New Structures, Spectrometric Quantification, and Inhibitory Properties of Cardenolides from Asclepias curassavica Seeds
Abstract
1. Introduction
2. Results and Discussion
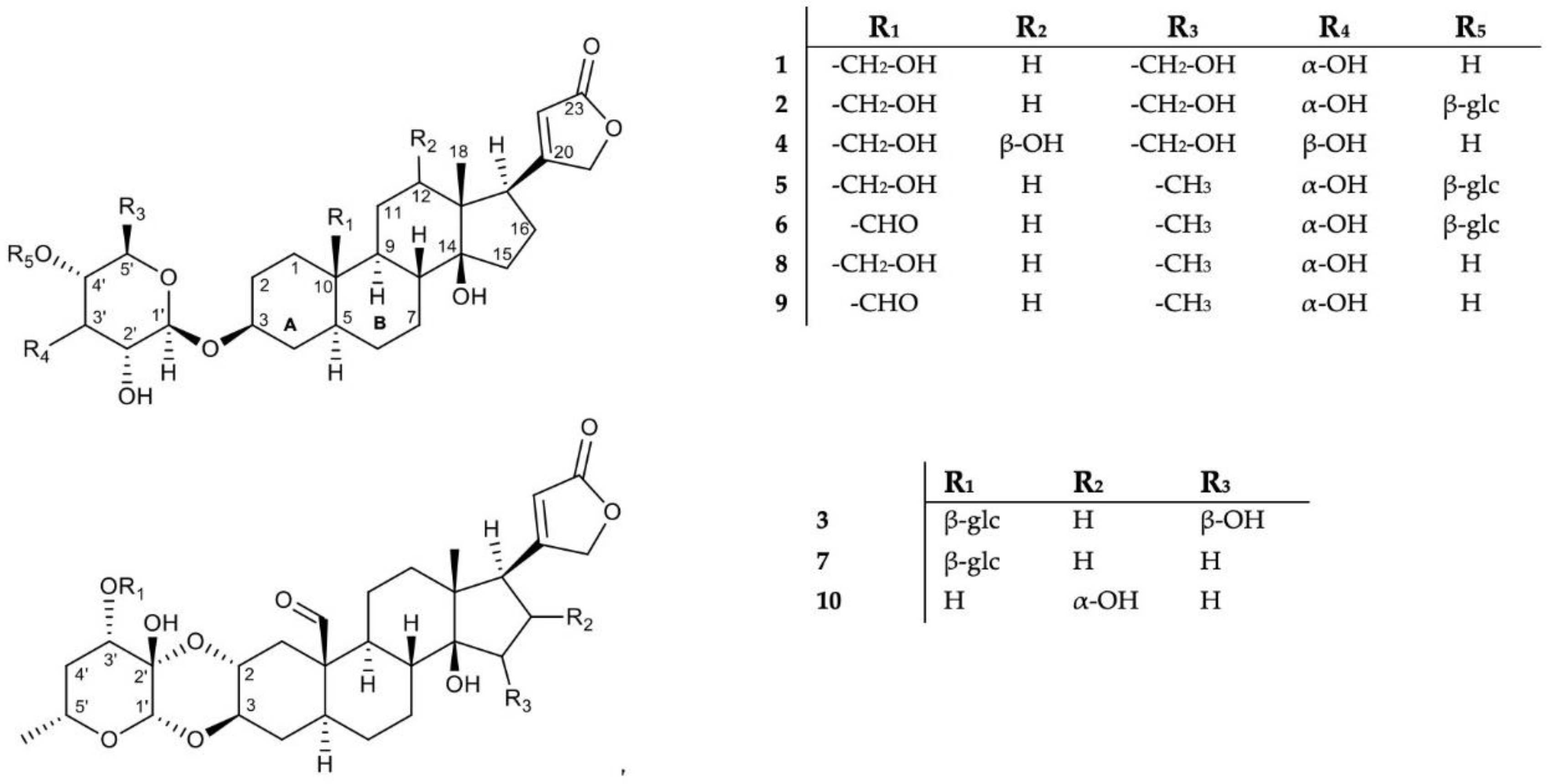
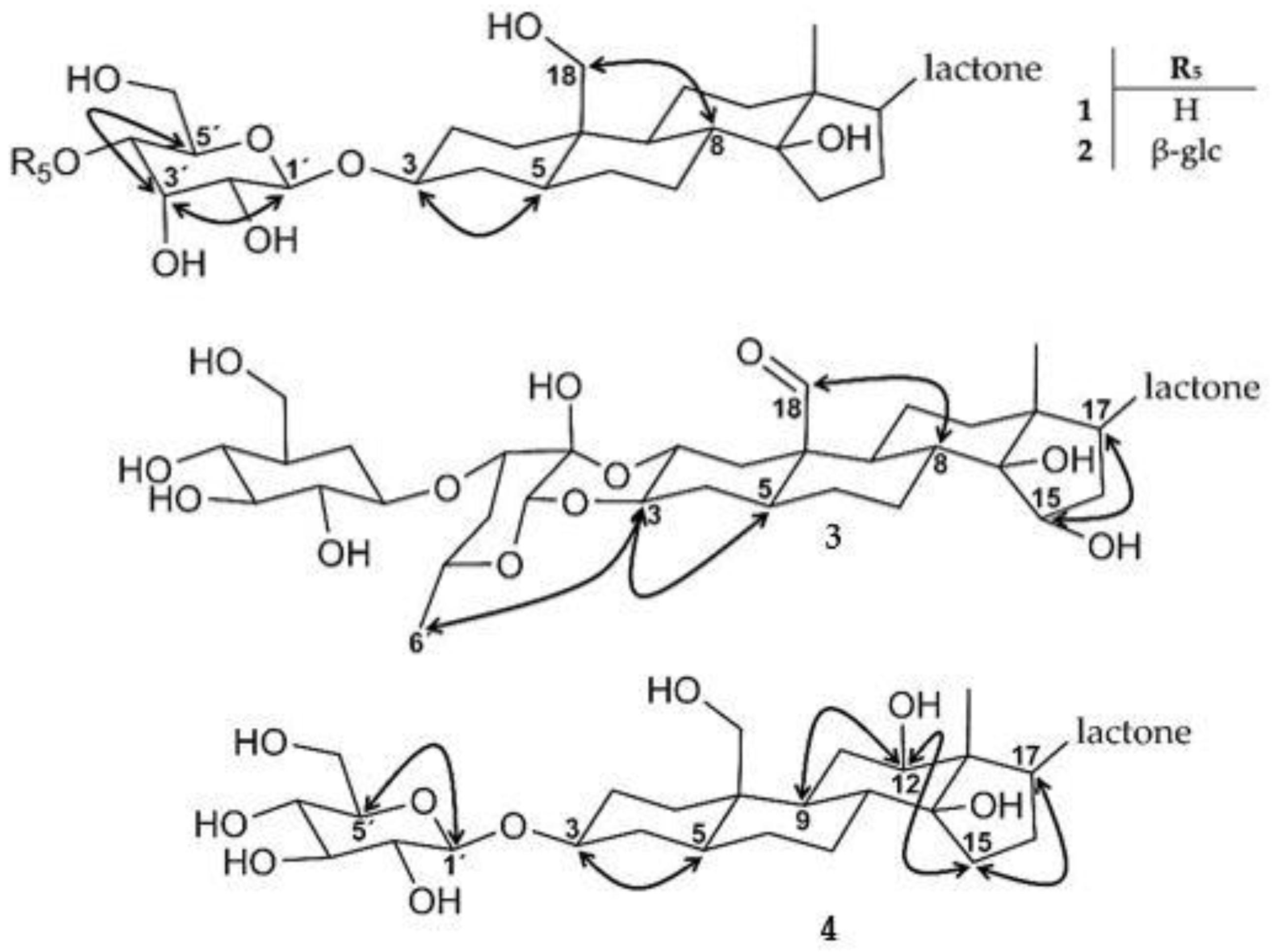
2.1. Isolation and Structure Elucidation
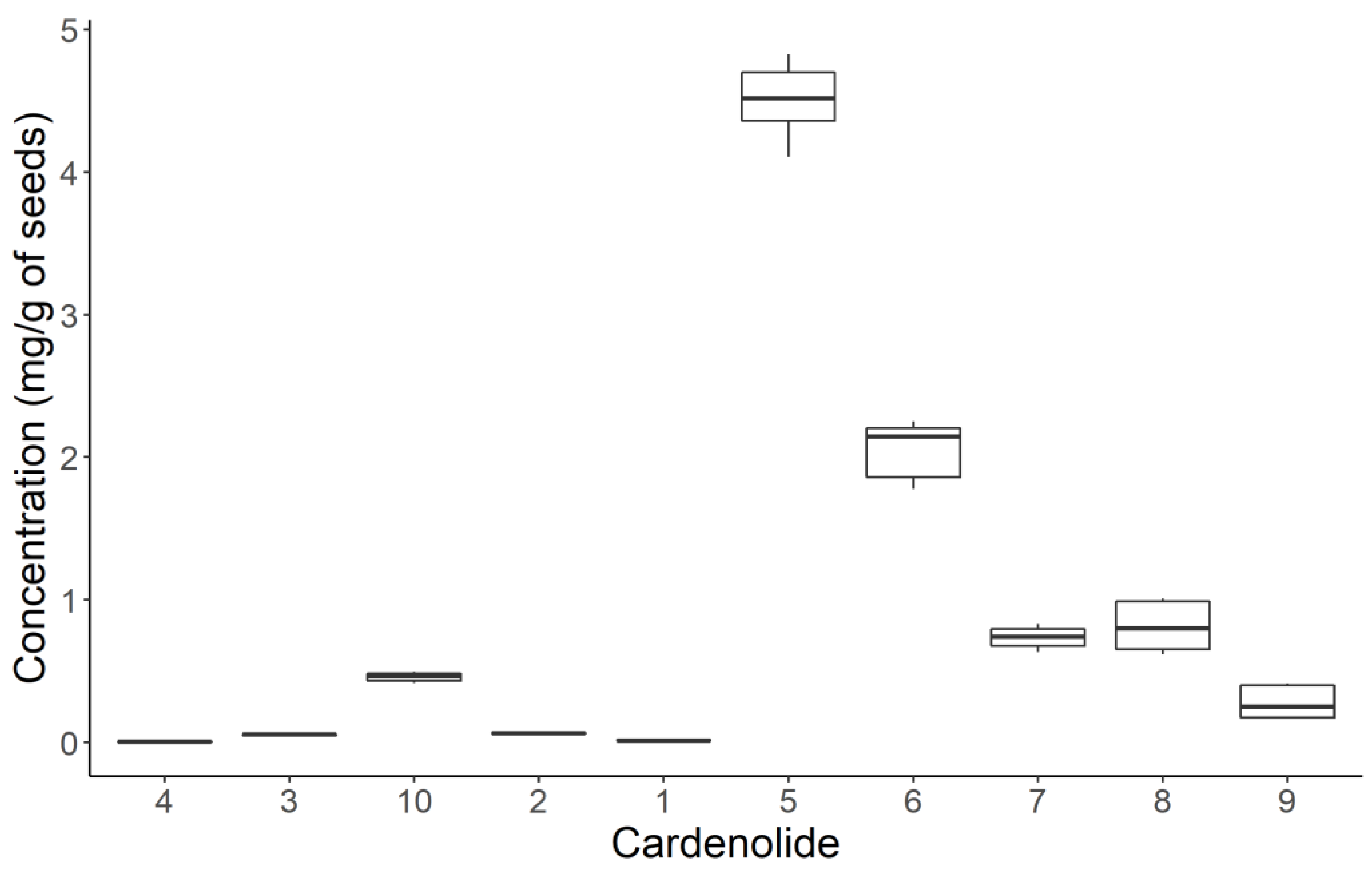
2.2. Quantification of Cardenolides
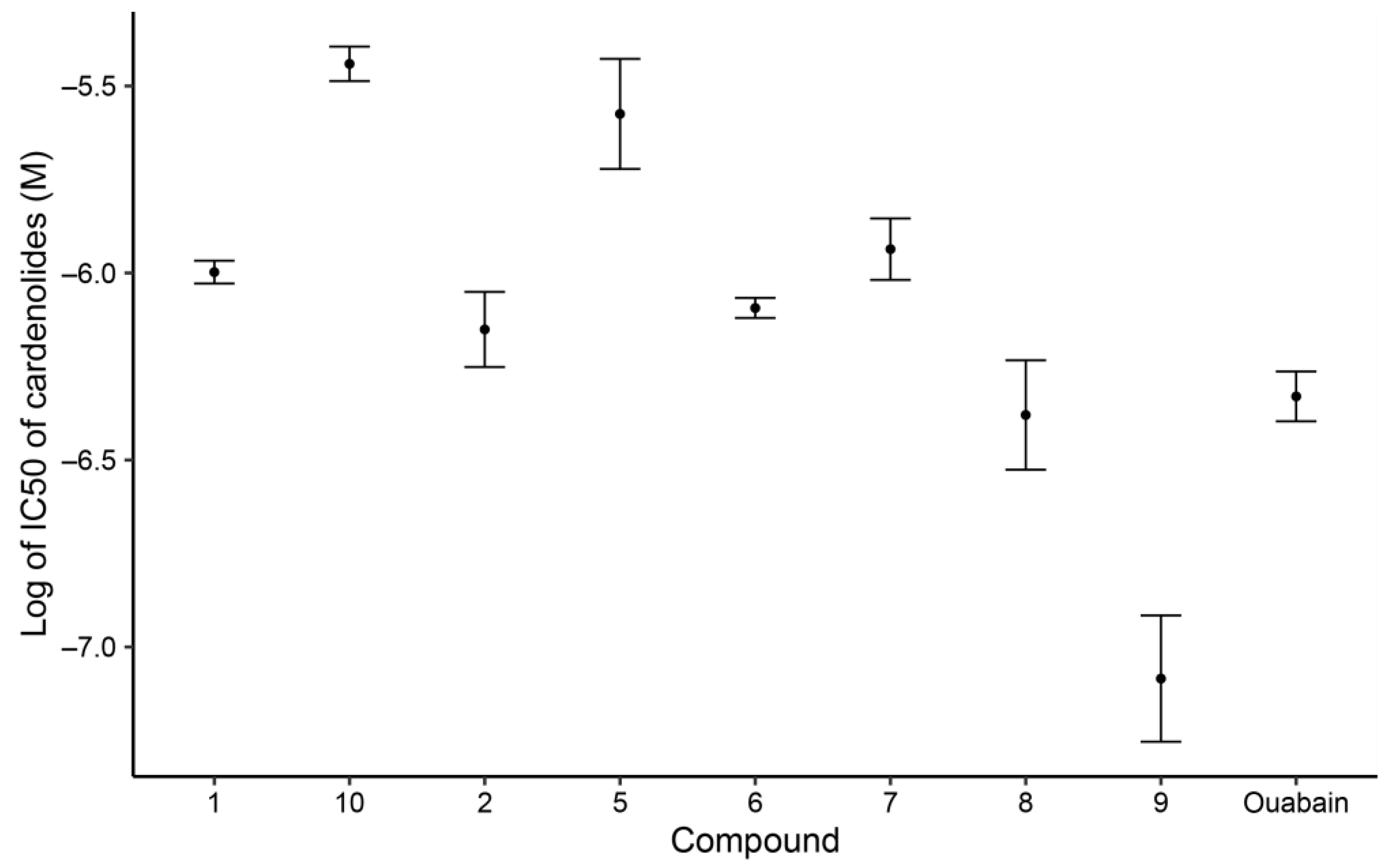
2.3. Na+/K+ ATPase (NKA) Inhibitory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Extraction and Isolation
3.3. Quantification of Cardenolides
3.4. Na+/K+ ATPase (NKA) Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Natural selection on common milkweed (Asclepias syriaca) by a community of specialized insect herbivores. Evol. Ecol. Res. 2005, 7, 651–667. [Google Scholar]
- Mauricio, R.; Rausher, M.D. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 1997, 51, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Schneider, G.F.; Dybzinski, R.; Nelson, A.S.; Gelambi, M.; Jos, E.; Beckman, N.G. Fruits, frugivores, and the evolution of phytochemical diversity. Oikos 2022, 2. [Google Scholar] [CrossRef]
- Seiber, J.N.; Lee, S.M.; Benson, J.M. Cardiac glycosides (cardenolides) in species of Asclepias (Asclepiadaceae). In Handbook of Natural Toxins; Richard, F.K., Anthony, T.T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1983; Volume 1, pp. 43–83. [Google Scholar]
- Agrawal, A.A.; Petschenka, G.; Bingham, R.A.; Weber, M.G.; Rasmann, S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 2012, 194, 28–45. [Google Scholar] [CrossRef]
- Brower, L.P.; van Brower, J.; Corvino, J.M. Plant poisons in a terrestrial food chain. Proc. Natl. Acad. Sci. USA 1967, 57, 893–898. [Google Scholar] [CrossRef]
- Rasmann, S.; Agrawal, A.A. Latitudinal patterns in plant defense: Evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 2011, 14, 476–483. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Fishbein, M. Phylogenetic escalation and decline of plant defense strategies. Proc. Natl. Acad. Sci. USA 2008, 105, 10057–10060. [Google Scholar] [CrossRef]
- López-Goldar, X.; Hastings, A.; Züst, T.; Agrawal, A. Evidence for tissue-specific defence-offence interactions between milkweed and its community of specialized herbivores. Mol. Ecol. 2022, 31, 3254–3265. [Google Scholar] [CrossRef]
- Nelson, C.J.; Seiber, J.N.; Brower, L.P. Seasonal and intraplant variation of cardenolide content in the California milkweed, Asclepias eriocarpa, and implications for plant defense. J. Chem. Ecol. 1981, 7, 981–1010. [Google Scholar] [CrossRef]
- Aperia, A. New roles for an old enzyme: Na+, K+-ATPase emerges as an interesting drug target. J. Intern. Med. 2007, 261, 44–52. [Google Scholar] [CrossRef]
- Köksoy, A.A. Na+/ K+-ATPase: A review. J. Ank. Med. Sch. 2002, 24, 73–82. [Google Scholar] [CrossRef]
- Petschenka, G.; Dobler, S. Target-site sensitivity in a specialized herbivore towards major toxic compounds of its host plant: The Na+/K+-ATPase of the oleander hawk moth (Daphnis nerii) is highly susceptible to cardenolides. Chemoecology 2009, 19, 235–239. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. Ren. Physiol. 1998, 275, 633–650. [Google Scholar] [CrossRef]
- Mobasheri, A.; Avila, J.; Cózar-Castellano, I.; Brownleader, M.D.; Trevan, M.; Francis, M.J.O.; Lamb, J.F.; Martín-Vasallo, P. Na+/K+-ATPase Isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 2000, 20, 51–91. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Böröczky, K.; Haribal, M.; Hastings, A.P.; White, R.A.; Jiang, R.W.; Duplais, C. Cardenolides, toxicity, and the costs of sequestration in the coevolutionary interaction between monarchs and milkweeds. Proc. Natl. Acad. Sci. USA 2021, 118, 2024463118. [Google Scholar] [CrossRef]
- Malcolm, S.B.; Cockrell, B.J.; Brower, L.P. Cardenolide fingerprint of monarch butterflies reared on common milkweed, Asclepias syriaca L. J. Chem. Ecol. 1989, 15, 819–853. [Google Scholar] [CrossRef]
- Jones, P.L.; Petschenka, G.; Flacht, L.; Agrawal, A.A. Cardenolide intake, sequestration, and excretion by the Monarch Butterfly along gradients of plant toxicity and larval ontogeny. J. Chem. Ecol. 2019, 45, 264–277. [Google Scholar] [CrossRef]
- Stenoien, C.M.; Meyer, R.A.; Nail, K.R.; Zalucki, M.P.; Oberhauser, K.S. Does chemistry make a difference? Milkweed butterfly sequestered cardenolides as a defense against parasitoid wasps. Arthropod. Plant. Interact. 2019, 13, 835–852. [Google Scholar] [CrossRef]
- Cheung, H.T.A.; Nelson, C.J.; Watson, T.R. New glucoside conjugates and other cardenolide glycosides from the monarch butterfly reared on Asclepias fruticosa L. J. Chem. Soc. Perkin Trans. 1 1988, 7, 1851–1857. [Google Scholar] [CrossRef]
- Burdfield-Steel, E.R.; Shuker, D.M. The evolutionary ecology of the Lygaeidae. Ecol. Evol. 2014, 4, 2278–2301. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, F.A. Effect of gross cardiac glycoside content of seeds of common milkweed, Asclepias syriaca, on cardiac glycoside uptake by the milkweed bug Oncopeltus fasciatus. J. Chem. Ecol. 1979, 5, 89–100. [Google Scholar] [CrossRef]
- Moore, L.V.; Scudder, G.G.E. Selective sequestration of milkweed (Asclepias sp.) cardenolides in Oncopeltus fasciatus (Dallas) (Hemiptera: Lygaeidae). J. Chem. Ecol. 1985, 11, 667–687. [Google Scholar] [CrossRef]
- Isman, M.B.; Duffey, S.S.; Scudder, G.G.E. Variation in cardenolide content of the lygaeid bugs, Oncopeltus fasciatus and Lygaeus kalmii kalmii and of their milkweed hosts (Asclepias spp.) in central California. J. Chem. Ecol. 1977, 3, 613–624. [Google Scholar] [CrossRef]
- Holzinger, F.; Wink, M. Mediation of cardiac glycoside insensitivity in the monarch butterfly (Danaus plexippus): Role of an amino acid substitution in the ouabain binding site of Na+/K+-ATPase. J. Chem. Ecol. 1996, 22, 1921–1937. [Google Scholar] [CrossRef]
- Holzinger, F.; Frick, C.; Wink, M. Molecular basis for the insensitivity of the Monarch (Danaus plexippus) to cardiac glycosides. FEBS Lett. 1992, 314, 477–480. [Google Scholar] [CrossRef]
- Taverner, A.M.; Yang, L.; Barile, Z.J.; Lin, B.; Peng, J.; Pinharanda, A.P.; Rao, A.S.; Roland, B.P.; Talsma, A.D.; Wei, D.; et al. Adaptive substitutions underlying cardiac glycoside insensitivity in insects exhibit epistasis in vivo. Elife 2019, 8, e48224. [Google Scholar] [CrossRef]
- Petschenka, G.; Agrawal, A.A. Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151865. [Google Scholar] [CrossRef]
- Petschenka, G.; Fandrich, S.; Sander, N.; Wagschal, V.; Boppré, M.; Dobler, S. Stepwise evolution of resistance to toxic cardenolides via genetic substitutions in the Na+/K+ATP ase of milkweed butterflies (lepidoptera: Danaini). Evolution 2013, 67, 2753–2761. [Google Scholar] [CrossRef]
- Petschenka, G.; Halitschke, R.; Züst, T.; Roth, A.; Stiehler, S.; Tenbusch, L.; Hartwig, C.; Moreno Gámez, J.F.; Trusch, R.; Deckert, J.; et al. Sequestration of defenses against predators drives specialized host plant associations in preadapted milkweed bugs (Heteroptera: Lygaeinae). Am. Nat. 2022, 199, E211–E228. [Google Scholar] [CrossRef]
- Woods, E.C.; Hastings, A.P.; Turley, N.E.; Heard, S.B.; Agrawal, A.A. Adaptive geographical clines in the growth and defense of a native plant. Ecol. Monogr. 2012, 82, 149–168. [Google Scholar] [CrossRef]
- Malcolm, S.B. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 1994, 5, 101–117. [Google Scholar] [CrossRef]
- Scudder, G.G.E.; Meredith, J. The permeability of the midgut of three insects to cardiac glycosides. J. Insect Physiol. 1982, 28, 689–694. [Google Scholar] [CrossRef]
- Malcolm, S.B. Cardenolide-mediated interactions between plants and herbivores. In Herbivores: Their Interactions with Secondary Plant Metabolites; Gerald, A.R., May, R.B., Eds.; Academic Press Inc.: London, UK, 1991; Volume 1, pp. 251–296. [Google Scholar]
- Krishna, A.B. Plant Cardenolides in Therapeutics. Int. J. Indig. Med. Plants 2015, 48, 1871–1896. [Google Scholar]
- Petschenka, G.; Fei, C.S.; Araya, J.J.; Schröder, S.; Timmermann, B.N.; Agrawal, A.A. Relative selectivity of plant cardenolides for Na+/K+-ATPases from the Monarch Butterfly and non-resistant insects. Front. Plant Sci. 2018, 9, 1424. [Google Scholar] [CrossRef]
- Zhang, R.R.; Tian, H.Y.; Tan, Y.F.; Chung, T.Y.; Sun, X.H.; Xia, X.; Ye, W.C.; Middleton, D.A.; Fedosova, N.; Esmann, M.; et al. Structures, chemotaxonomic significance, cytotoxic and Na+/K+-ATPase inhibitory activities of new cardenolides from Asclepias curassavica. Org. Biomol. Chem. 2014, 12, 8919–8929. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Espinosa del Alba, L.; López-Goldar, X.; Hastings, A.P.; White, R.A.; Halitschke, R.; Dobler, S.; Petschenka, G.; Duplais, C. Functional evidence supports adaptive plant chemical defense along a geographical cline. Proc. Natl. Acad. Sci. USA 2022, 119, e2205073119. [Google Scholar] [CrossRef]
- Warashina, T.; Noro, T. Steroidal Glycosides from the Roots of Asclepias curassavica. Chem. Pharm. Bull. 2008, 56, 315–322. [Google Scholar] [CrossRef][Green Version]
- Li, J.-Z.; Liu, H.-Y.; Lin, Y.-J.; Hao, X.-J.; Ni, W.; Chen, C.-X. Six new C21 steroidal glycosides from Asclepias curassavica L. Steroids 2008, 73, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Z.; Qing, C.; Chen, C.-X.; Hao, X.-J.; Liu, H.-Y. Cytotoxicity of cardenolides and cardenolide glycosides from Asclepias curassavica. Bioorg. Med. Chem. Lett. 2009, 19, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Mori, Y.; Yamauchi, T. Cardenolide glycosides from the seeds of Asclepias curassavica. Chem. Pharm. Bull. 1992, 40, 2917–2920. [Google Scholar] [CrossRef][Green Version]
- Ji, A.-J.; Ma, Q.; Kong, M.-Y.; Li, L.-Y.; Chen, X.-L.; Liu, Z.-Q.; Wu, J.-J.; Zhang, R.R. Two cardenolide glycosides from the seed fairs of Asclepias curassavica and their cytotoxic activities. Chin. J. Nat. Med. 2022, 20, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Gopal Ravi, B.; Grace Guardian, M.E.; Dickman, R.; Wang, Z.Q. Profiling and structural analysis of cardenolides in two species of Digitalis using liquid chromatography coupled with high-resolution mass spectrometry. J. Chromatogr. A 2020, 1618, 460903. [Google Scholar] [CrossRef]
- Münger, L.H.; Boulos, S.; Nyström, L. UPLC-MS/MS based identification of dietary steryl glucosides by investigation of corresponding free sterols. Front. Chem. 2018, 6, 342. [Google Scholar] [CrossRef]
- Kiuchi, F.; Fukao, Y.; Maruyama, T.; Obata, T.; Tanaka, M.; Sasaki, T.; Mirage, M.; Haque, M.E.; Tsuda, Y. Cytotoxic principles of a Bangladeshi crude drug, akond mul (roots of Calotropis gigantea L.). Chem. Pharm. Bull. 1998, 46, 528–530. [Google Scholar] [CrossRef]
- Hunger, A.; Reichstein, T. Die Konstitution von Gofrusid und Frugosid. Glykoside und Aglykone 98. Mitteilung. Helv. Chim. Acta 1952, 35, 1073–1103. [Google Scholar] [CrossRef]
- Keller, M.; Reichstein, T. Gofrusid, ein Krystallisiertes Glykosid aus den Samen von Gomphocarpus fructicosus (L.) R. Br. Helv. Chim. Acta 1949, 32, 1607–1612. [Google Scholar] [CrossRef]
- Hernández-Quiroz, T.; Soriano-García, M.; Rodríguez-Romero, A.; Valencia, C.; Hernandez, L.; Aguirre-García, F. [2α(2S,3S,4R,6R),3β,5α]-14-Hydroxy-19-oxo-3,2-[(tetrahydro-3,4-dihydroxy-6-methyl-2H-pyran-2,3-diyl)bis(oxy)]card-20(22)-enolide dihydrate (calactin), C29H39O9.2H2O, a cardenolide from Asclepias linaria. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 935–938. [Google Scholar] [CrossRef]
- Nishio, S.; Blum, M.S.; Silverton, J.V.; Highet, R.J. Structure of humistratin: A novel cardenolide from the sandhill milkweed Asclepias humistrata. J. Org. Chem. 1982, 47, 2154–2157. [Google Scholar] [CrossRef]
- Pederson, P.J.; Cai, S.; Carver, C.; Powell, D.R.; Risinger, A.L.; Grkovic, T.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H. Triple-Negative breast cancer cells exhibit differential sensitivity to cardenolides from Calotropis gigantea. J. Nat. Prod. 2020, 83, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.E.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from chemical structure and pharmacological utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Naha, N.; Lee, H.Y.; Jo, M.J.; Chung, B.C.; Kim, S.H.; Kim, M.O. Rare sugar D-allose induces programmed cell death in hormone refractory prostate cancer cells. Apoptosis 2008, 13, 1121–1134. [Google Scholar] [CrossRef]
- Meneses-Sagrero, S.E.; Rascón-Valenzuela, L.A.; García-Ramos, J.C.; Vilegas, W.; Arvizu-Flores, A.A.; Sotelo-Mundo, R.R.; Robles-Zepeda, R.E. Calotropin and corotoxigenin 3-O-glucopyranoside from the desert milkweed Asclepias subulata inhibit the Na+/K+-ATPase activity. PeerJ 2022, 10, e13524. [Google Scholar] [CrossRef]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical diversity drives plant-insect community diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef]
- Salazar, D.; Lokvam, J.; Mesones, I.; Pilco, M.V.; Milagros, J.; Zuñiga, A.; De Valpine, P.; Fine, P.V.A. Origin and maintenance of chemical diversity in a species-rich tropical tree lineage. Nat. Ecol. Evol. 2018, 2, 983–990. [Google Scholar] [CrossRef]
- Volf, M.; Segar, S.T.; Miller, S.E.; Isua, B.; Sisol, M.; Aubona, G.; Šimek, P.; Moos, M.; Laitila, J.; Kim, J.; et al. Community structure of insect herbivores is driven by conservatism, escalation and divergence of defensive traits in Ficus. Ecol. Lett. 2018, 21, 83–92. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 1 October 2022).
- Petschenka, G.; Offe, J.K.; Dobler, S. Physiological screening for target site insensitivity and localization of Na+/K+-ATPase in cardenolide-adapted Lepidoptera. J. Insect Physiol. 2012, 58, 607–612. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 2.15 β brd (13.4) 0.81 α dd (13.4, 11.1) | 30.7 CH2 | 2.23 br, 0.89 br | 30.6 CH2 | 2.45 β dd (12.6, 4.4) 1.19 α brt (12.6) | 34.4 CH2 | 2.15 α dt (13.5, 3.1) 0.83 α brt (13.4) | 30.5 CH2 |
| 2 | 1.85 brd, (11.1), 1.35 br | 29.1 CH2 | 1.92 br, 1.43 br | 29.2 CH2 | 3.91 β br | 69.1 CH | 1.86 br, 1.37 br | 28.9 CH2 |
| 3 | 3.77 m | 79.1 CH | 3.85 m | 79.1 CH | 4.02 α td (10.6, 4.0) | 72.1 CH | 3.78 α m | 79.0 CH |
| 4 | 1.72 brd (12.2), 1.31 br | 34.0 CH2 | 1.81 br, 1.41 br | 34.0 CH2 | 1.76 α br, 1.31 β br | 32.6 CH2 | 1.72 α br, 1.31 β q (12.2) | 33.8 CH2 |
| 5 | 1.19 brt (11.5) | 43.7 CH | 1.28 br | 43.8 CH | 1.70 α br | 42.2 CH | 1.20 α br | 43.8 CH |
| 6 | 1.26 m, 1.26 m | 27.3 CH2 | 1.34 m, 1.34 m | 27.4 CH2 | 2.16 m, 1.57 m | 26.1 CH2 | 1.25 m, 1.25 m | 27.3 CH2 |
| 7 | 1.88 m, 1.07 m | 26.9 CH2 | 1.96 m, 1.16 m | 26.9 CH2 | 1.90 β m, 1.74 α m | 26.9 CH2 | 1.86 m, 1.06 m | 27.1 CH2 |
| 8 | 1.63 br | 41.2 CH | 1.71 br | 41.3 CH | 1.72 β br | 41.8 CH | 1.63 β br | 40.6 CH |
| 9 | 1.00 br | 49.1 CH | 1.08 br | 48.9 CH | 1.64 α br | 46.9 CH | 1.02 α brt (13.8) | 45.3 CH |
| 10 | - | 38.7 C | - | 38.7 C | - | 53.3 C | - | 38.6 C |
| 11 | 1.55 m, 1.33 m | 22.5 CH2 | 1.64 m, 1.42 m | 22.5 CH2 | 1.73 α m, 1.12 β m | 21.5 CH2 | 1.76 α m, 1.46 β q (12.5) | 30.6 CH2 |
| 12 | 1.44 m, 1.33 m | 39.7 CH2 | 1.53 m, 1.42 m | 39.7 CH2 | 1.53 m, 1.46 m | 37.2 CH2 | 3.32 α dd (12.2, 1.7) | 74.7 CH |
| 13 | - | 49.8 C | - | 49.5 C | - | 48.3 C | - | 55.8 C |
| 14 | - | 86.3 C | - | 85.7 C | - | 82.1 C | - | 86.4 C |
| 15 | 2.09 m, 1.63 m | 31.7 CH2 | 2.17 m, 1.72 m | 31.8 CH2 | 4.66 α brd (8.4) | 71.8 CH | 1.88 α m, 1.67 β m | 31.7 CH2 |
| 16 | 2.09 m, 1.73 m | 26.5 CH2 | 2.19 m, 1.82 m | 26.6 CH2 | 2.68 m, 1.66 m | 36.0 CH2 | 2.10 m, 1.79 m | 26.8 CH2 |
| 17 | 2.81 α br | 50.2 CH | 2.89 α br | 50.2 CH | 2.77 α dd (9.8, 4.9) | 47.3 CH | 3.20 br | 45.4 CH |
| 18 | 0.82 β s | 15.2 CH3 | 0.91 β s | 15.3 CH3 | 0.85 β s | 16.1 CH3 | 0.73 β s | 8.9 CH3 |
| 19 | 3.82, br 3.67 br | 59.0 CH2 | 3.90 br, 3.76 br | 59.0 CH2 | 10.10 s | 213.1 CH | 3.81 d (12.2) 3.68 d (12.2) | 58.6 CH2 |
| 20 | - | 178.5 C | - | 178.2 C | - | 177.6 C | - | 178.4 C |
| 21 | 4.99 d (18.8) 4.93 d (18.8) | 75.2 CH2 | 5.06 d (18.7) 5.00 d (18.7) | 75.1 CH2 | 5.09 d (18.3) 5.02 d (18.3) | 75.0 CH2 | 4.95 br, 4.95 br | 75.1 CH2 |
| 22 | 5.89 s | 115.9 CH | 5.98 s | 115.7 CH | 6.02 s | 116.2 CH | 5.92 s | 116.2 CH |
| 23 | - | 179.3 C | - | 178.9 C | - | 178.2 C | - | 178.5 C |
| 1′ | 4.77 d (8.3) | 98.0 CH | 4.87 d (8.2) | 97.8 CH | 4.63 s | 94.8 CH | 4.51 α d (7.8) | 100.3 CH |
| 2′ | 3.32 dd (8.3, 3) | 70.2 CH | 3.43 dd (8.7, 2.7) | 70.0 CH | - | 91.6 C | 3.14 β brt (8.7) | 73.1 CH |
| 3′ | 4.09 t (3) | 71.3 CH | 4.45 t (3.1) | 70.9 CH | 3.95 br | 81.5 CH | 3.39 α t (9.3) | 75.8 CH |
| 4′ | 3.53 dd (10, 3) | 66.9 CH | 3.78 dd (10, 2.7) | 76.2 CH | 2.13 β br, 1.71 α br | 37.1 CH2 | 3.29 β t (9.3) | 69.6 CH |
| 5′ | 3.68 brddd (10.6, 1.5) | 73.6 CH | 3.88 brd (10.3, 2.2) | 72.4 CH | 3.82 β q (5.8) | 68.7 CH | 3.36 α ddd (9.3, 5.8, 1.7) | 75.9 CH |
| 6′ | 3.82 dd (12.0, 1.5) 3.61 dd, (12.0, 6.0) | 61.2 CH2 | 3.90 brd (12.1) 3.76 dd (12.5, 4.2) | 60.6 CH2 | 1.29 α d (6.3) | 19.8 CH3 | 3.82 dd (1.7, 12.2) 3.63 dd (5.8, 12.2) | 60.7 CH2 |
| 1” | 4.56 d (7.9) | 103.6 CH | 4.64 d (7.7) | 104 CH | ||||
| 2” | 3.32 brt (8.9) | 73.2 CH | 3.37 t (9.1) | 73.4 CH | ||||
| 3” | 3.48 t (9.2) | 75.7 CH | 3.51 t (9.1) | 75.5 CH | ||||
| 4” | 3.42 br | 69.3 CH | 3.43 br | 69.4 CH | ||||
| 5” | 3.44 br | 75.7 CH | 3.44 br | 75.8 CH | ||||
| 6” | 3.90,br, 3.76 br | 60.6 CH2 | 3.90 dd (12.2, 1.5) 3.74 dd (12.2, 5.7) | 60.2 CH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiano-Buitrago, P.; Pradhan, S.; Paetz, C.; Rowland, H.M. New Structures, Spectrometric Quantification, and Inhibitory Properties of Cardenolides from Asclepias curassavica Seeds. Molecules 2023, 28, 105. https://doi.org/10.3390/molecules28010105
Rubiano-Buitrago P, Pradhan S, Paetz C, Rowland HM. New Structures, Spectrometric Quantification, and Inhibitory Properties of Cardenolides from Asclepias curassavica Seeds. Molecules. 2023; 28(1):105. https://doi.org/10.3390/molecules28010105
Chicago/Turabian StyleRubiano-Buitrago, Paola, Shrikant Pradhan, Christian Paetz, and Hannah M. Rowland. 2023. "New Structures, Spectrometric Quantification, and Inhibitory Properties of Cardenolides from Asclepias curassavica Seeds" Molecules 28, no. 1: 105. https://doi.org/10.3390/molecules28010105
APA StyleRubiano-Buitrago, P., Pradhan, S., Paetz, C., & Rowland, H. M. (2023). New Structures, Spectrometric Quantification, and Inhibitory Properties of Cardenolides from Asclepias curassavica Seeds. Molecules, 28(1), 105. https://doi.org/10.3390/molecules28010105






