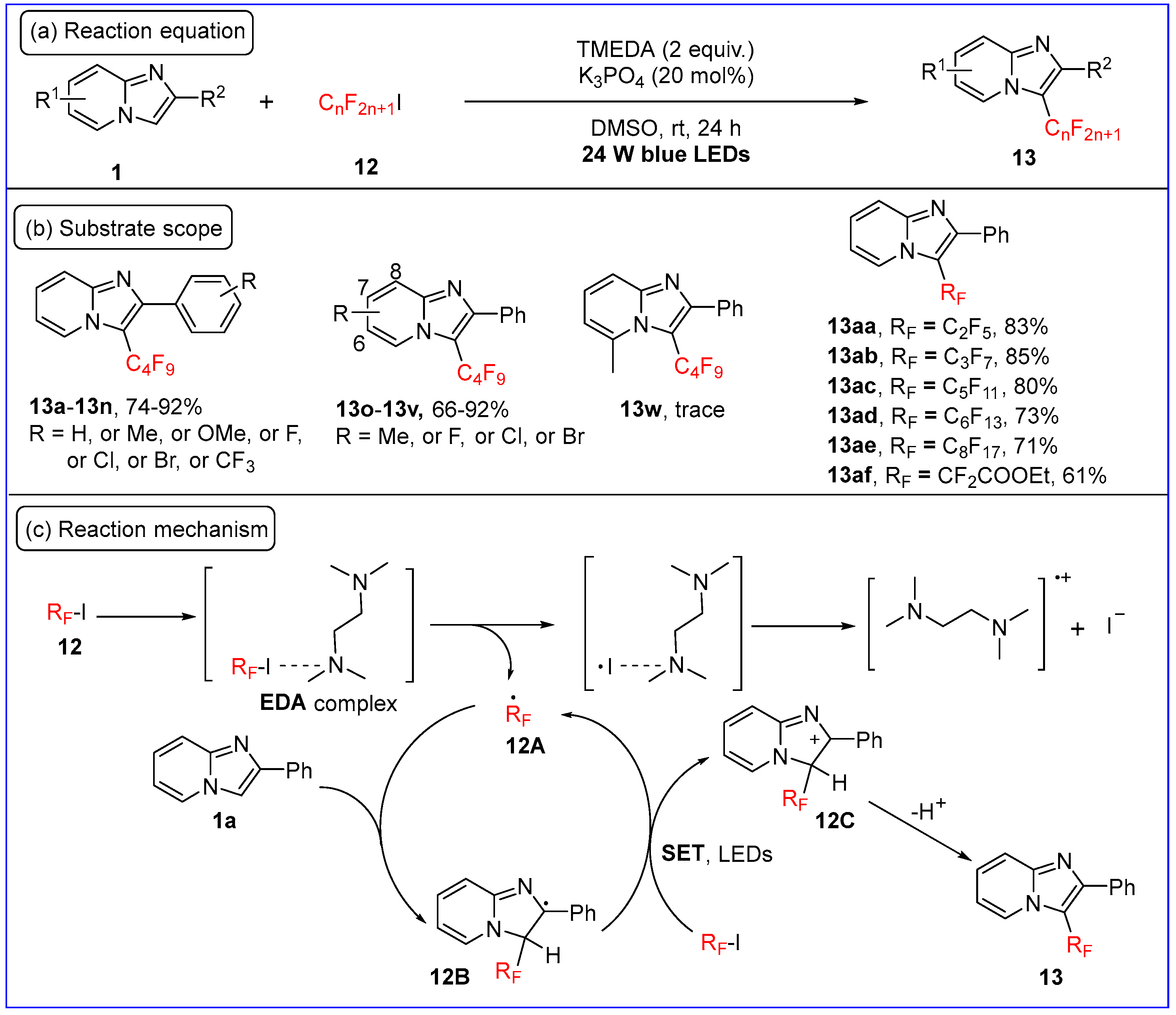
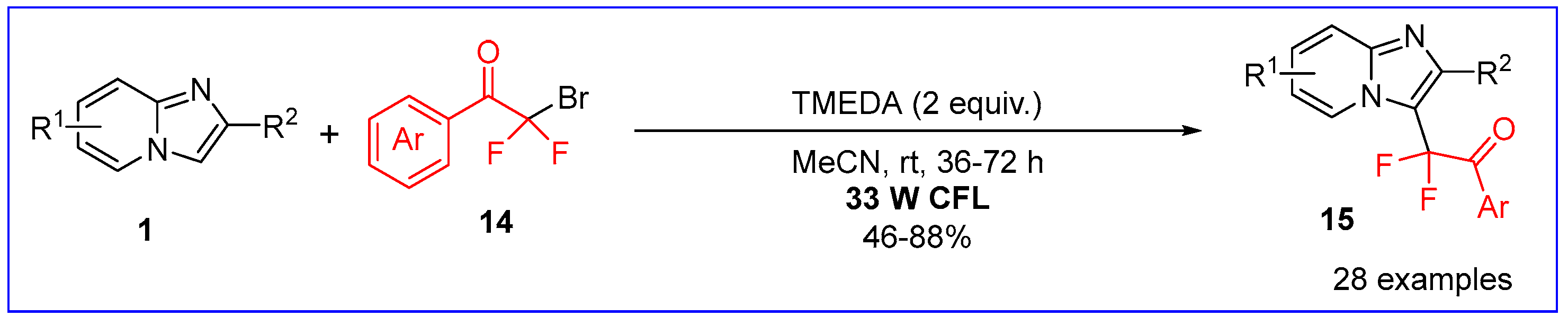
2.1. C3-Fluoroalkylation of Imidazo[1,2-a]pyridines
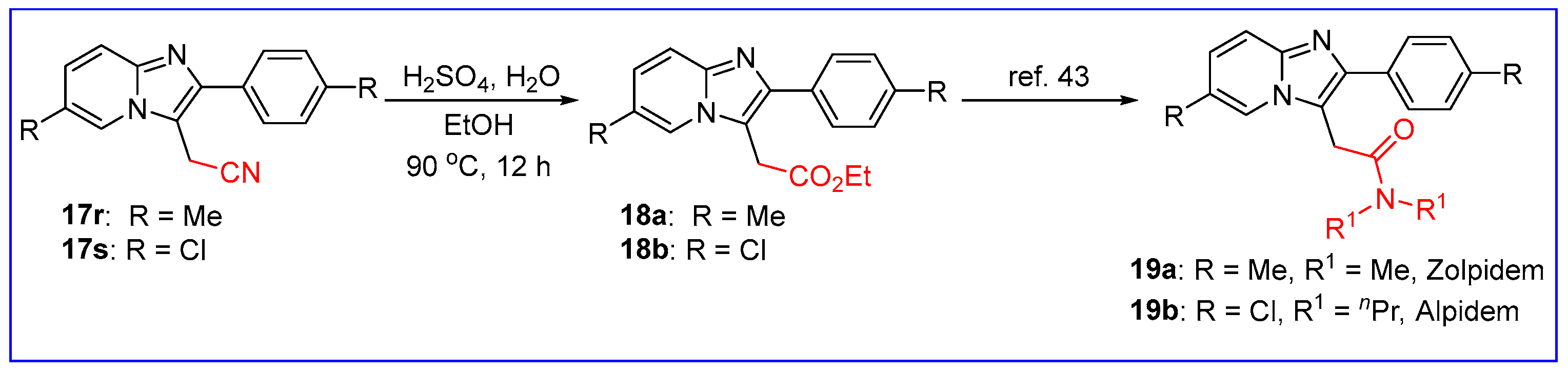
2.2. C3-Cyanomethylation of Imidazo[1,2-a]pyridines
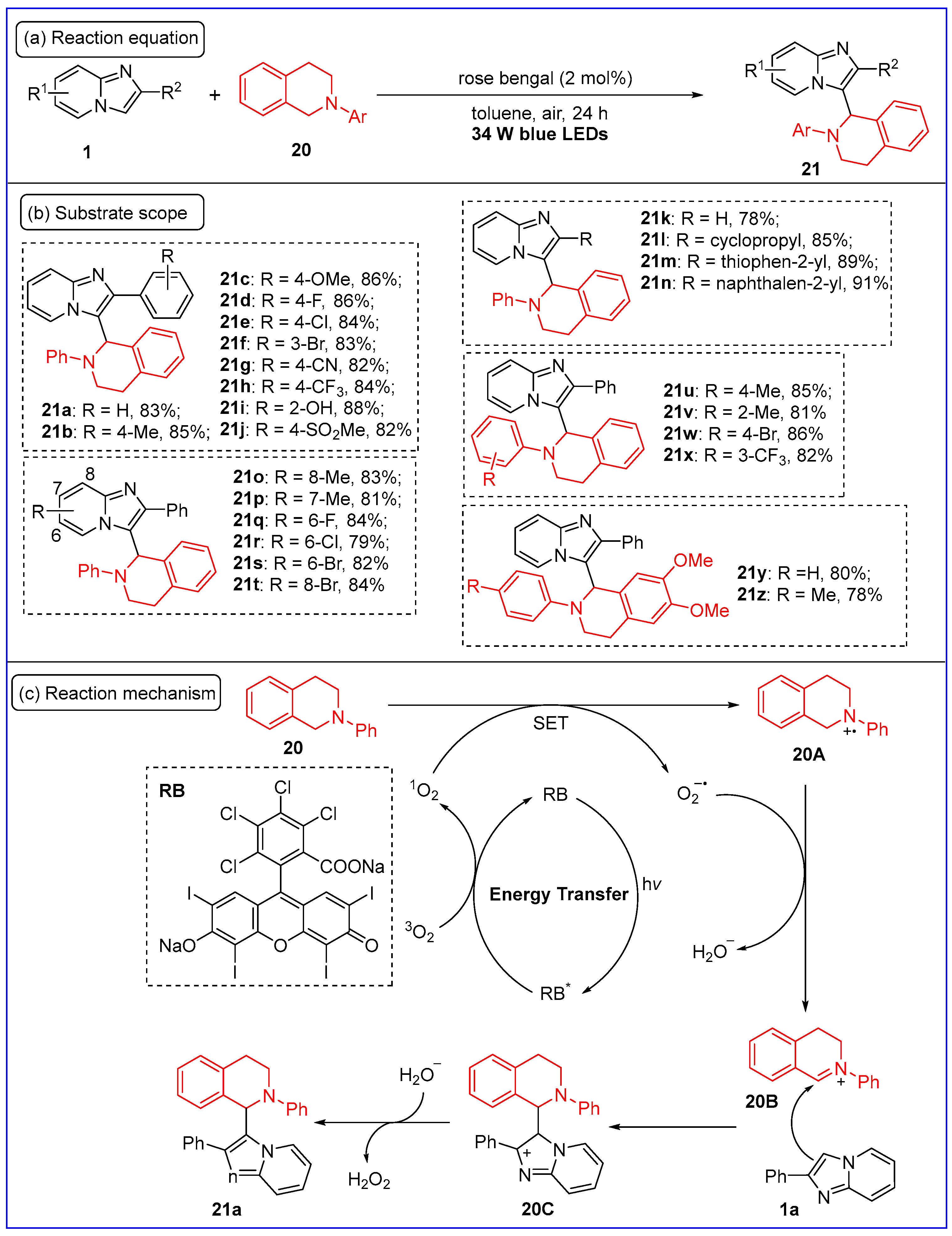
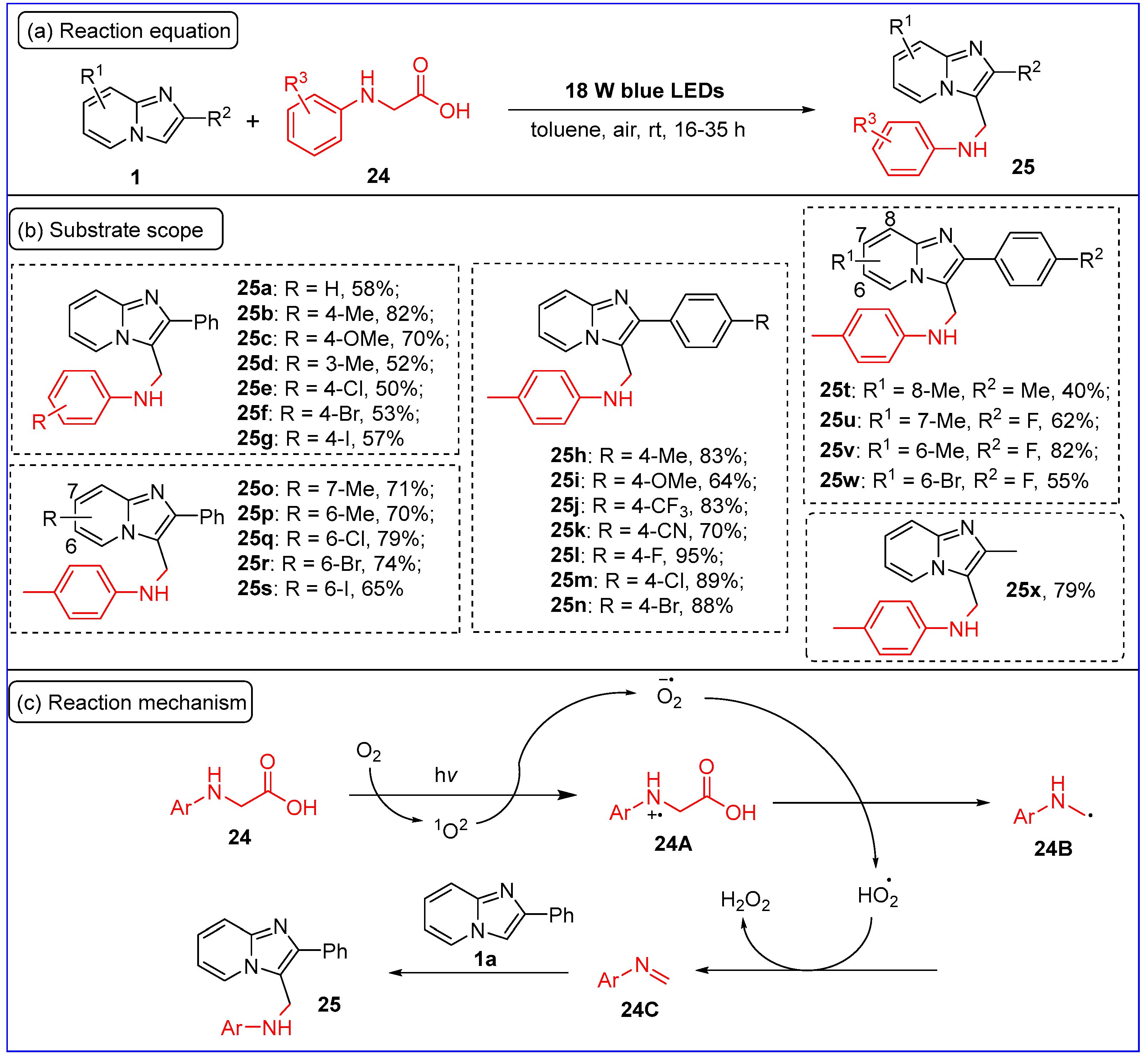
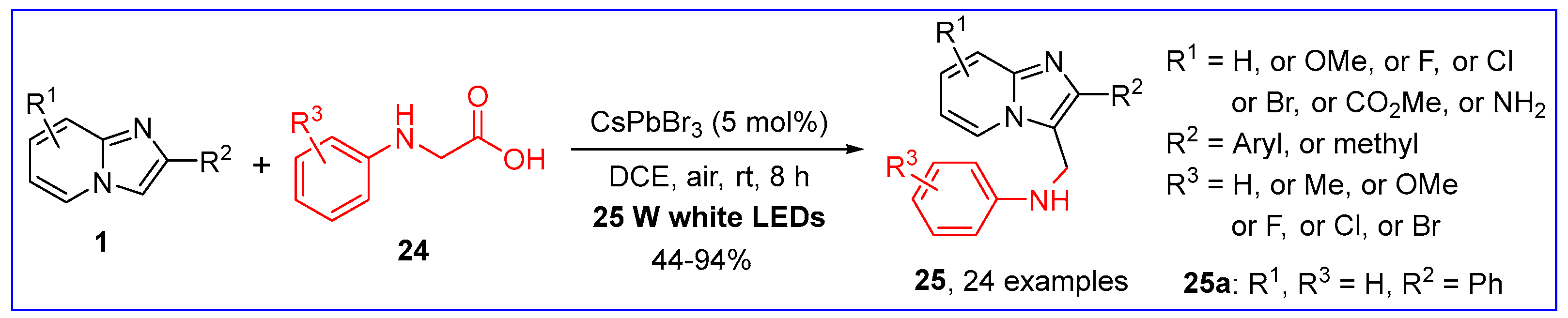
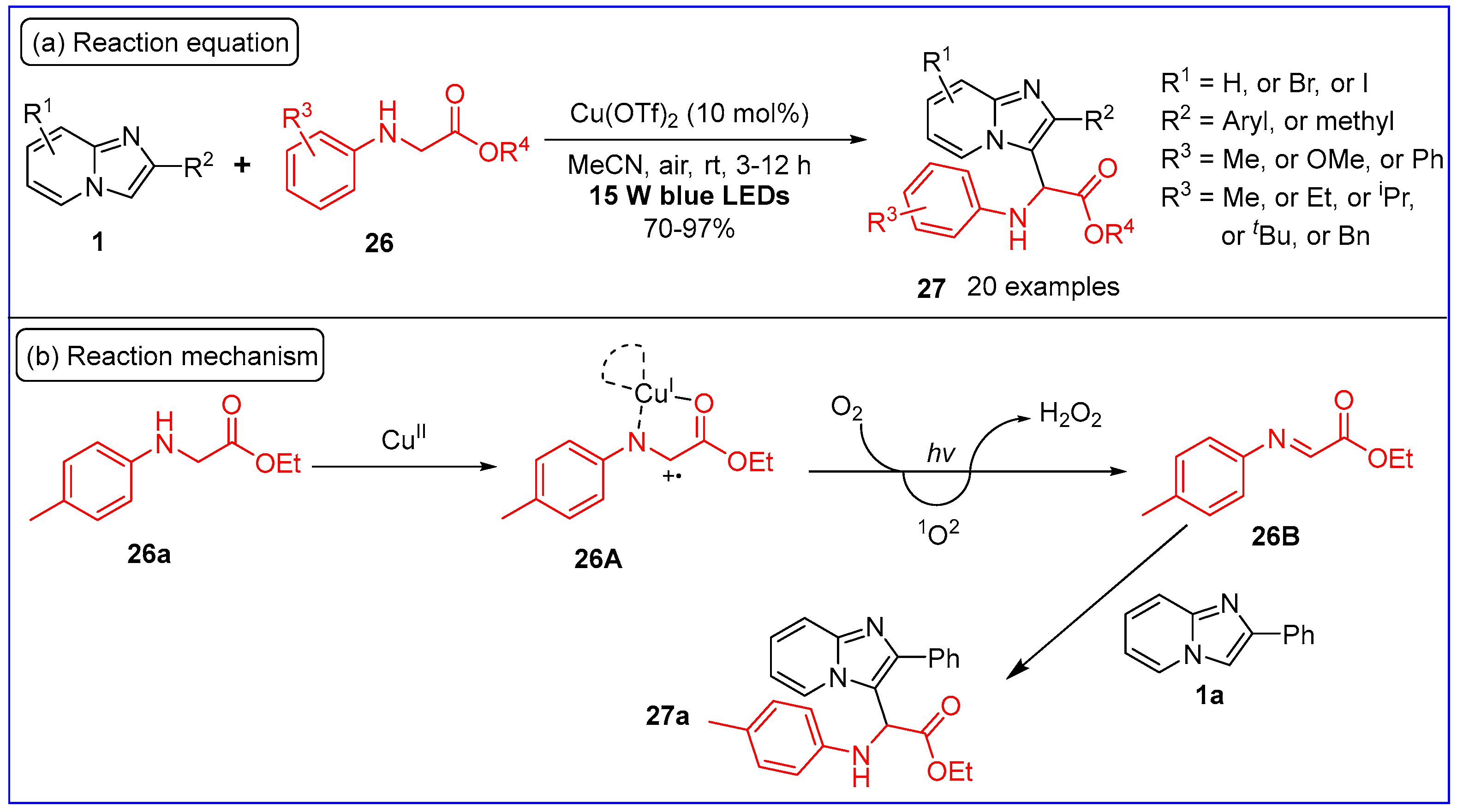
2.3. C3-Aminoalkylation of Imidazo[1,2-a]pyridines
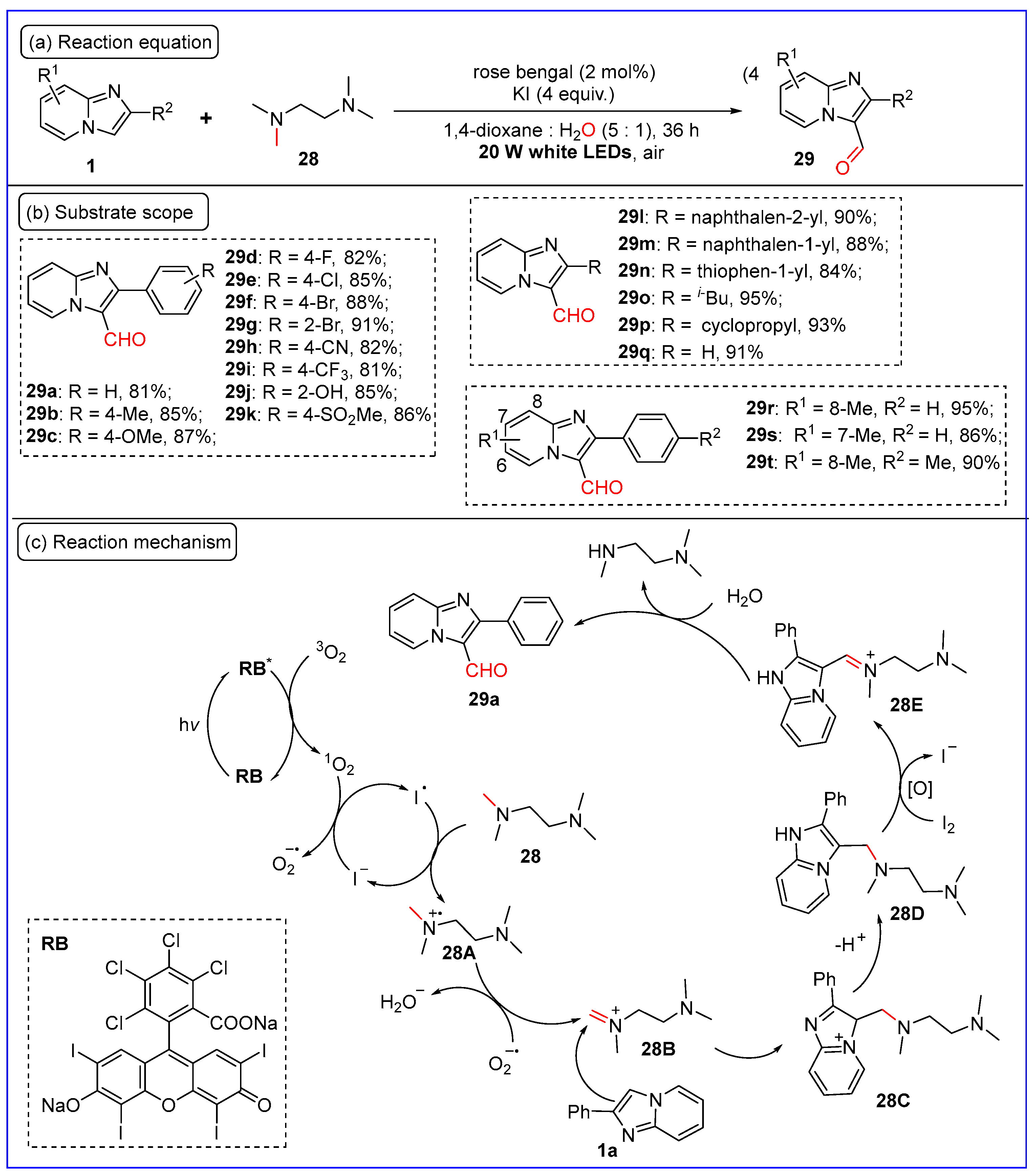
2.4. C3-Formylation of Imidazo[1,2-a]pyridines
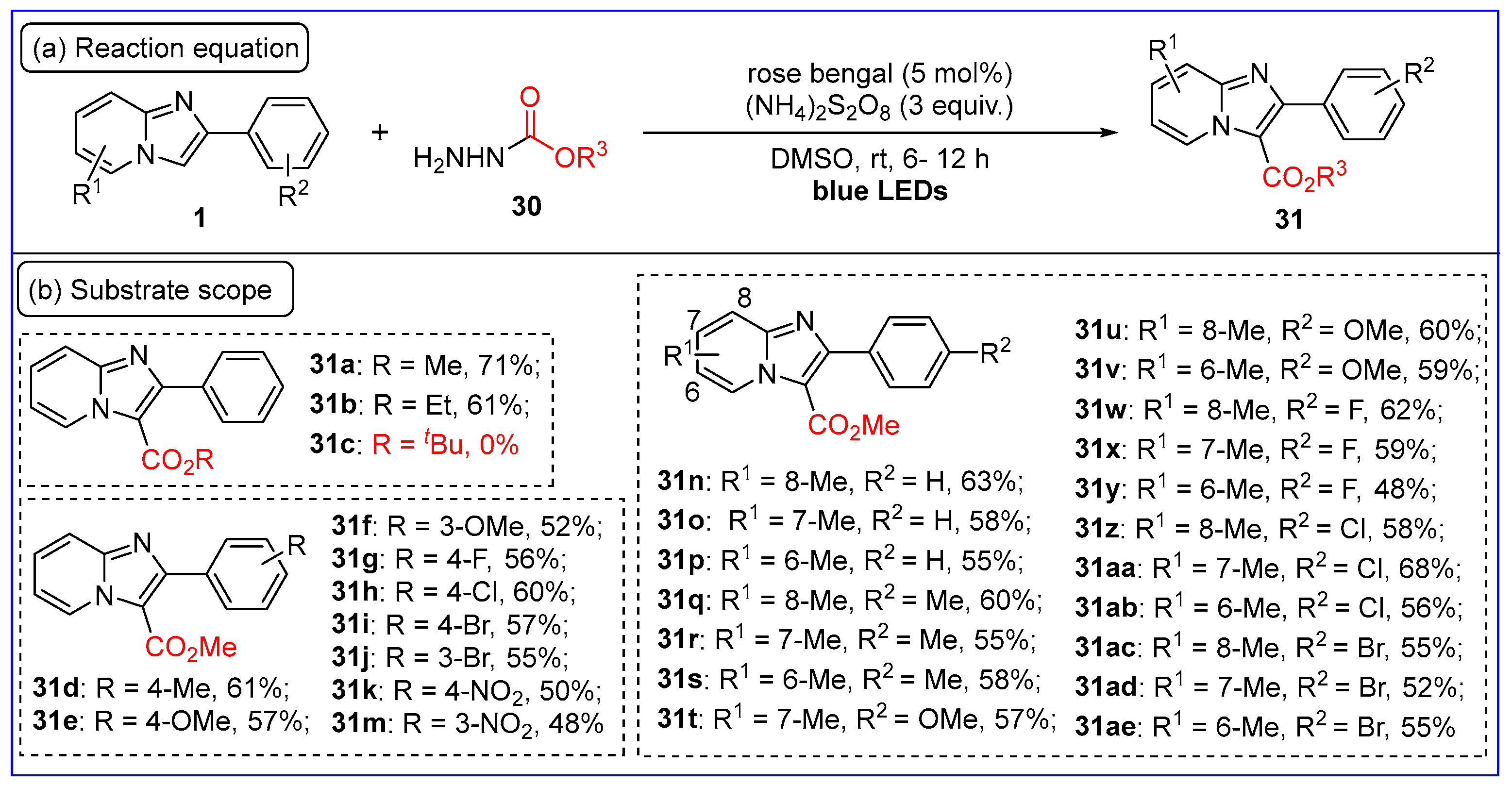
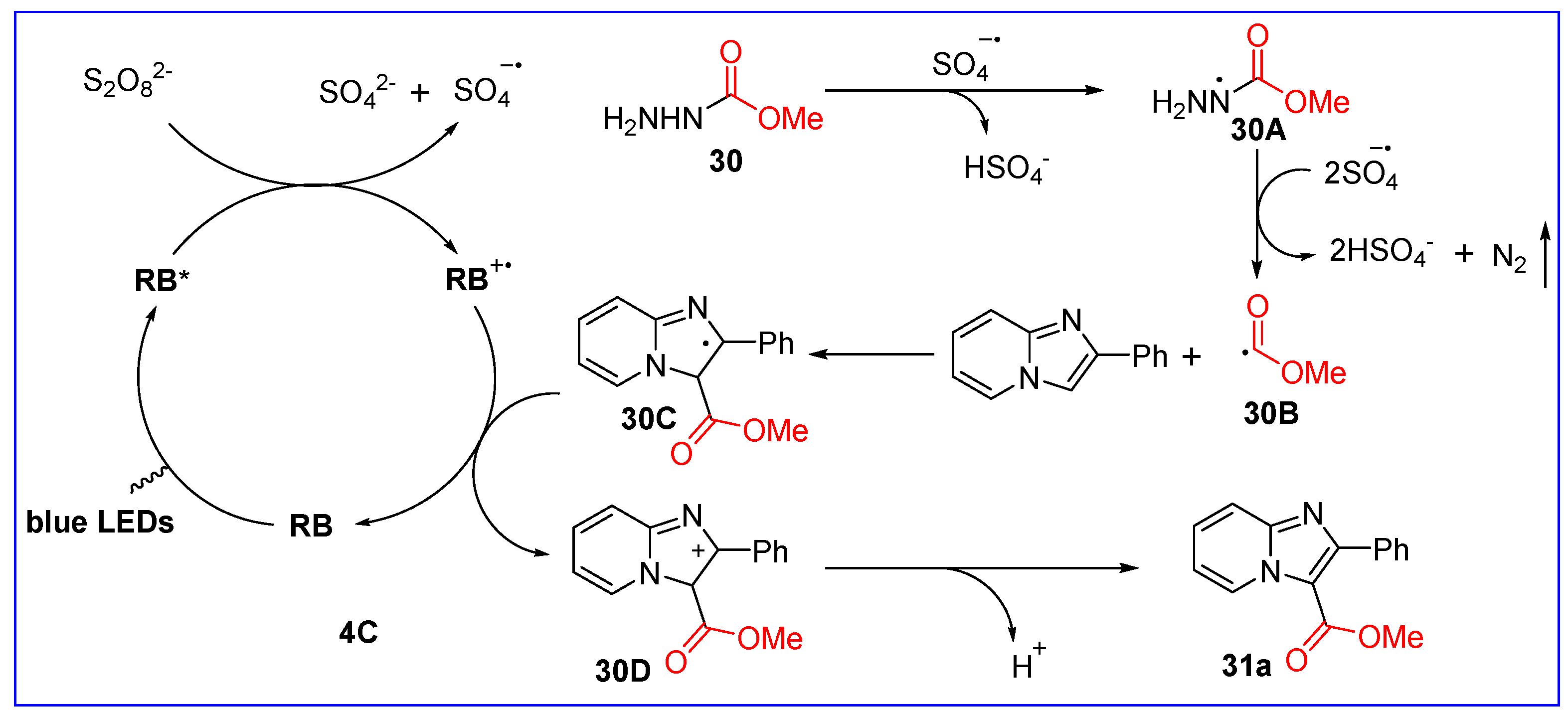
2.5. C3-Alkoxycarbonylation of Imidazo[1,2-a]pyridines
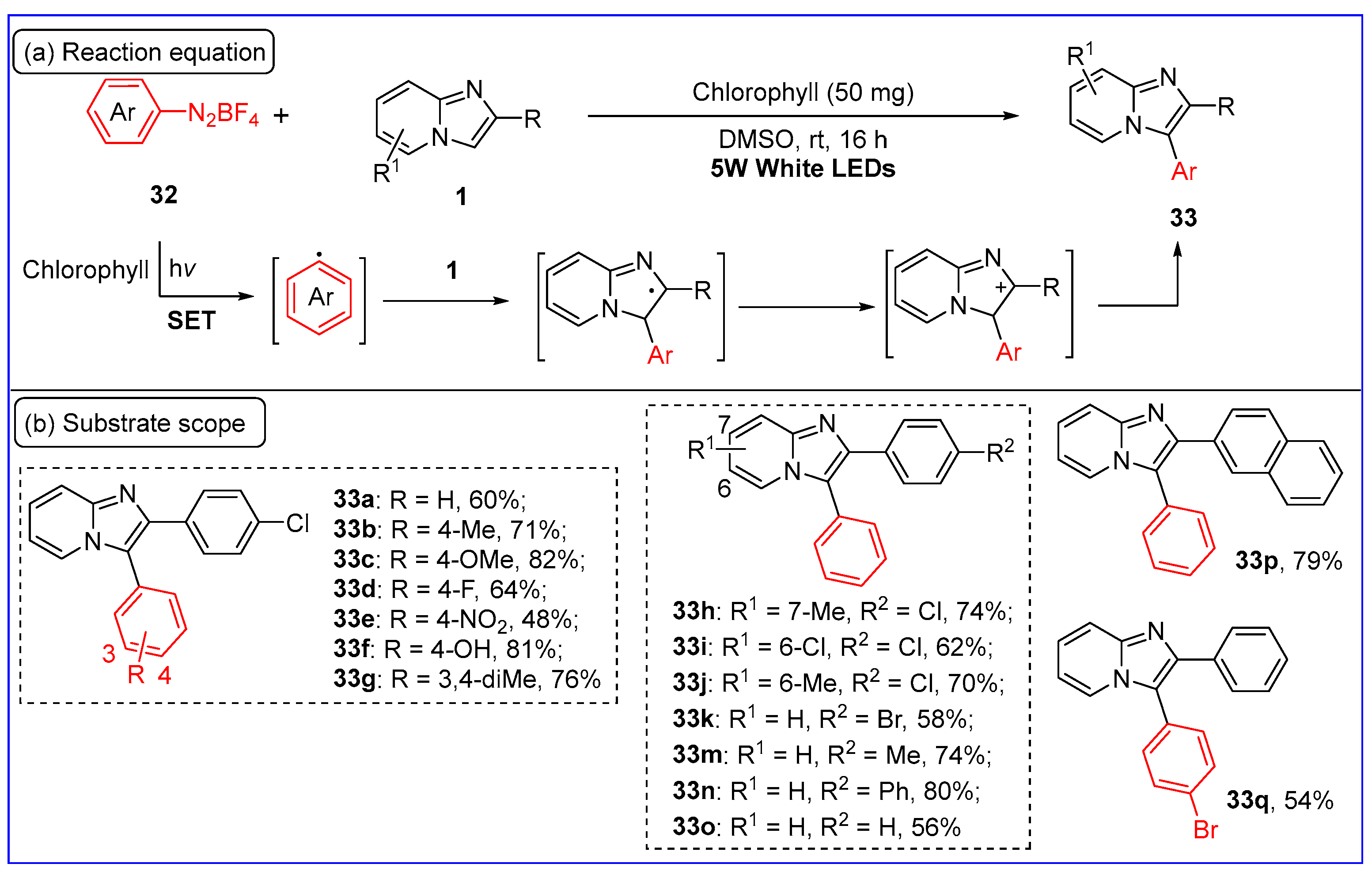
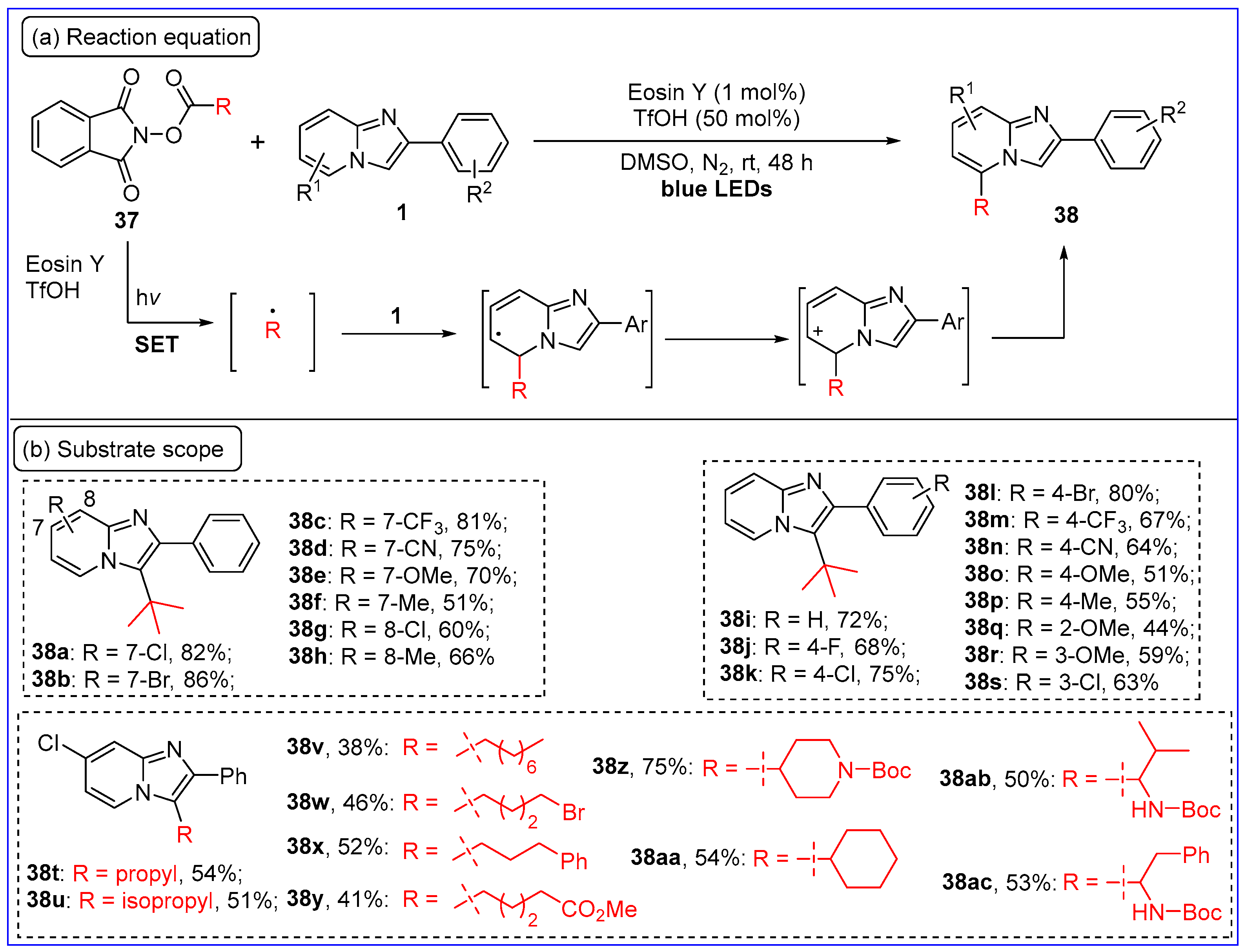
2.6. C3-Arylation of Imidazo[1,2-a]pyridines
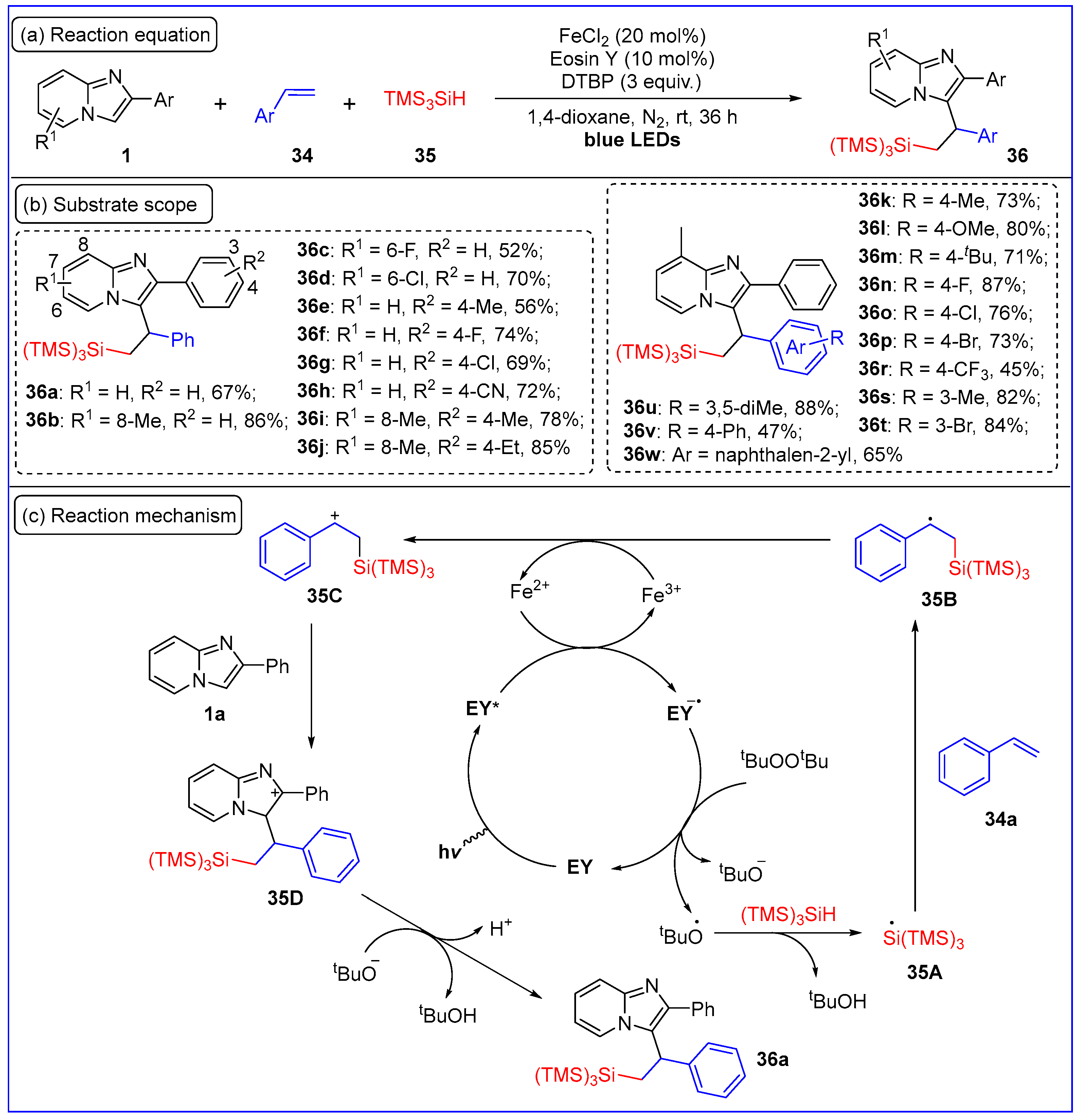
2.7. C3-Carbosilylation of Imidazo[1,2-a]pyridines
2.8. C5-Alkylation of Imidazo[1,2-a]pyridines
3. C-N Bond Formation
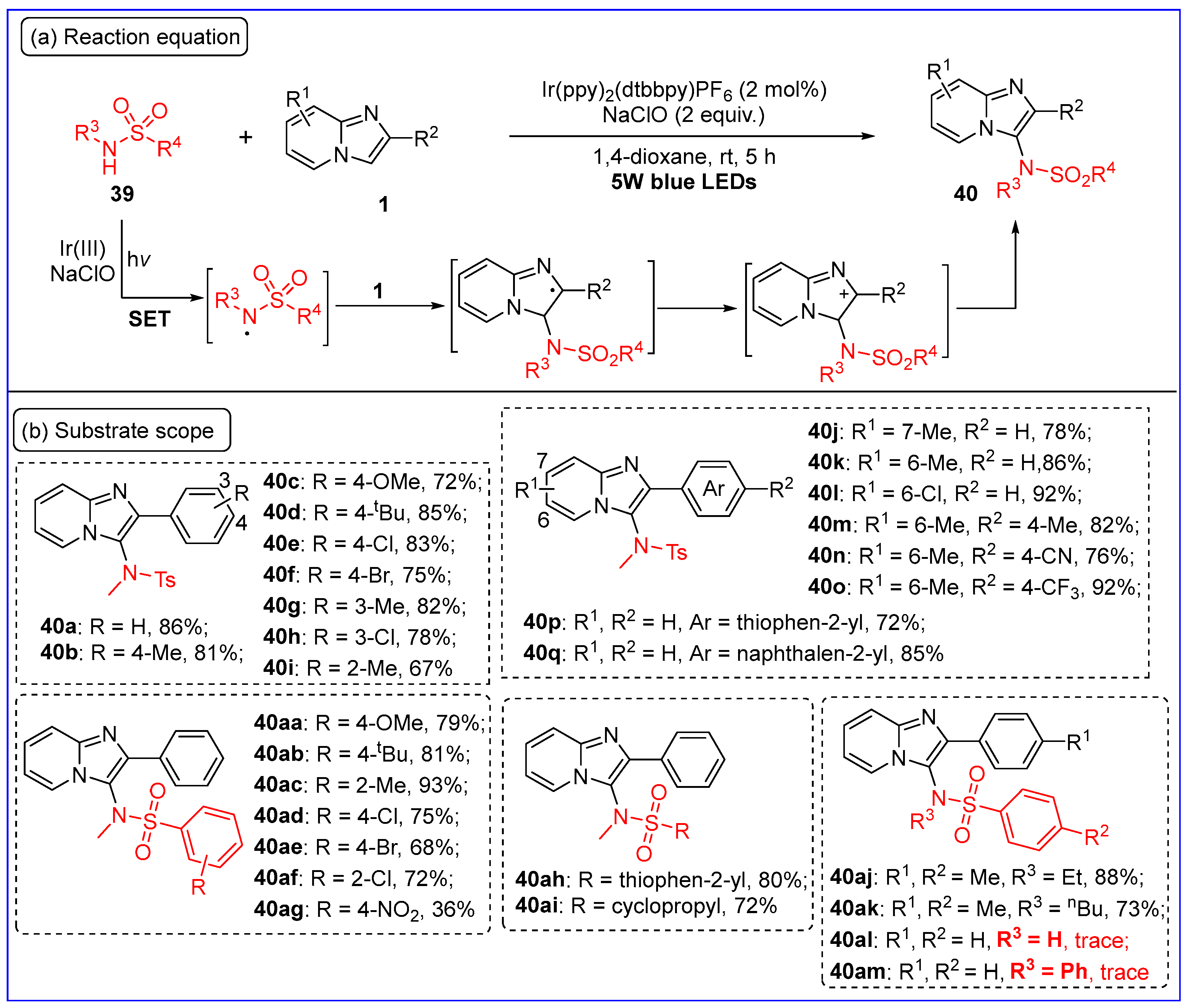
3.1. C3-Sulfonamidation of Imidazo[1,2-a]pyridines
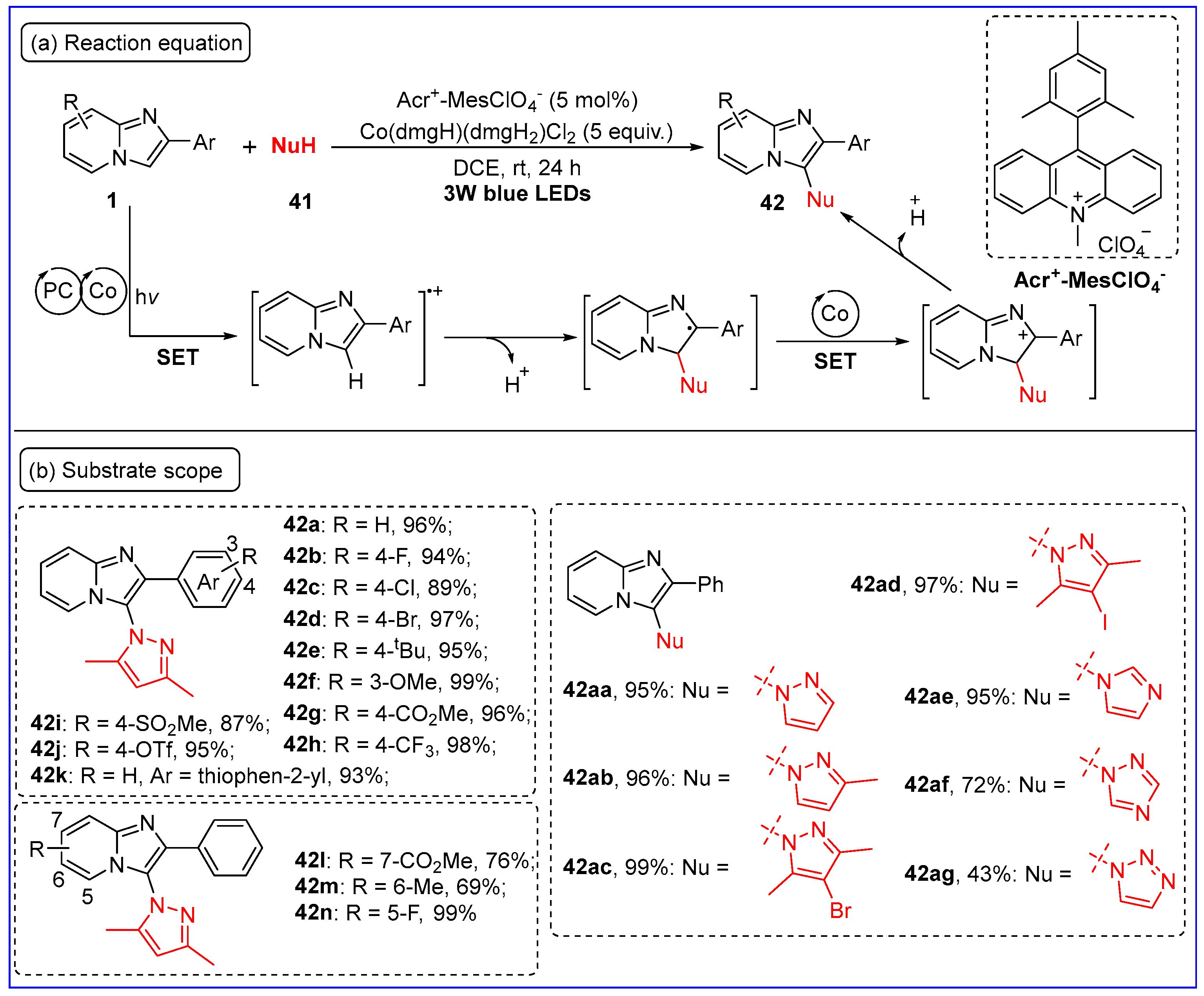
3.2. C3-Amination of Imidazo[1,2-a]pyridines
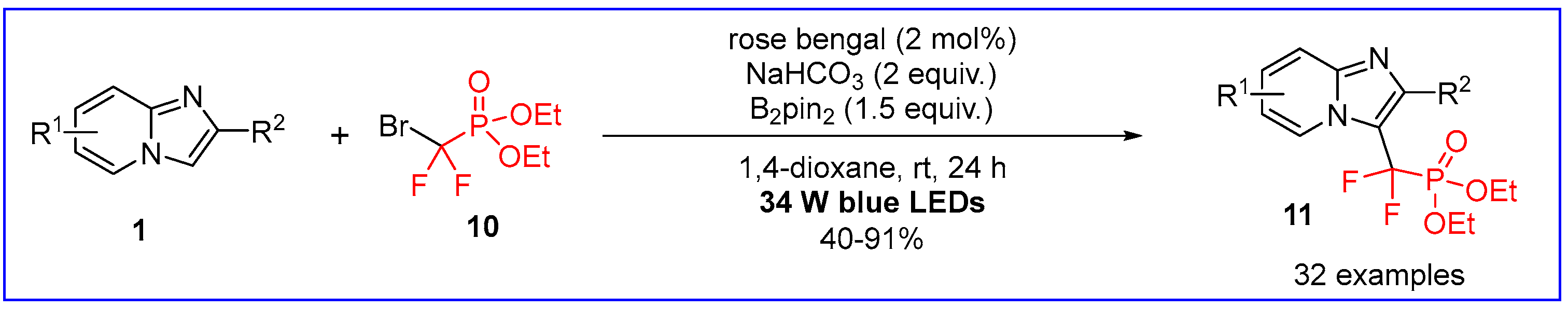
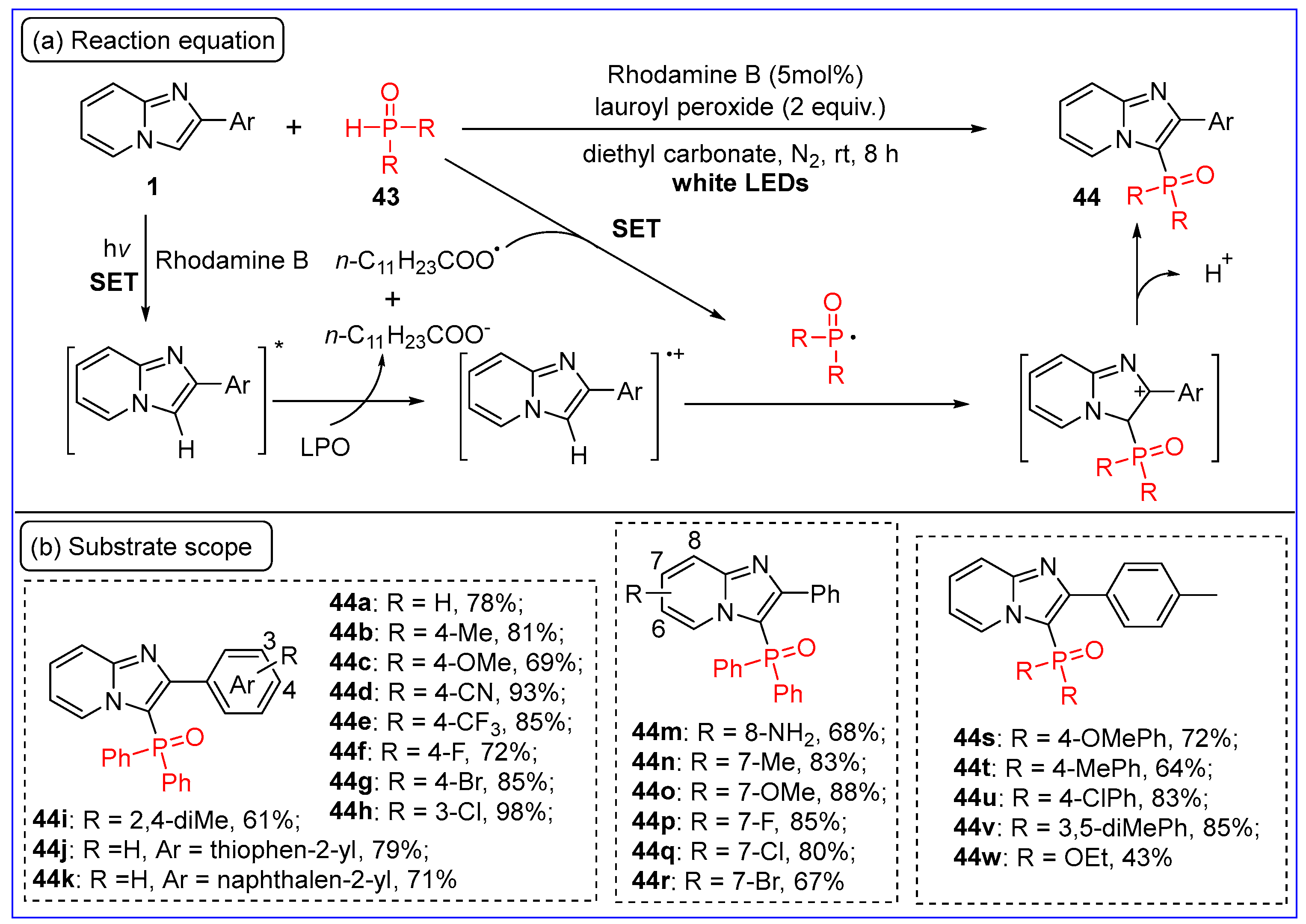
4. C-P Bond Formation
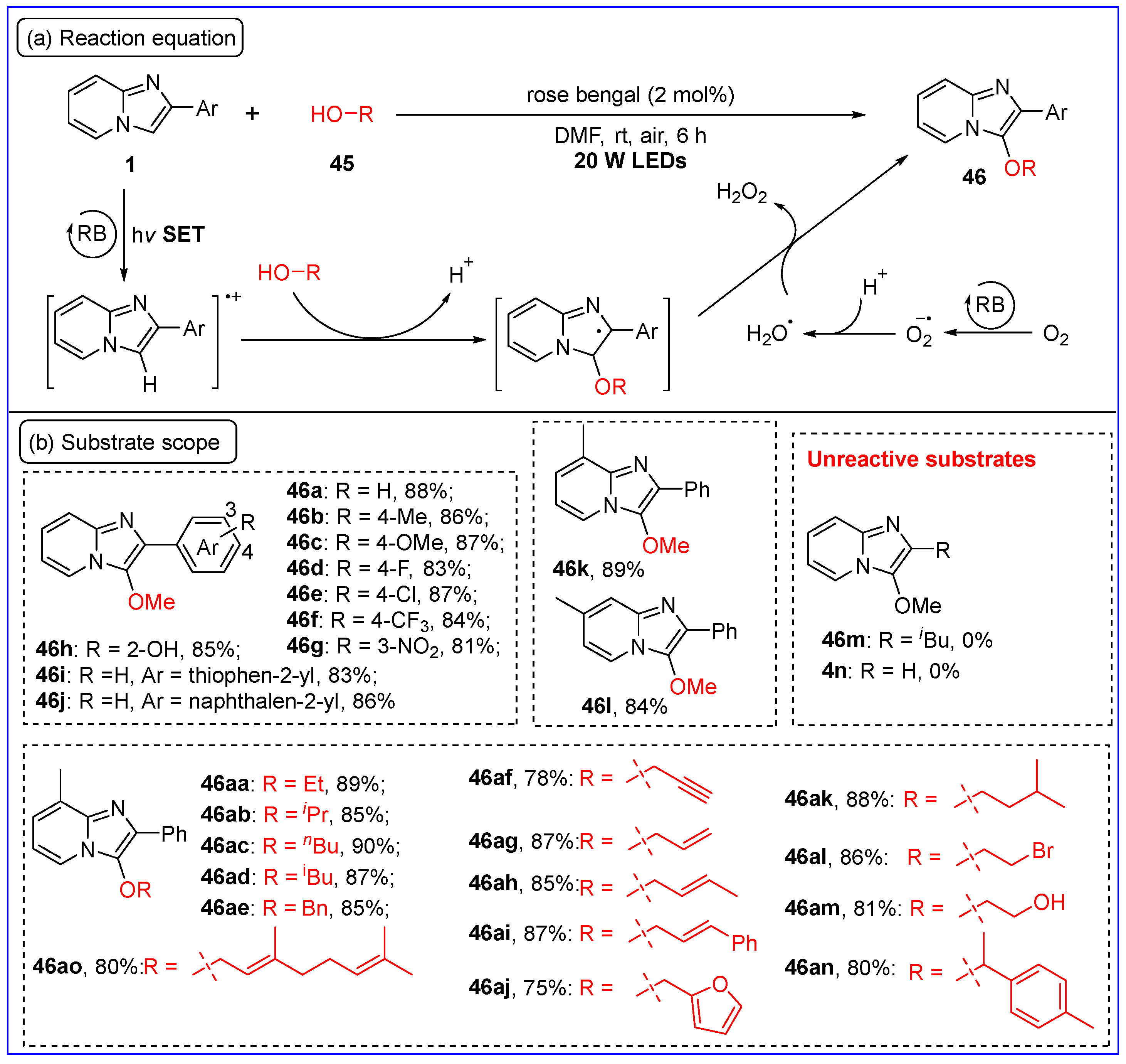
5. C-O Bond Formation
6. C-S Bond Formation
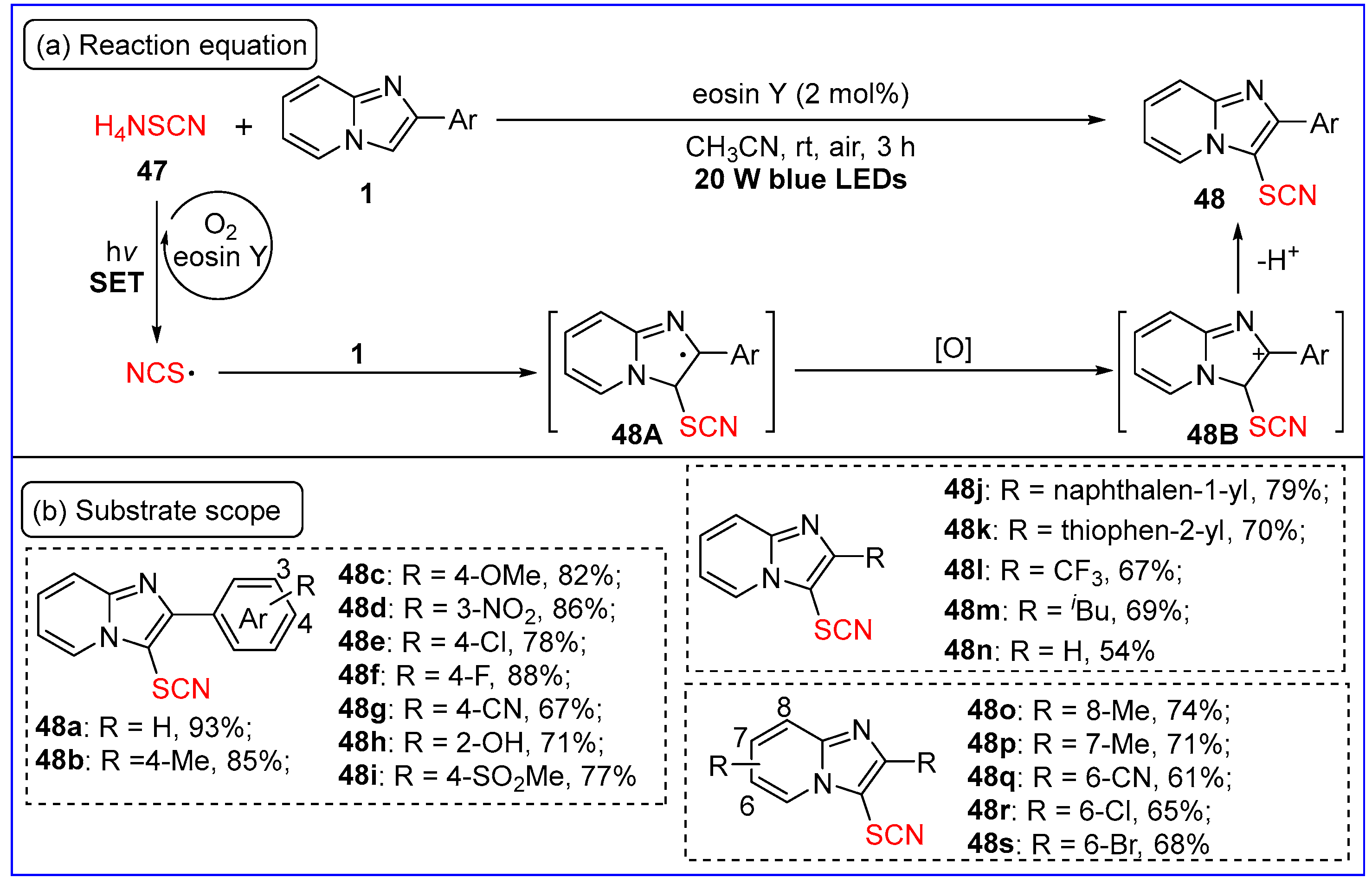
6.1. C3-Thiocyanation of Imidazo[1,2-a]pyridines
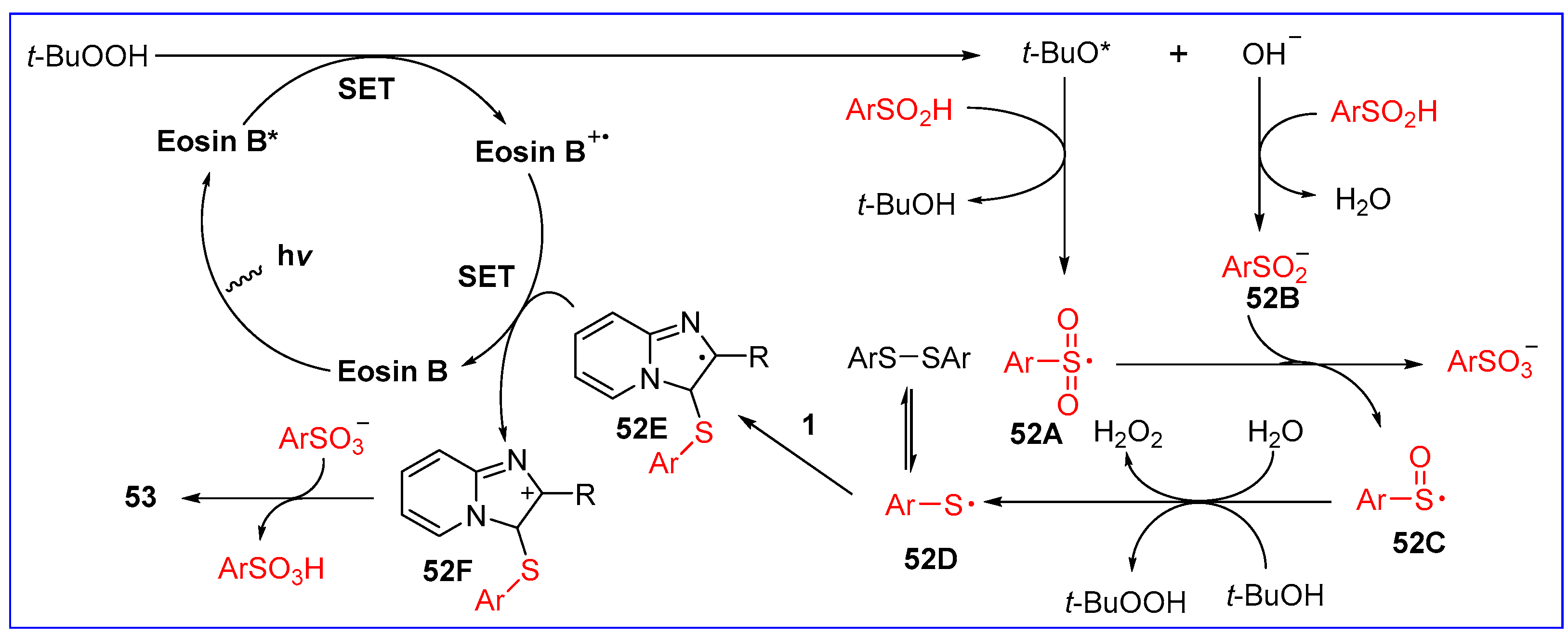
6.2. C3-Sulfonylation of Imidazo[1,2-a]pyridines
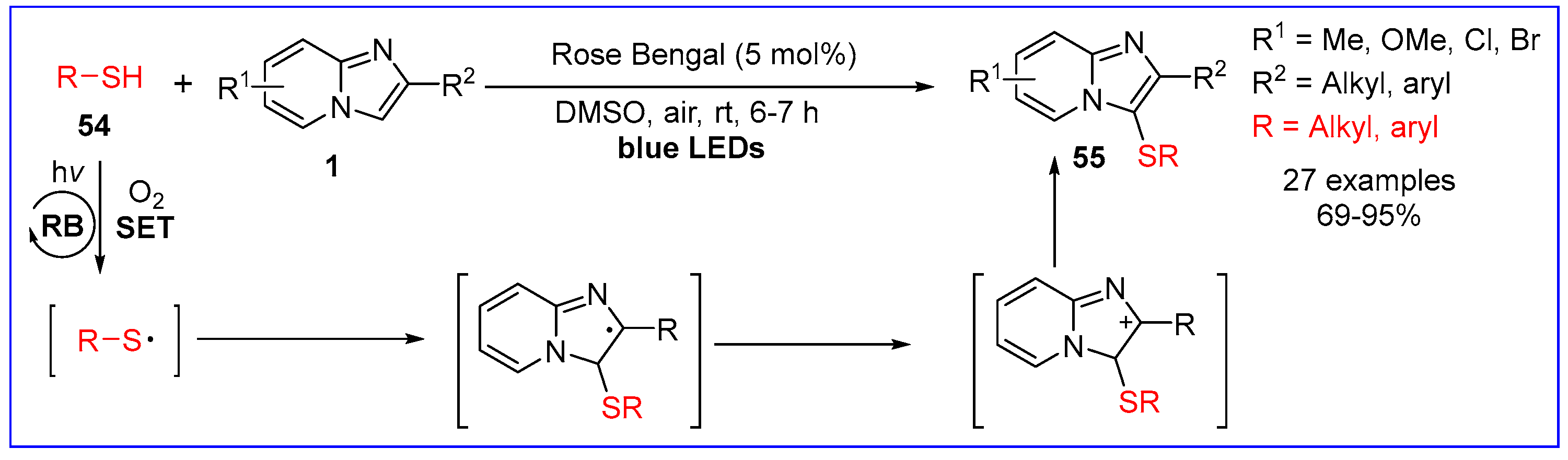
6.3. C3-Sulfenylation of Imidazo[1,2-a]pyridines
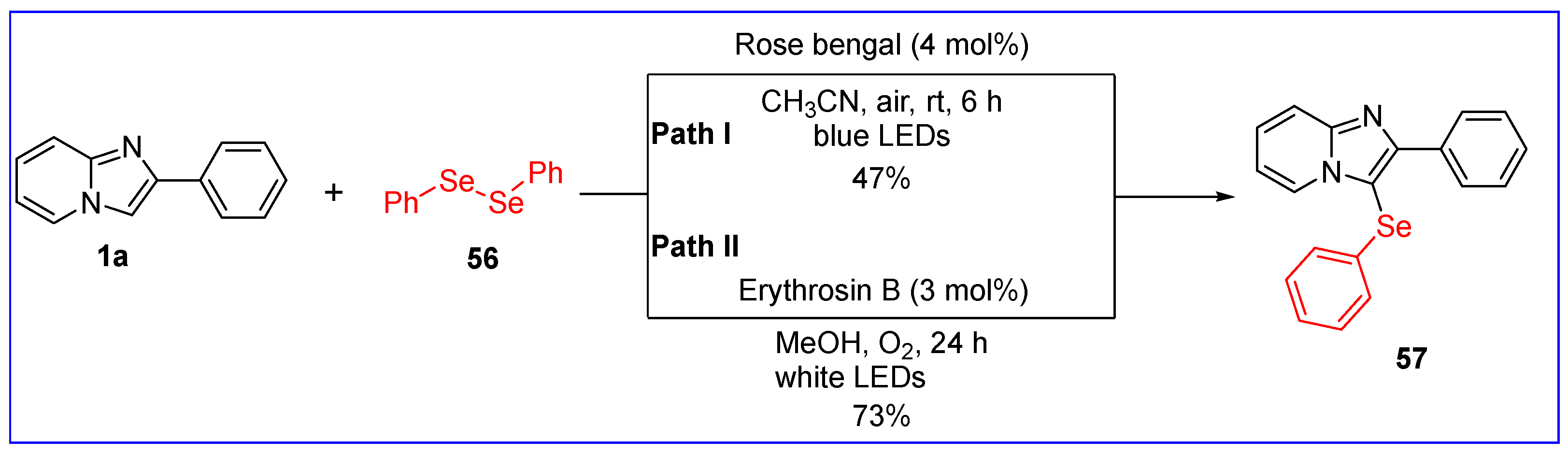
7. C-Se Bond Formation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Deep, A.; Bhatia, R.K.; Kaur, R.; Kumar, S.; Jain, U.K.; Singh, H.; Batra, S.; Kaushik, D.; Deb, P.K. Imidazo[1,2-a]pyridine scaffold as prospective therapeutic agents. Curr. Top. Med. Chem. 2017, 17, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yao, C.; Gao, J.J.; Chen, S.H.; Zheng, X.J.; Deng, L.X.; Zhang, Y.N.; Liu, M.J.; Wang, Z.Y. Progress on the Synthesis of Pyrido[1,2-a]benzimidazoles. Chin. J. Org. Chem. 2020, 40, 4168–4183. [Google Scholar] [CrossRef]
- Ismael, A.S.; Amin, N.H.; Elsaadi, M.T.; Abdel-Rahman, H.M. Design, synthesis and mechanistic studies of novel imidazo[1,2-a]pyridines as anticancer agents. Bioorg. Chem. 2023, 128, 106042. [Google Scholar]
- Samanta, S.; Kumar, S.; Aratikatla, E.K.; Ghorpade, S.R.; Singh, V. Recent developments of imidazo[1,2-a]pyridine analogues as antituberculosis agents. RSC Med. Chem. 2023, 14, 644–657. [Google Scholar] [CrossRef]
- Mishra, N.P.; Mohapatra, S.; Das, T.; Nayak, S. Imidazo[1,2-a]pyridine as a promising scaffold for the development of antibacterial agents. J. Heterocycl. Chem. 2022, 59, 2051–2075. [Google Scholar] [CrossRef]
- Zhang, W.B.; Jin, F.R.; Wu, S.; Zhao, C.N.; He, H.F.; Wu, Z.X.; Hu, D.Y.; Song, B.A. Development of imidazo[1,2-a]pyridines containing sulfonyl piperazines as potential antivirals against tomato spotted wilt virus. J. Agric. Food Chem. 2024, 72, 23118–23130. [Google Scholar] [CrossRef]
- Shrivastava, S.K.; Srivastava, P.; Bandresh, R.; Tripathi, P.N.; Tripathi, A. Design, synthesis, and biological evaluation of some novel indolizine derivatives as dual cyclooxygenase and lipoxygenase inhibitor for anti-inflammatory activity. Bioorg. Med. Chem. 2017, 25, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Liu, C.X.; Zou, F.; Cai, Y.; Chen, B.; Zou, Y.X.; Mo, J.X.; Han, T.; Huang, W.L.; Qiu, Q.Q.; et al. Discovery of novel potent GPR40 agonists containing imidazo[1,2-a ] pyridine core as antidiabetic agents. Bioorg. Med. Chem. 2020, 28, 115574. [Google Scholar] [CrossRef] [PubMed]
- Raiguru, B.P.; Panda, J.; Mohapatra, S.; Nayak, S. Palladium-catalyzed facile synthesis of imidazo[1,2-a]pyridine-flavone hybrids and evaluation of their antiplasmodial activity. J. Mol. Struct. 2023, 1294, 136282. [Google Scholar] [CrossRef]
- Rahaman, S.; Sahay, S.S.; Kumari, A.; Dey, S. Multicomponent cross-dehydrogenative coupling of imidazo[1,2-a]pyridine: Access to abnormal Mannich and Mannich-type reaction. J. Org. Chem. 2024, 89, 10773–10784. [Google Scholar] [CrossRef]
- Nagy, E.; Máriás, A.; Kovács, M.; Skoda-Földes, R. Synthesis of 6-or 8-carboxamido derivatives of imidazo[1,2-a]pyridines via a heterogeneous catalytic aminocarbonylation reaction. Molecules 2024, 29, 5048. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Su, Z.Q.; Cao, H. Strategies for synthesis of imidazo[1,2-a]pyridine derivatives: Carbene transformations or C-H functionalizations. Chem. Rec. 2019, 19, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, D.; Wang, Z. Recent progress in transition metal-free C-heteroatom bond formation by functionalization of C-H bond in imidazole-fused heterocycles. Chin. J. Org. Chem. 2019, 39, 3338–3352. [Google Scholar] [CrossRef]
- Patel, O.P.S.; Nandwana, N.K.; Legoabe, L.J.; Das, B.C.; Kumar, A. Recent advances in radical C-H bond functionalization of imidazoheterocycles. Adv. Synth. Catal. 2020, 362, 4226–4255. [Google Scholar] [CrossRef]
- Tali, J.A.; Kumar, G.; Sharma, B.K.; Rasool, Y.; Sharma, Y.; Shankar, R. Synthesis and site selective C-H functionalization of imidazo-[1,2-a]pyridines. Org. Biomol. Chem. 2023, 21, 7267–7289. [Google Scholar] [CrossRef] [PubMed]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdavi, M. C3-functionalization of imidazo[1,2-a]pyridines. Eur. J. Org. Chem. 2020, 2020, 269–284. [Google Scholar] [CrossRef]
- Ma, C.H.; Chen, M.; Feng, Z.W.; Zhang, Y.; Wang, J.; Jiang, Y.Q.; Yu, B. Functionalization of imidazo[1,2-a]pyridines via radical reactions. New J. Chem. 2021, 45, 9302–9314. [Google Scholar] [CrossRef]
- Wu, Y.R.; Li, L.; Wen, K.M.; Deng, J.; Chen, J.W.; Shi, J.; Wu, T.; Pang, J.X.; Tang, X.D. Copper-catalyzed C-3 functionalization of imidazo[1,2-a]pyridines with 3-indoleacetic acids. J. Org. Chem. 2021, 86, 12394–12402. [Google Scholar] [CrossRef] [PubMed]
- Tali, J.A.; Shankar, R. Ru(II)-catalyzed synthesis of fused imidazo[1,2-a]pyridine-chromenones and methylene-tethered bis-imidazo[1,2-a]pyridines and regioselective o-acetoxylation of imidazo[1,2-a]pyridines. Org. Lett. 2023, 25, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Bagdi, A.K.; Hajra, A. Visible light promoted C-H functionalization of imidazoheterocycles. Org. Biomol. Chem. 2020, 18, 2611–2631. [Google Scholar] [CrossRef] [PubMed]
- Holmberg-Douglas, N.; Nicewicz, D.A. Photoredox-catalyzed C-H functionalization reactions. Chem. Rev. 2022, 122, 1925–2016. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.L.; Sharma, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.A.; Gogde, K.; Mukherjee, D.; Masoodi, M.H. Sustainable approaches towards the synthesis of functionalized imidazo[1,2-a]pyridines: Recent advancements. J. Mol. Struct. 2024, 1297, 137012. [Google Scholar] [CrossRef]
- Ka, C.H.; Kim, S.; Cho, E.J. Visible light-induced metal-free fluoroalkylations. Chem. Rec. 2023, 23, e202300036. [Google Scholar]
- Zhang, B.; Zhang, X.; Wang, J. Recent progress in carbene-catalyzed fluoroalkylation. Sci. China Chem. 2024, 67, 2448–2460. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Chen, Z.; Wu, L.; Yang, K. Metal-free synthesis of trifluoromethyl carbinol-containing imidazo[1,2-a]pyridines via dehydrative coupling of imidazo[1,2-a]pyridines with trifluoroacetaldehyde. Synthesis 2024, 56, 1756–1764. [Google Scholar]
- Gao, J.; Liu, Z.; Guo, X.; Wu, L.; Chen, Z.; Yang, K. 1,1,1,3,3,3-Hexafluoro-2-propanol-promoted Friedel-Crafts reaction: Metal-free synthesis of C3-difluoromethyl carbinol-containing imidazo[1,2-a]pyridines at room temperature. Molecules 2023, 28, 7522. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, X.; Liu, Z.; Cao, Y. HFIP-promoted synthesis of imidazo[1,2-a]pyridines containing CF3-substituted tertiary alcohols at room temperature. Tetrahedron 2024, 156, 133943. [Google Scholar] [CrossRef]
- Han, S.; Gao, X.; Wu, Q.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Transition-metal-free direct trifluoromethylation and perfluoroalkylation of imidazopyridines under mild conditions. Adv. Synth. Catal. 2019, 361, 1559–1563. [Google Scholar] [CrossRef]
- Lefebvre, Q.; Hoffmann, N.; Rueping, M. Photoorganocatalysed and visible light photoredox catalysed trifluoromethylation of olefins and (hetero)aromatics in batch and continuous flow. Chem. Commun. 2016, 52, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, S.; Zhang, R. Transition-metal-free, visible-light-mediated regioselective C-H trifluoromethylation of imidazo[1,2-a]pyridines. Tetrahedron Lett. 2019, 60, 734–738. [Google Scholar] [CrossRef]
- Mi, X.; Kong, Y.F.; Yang, H.X.; Zhang, J.Y.; Pi, C.; Cui, X.L. Visible-light-promoted metal-free C-H trifluoromethylation of imidazopyridines. Eur. J. Org. Chem. 2020, 2020, 1019–1022. [Google Scholar] [CrossRef]
- Zhu, M.; Han, X.; Fu, W.J.; Wang, Z.Q.; Hao, B.M.; Hao, X.Q.; Song, M.P.; Xu, C. Regioselective 2,2,2-trifluoroethylation of imidazopyridines by visible light photoredox catalysis. J. Org. Chem. 2016, 81, 7282–7287. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.J.; Zhu, M.; Fu, W.J. Visible-light-induced photocatalytic difluoroacetylation of imidazopyridines via direct and regioselective C-H functionalization. J. Fluorine Chem. 2017, 199, 14–19. [Google Scholar] [CrossRef]
- Yin, G.J.; Zhu, M.; Fu, W.J. Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone. Heterocycl. Commun. 2017, 23, 275–279. [Google Scholar] [CrossRef]
- Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Organophotoredox-catalyzed C(sp2)-H difluoromethylenephosphonation of imidazoheterocycles. Org. Lett. 2019, 21, 5606–5610. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; Wu, D.D.; Bai, X.K.; Cai, P.Y.; Zhu, W.G. Catalyst-free, visible-light-induced direct radical cross-coupling perfluoroalkylation of the imidazo[1,2-a]pyridines with perfluoroalkyl iodides. New J. Chem. 2021, 45, 4925–4929. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Mzaaarella, D.; Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef]
- Zheng, D.; Studer, A. Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis. Org. Lett. 2019, 21, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Chen, X.Y.; Pei, C.C.; Li, J.Y.; Zou, D.P.; Wu, Y.J.; Wu, Y.S. Visible-light-mediated direct C-H perfluoroalkylation of imidazoheterocycles. Tetrahedron Lett. 2021, 83, 153407. [Google Scholar] [CrossRef]
- Qu, C.H.; Song, G.T.; Xu, J.; Yan, W.; Zhou, C.H.; Li, H.Y.; Chen, Z.Z.; Xu, Z.G. Merging visible light with cross-coupling: The photochemical direct C-H difluoroalkylation of imidazopyridines. Org. Lett. 2019, 21, 8169–8173. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Zhu, C.; Huang, K.; Bau, J.A.; Jia, J.Q.; Yue, H.F.; Rueping, M. Photoinduced nickel-catalyzed demethylative cyanation and decarboxylative cyanomethylation of aryl halides. ACS Catal. 2023, 13, 16279–16285. [Google Scholar] [CrossRef]
- Donald, J.R.; Berrell, S.L. Radical cyanomethylation via vinyl azide cascade-fragmentation. Chem. Sci. 2019, 10, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Liu, Z.Y.; Liu, P.; Yu, L.; Sun, P.P. Visible-light-induced regioselective cyanomethylation of imidazopyridines and its application in drug synthesis. J. Org. Chem. 2017, 82, 5391–5397. [Google Scholar] [CrossRef]
- Nair, D.K.; Mobin, S.M.; Namboothiri, I.N.N. Synthesis of imidazopyridines from the Morita-Baylis-Hillman acetates of nitroalkenes and convenient access to Alpidem and Zolpidem. Org. Lett. 2012, 14, 4580–4583. [Google Scholar] [CrossRef] [PubMed]
- Kibriya, G.; Bagdi, A.K.; Hajra, A. Visible-Light-Promoted C(sp3)-C(sp2) Cross-Dehydrogenative Coupling of Tertiary Amine with Imidazopyridine. J. Org. Chem. 2018, 83, 10619–10626. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; König, B. Decarboxylative reactions with and without light—A comparison. Green Chem. 2018, 20, 323–361. [Google Scholar] [CrossRef]
- Ji, J.J.; Zhu, Z.Q.; Xiao, L.J.; Guo, D.; Zhu, X.; Tang, J.; Wu, J.; Xie, Z.B.; Le, Z.G. Photocatalyst-free decarboxylative aminoalkylation of imidazo[1,2-a]pyridines with N-aryl glycines enabled by visible light. Org. Chem. Front. 2019, 6, 3693–3697. [Google Scholar] [CrossRef]
- Shi, T.; Sun, K.; Chen, X.L.; Zhang, Z.X.; Huang, X.Q.; Peng, Y.Y.; Qu, L.B.; Yu, B. Recyclable perovskite as heterogeneous photocatalyst for aminomethylation of imidazo-fused heterocycles. Adv. Synth. Catal. 2020, 362, 2143–2149. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Xiao, L.J.; Zhou, C.C.; Song, H.L.; Xie, Z.B.; Le, Z.G. A Visible-light-promoted cross-dehydrogenative-coupling reaction of N arylglycine esters with imidazo[1,2-a]pyridines. Tetrahedron Lett. 2018, 59, 3326–3331. [Google Scholar] [CrossRef]
- Meng, Q.-Y.; Gao, X.-W.; Lei, T.; Liu, Z.; Zhan, F.; Li, Z.-J.; Zhong, J.-J.; Xiao, H.; Feng, K.; Chen, B.; et al. Identifying key intermediates generated in situ from Cu(II) sal-catalyzed C-H functionalization of aromatic amines under illumination. Sci. Adv. 2017, 3, e1700666. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.J.; Chen, H.J.; Liu, Q. Aerobic iron(III)-catalyzed direct formylation of imidazo[1,2-a] pyridine using DMSO as carbon source. Tetrahedron Lett. 2016, 57, 3870–3872. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.C.; Zang, J.W.; Liu, M.C.; Jiang, G.B.; Ji, F.H. Copper- and DMF-mediated switchable oxidative C-H cyanation and formylation of imidazo[1,2-a]pyridines using ammonium iodide. Org. Biomol. Chem. 2020, 18, 9100–9108. [Google Scholar] [CrossRef] [PubMed]
- Kibriya, G.; Bagdi, A.K.; Hajra, A. Visible light induced tetramethylethylenediamine assisted formylation of imidazopyridines. Org. Biomol. Chem. 2018, 16, 3473–3478. [Google Scholar] [CrossRef]
- Tanbakouchian, A.; Kianmehr, E. Palladium-catalyzed regioselective direct C-H bond alkoxycarbonylation of 2-arylimidazo[1,2-a]pyridines. New J. Chem. 2021, 45, 12145–12149. [Google Scholar] [CrossRef]
- Kshirsagar, U.A.; Sarothiya, D.; Bhawale, R.T. Organic-dye-catalyzed visible-light-mediated regioselective C-3 alkoxycarbonylation of imidazopyridines by carbazates. J. Org. Chem. 2022, 87, 14915–14922. [Google Scholar]
- Gudmundsson, K.S.; Johns, B.A. Imidazo[1,2-a]pyridines with potent activity against herpesviruses. Bioorg. Med. Chem. Lett. 2007, 17, 2735. [Google Scholar] [CrossRef]
- Singhaus, R.R.; Bernotas, R.C.; Steffan, R.; Matelan, E.; Quinet, E.; Nambi, P.; Feingold, I.; Huselton, C.; Wilhelmsson, A.; Goos Nilsson, A.; et al. 3-(3-Aryloxyaryl)imidazo[1,2-a]pyridine sulfones as liver X receptor agonists. Bioorg. Med. Chem. Lett. 2010, 20, 521–525. [Google Scholar] [CrossRef]
- Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Direct arylation of imidazo[1,2-a]pyridine at C-3 with aryl iodides, bromides, and triflates via Copper(I)-catalyzed C-H bond functionalization. Org. Lett. 2012, 14, 1688–1691. [Google Scholar] [CrossRef]
- Moazzam, A.; Farid, S.M.; Alizadeh, N.; Mahdavi, M. Photochemical regioselective C-H arylation of imidazo[1,2-a]pyridine derivatives using chlorophyll as a biocatalyst and diazonium salts. New J. Chem. 2022, 46, 10814–10819. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, A.K.; Mandal, S.; Ghosh, D.; Ghosh, S.; Hajra, A. Three-component carbosilylation of alkenes by merging iron and visible-light photocatalysis. Org. Lett. 2021, 23, 6510–6514. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Di Monaco, S.; Thai, A.N.; Rahman, M.S.; Pang, B.P.; Wang, C.; Yoshikai, N. Cobalt/Lewis acid catalysis for hydrocarbofunctionalization of alkynes via cooperative C-H activation. J. Am. Chem. Soc. 2020, 142, 12878–12889. [Google Scholar] [CrossRef]
- Sun, B.; Xu, T.W.; Zhang, L.; Zhu, R.; Yang, J.; Xu, M.; Jin, C. Metal-free regioselective alkylation of imidazo[1,2-a]pyridines with N-hydroxyphthalimide esters under organic photoredox catalysis. Synlett 2020, 31, 363–368. [Google Scholar]
- Supuran, C.T. Special issue: Sulfonamides. Molecules 2017, 22, 1642. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pal, P.P.; Hajra, A. N-heteroarylation of sulfonamides: An overview. Adv. Synth. Catal. 2023, 365, 3020–3043. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Chen, S.; Lu, W.Y.; Gu, W.J.; Liu, P.; Sun, P.P. Visible light-induced C3-sulfonamidation of imidazopyridines with sulfamides. Org. Biomol. Chem. 2017, 15, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lei, T.; Wei, X.Z.; Ye, C.; Liu, Z.; Chen, B.; Tung, C.H.; Wu, L.Z. Metal-free, redox-neutral, site-selective access to heteroarylamine via direct radical-radical cross-coupling powered by visible light photocatalysis. J. Am. Chem. Soc. 2020, 142, 16805–16813. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yi, H.; Tang, Z.L.; Bian, C.L.; Zhang, H.; Lei, A.W. External oxidant-free regioselective cross dehydrogenative coupling of 2-arylimidazoheterocycles and azoles with H2 evolution via photoredox catalysis. Adv. Synth. Catal. 2018, 360, 3220–3227. [Google Scholar] [CrossRef]
- Samanta, S.; Ravi, C.; Rao, S.N.; Joshi, A.; Adimurthy, S. Visible-light-promoted selective C-H amination of heteroarenes with heteroaromatic amines under metal-free conditions. Org. Biomol. Chem. 2017, 15, 9590–9594. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Chatterjee, N.; Das, S.; Das Saha, K.; Bhaumik, A. Anthracene-bisphosphonate based novel fluorescent organic nanoparticles explored as apoptosis inducers of cancer cells. Chem. Commun. 2013, 49, 9461–9463. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiao, J.; Cao, Y.M.; Tang, J.M.; Wang, M.Q.; Ke, G.J.; Lu, Y.C.; Liu, X.; Lei, A.W. Exogenous-oxidant-free electrochemical oxidative C-H phosphonylation with hydrogen evolution. Chem. Commun. 2019, 55, 4230–4233. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sun, K.; Chen, X.L.; Shi, T.; Li, X.Y.; Qu, L.B.; Zhao, Y.F.; Yu, B. Visible-light-induced phosphorylation of imidazo-fused heterocycles under metal-free conditions. J. Org. Chem. 2020, 85, 14744–14752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ruiz-Castillo, P.; Buchwald, S.L. Palladium-catalyzed C-O cross-coupling of primary alcohols. Org. Lett. 2018, 20, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Kibriya, G.; Samanta, S.; Jana, S.; Mondal, S.; Hajra, A. Visible light organic photoredox-catalyzed C-H alkoxylation of imidazopyridine with alcohol. J. Org. Chem. 2017, 82, 13722–13727. [Google Scholar] [CrossRef] [PubMed]
- Kokorekin, V.A.; Terentev, A.O.; Ramenskaya, G.V.; Grammatikova, N.É.; Rodionova, G.M.; Ilovaiskii, A.I. Synthesis and antifungal activity of arylthiocyanates. Pharm. Chem. J. 2013, 47, 422–425. [Google Scholar] [CrossRef]
- Wu, H.; Yang, K.; Luo, S.; Wu, X.; Wang, N.; Chen, S.; Wang, Z.-Y. C4-Selective synthesis of vinyl thiocyanates and selenocyanates through 3,4-dihalo-2(5H)-furanones. Eur. J. Org. Chem. 2019, 2019, 4572–4580. [Google Scholar] [CrossRef]
- Mitra, S.; Ghosh, M.; Mishra, S.; Hajra, A. Metal-free thiocyanation of imidazoheterocycles through visible light photoredox catalysis. J. Org. Chem. 2015, 80, 8275–8281. [Google Scholar] [CrossRef]
- Saha, P.; Kumar, R.; Das, S.; Ansari, T.; Indra, A.; Sharma, D.K. Visible light induced regioselective C-3 thiocyanation of imidazoheterocycles through naphthalimide dye based photoredox catalysis. Org. Biomol. Chem. 2023, 21, 8471–8476. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Huang, M.M.; Li, Y.B.; Zhang, J.Y.; Zhu, Y.; Kim, J.K.; Wu, Y.J. An electrolyte- and catalyst-free electrooxidative sulfonylation of imidazo[1,2-a]pyridines. Org. Chem. Front. 2021, 8, 3110–3117. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Chen, Z.Y.; He, M.; Wang, D.X.; Li, L.S.; Qi, J.C.; Shi, R.Y.; Lei, A.W. Metal-free electrochemical C3-sulfonylation of imidazo[1,2-a]pyridines. Org. Chem. Front. 2021, 8, 3815–3819. [Google Scholar] [CrossRef]
- Breton-Patient, C.; Naud-Martin, D.; Mahuteau-Betzer, F.; Piguel, S. Three-component C-H bond sulfonylation of imidazoheterocycles by visible-light organophotoredox catalysis. Eur. J. Org. Chem. 2020, 2020, 6653–6660. [Google Scholar] [CrossRef]
- Guo, Y.J.; Lu, S.; Tian, L.L.; Huang, E.L.; Hao, X.Q.; Zhu, X.J.; Shao, T.; Song, M.P. Iodine-mediated difunctionalization of imidazopyridines with sodium sulfinates: Synthesis of sulfones and sulfides. J. Org. Chem. 2018, 83, 338–349. [Google Scholar] [CrossRef]
- Iida, H.; Demizu, R.; Ohkado, R. Tandem flavin-iodine-catalyzed aerobic oxidative sulfenylation of imidazo[1,2-a]pyridines with thiols. J. Org. Chem. 2018, 83, 12291–12296. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.F.; Yang, D.S.; Wei, W.; Jiang, M.; Wang, Z.L.; Zhang, L.Y.; Zhang, H.; Zhang, Z.Z.; Wang, Y.; Wang, H. Visible light-induced C-H sulfenylation using sulfinic acids. Green Chem. 2017, 19, 4785–4791. [Google Scholar] [CrossRef]
- Rahaman, R.; Das, S.; Barman, P. Visible-light-induced regioselective sulfenylation of imidazopyridines with thiols under transition metal-free conditions. Green Chem. 2018, 20, 141–147. [Google Scholar] [CrossRef]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardao, E.J.; Savegnago, L.; Davies, M.J. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free. Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Chakraborty, A.; Mondal, S.; Hajra, A. Catalyst-free selenylation of imidazoheterocycles. RSC Adv. 2015, 5, 77534–77537. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espindola, L.; Silva, D.O.; Braga, A.L. Rose Bengal catalysed photo-induced selenylation of indoles, imidazoles and arenes: A metal free approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, T.; Paul, S.; Das, S.; Pathak, D.D.; Bagdi, A.K. Visible-light-mediated regioselective C3-H selenylation of pyrazolo[1,5-a]pyrimidines using erythrosine b as photocatalyst. J. Org. Chem. 2023, 88, 8992–9003. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Fu, X.; Yang, K.; Liu, Z. Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines. Molecules 2025, 30, 607. https://doi.org/10.3390/molecules30030607
Gao J, Fu X, Yang K, Liu Z. Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines. Molecules. 2025; 30(3):607. https://doi.org/10.3390/molecules30030607
Chicago/Turabian StyleGao, Juanjuan, Xinlei Fu, Kai Yang, and Zhaowen Liu. 2025. "Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines" Molecules 30, no. 3: 607. https://doi.org/10.3390/molecules30030607
APA StyleGao, J., Fu, X., Yang, K., & Liu, Z. (2025). Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines. Molecules, 30(3), 607. https://doi.org/10.3390/molecules30030607




