Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications
Abstract
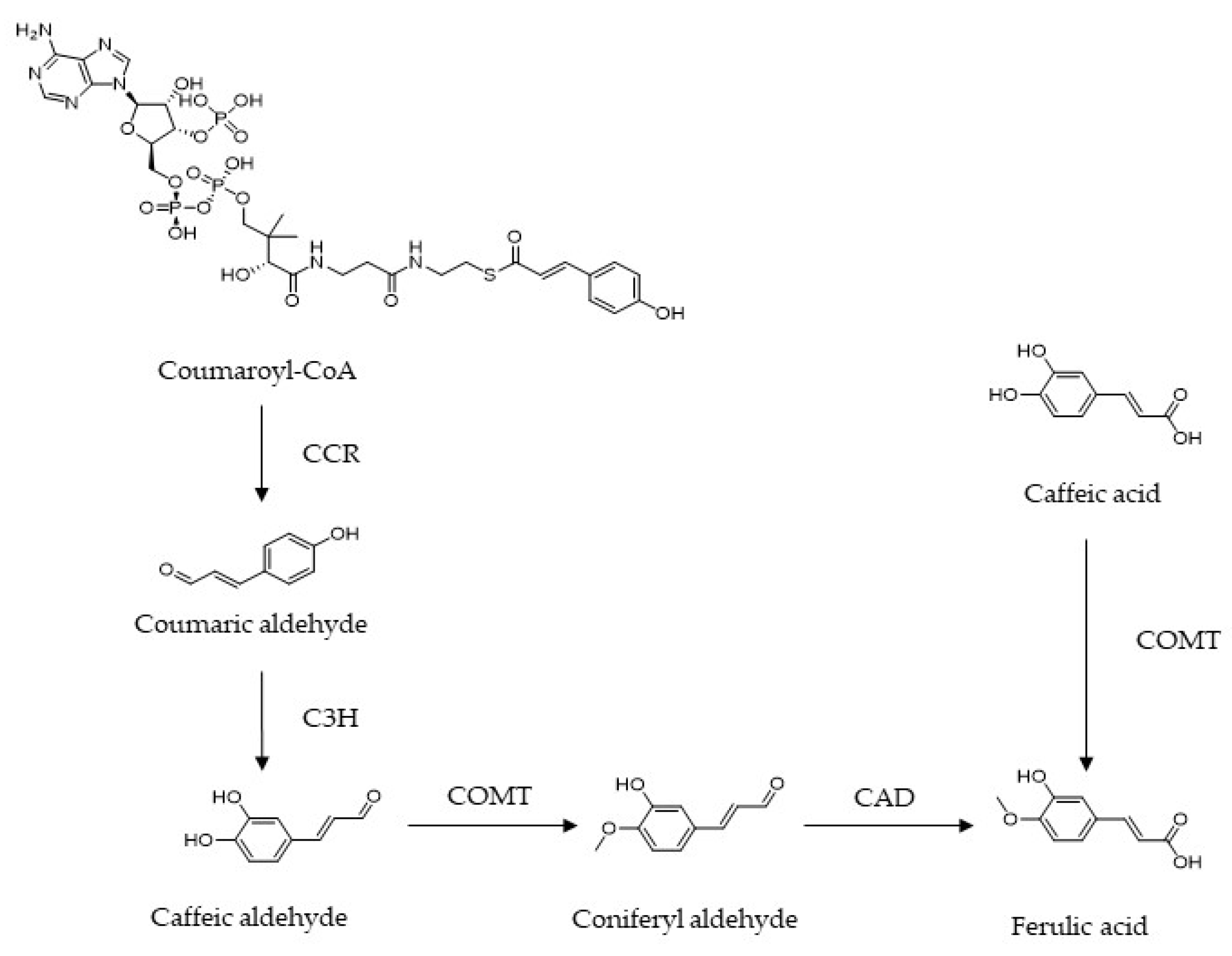
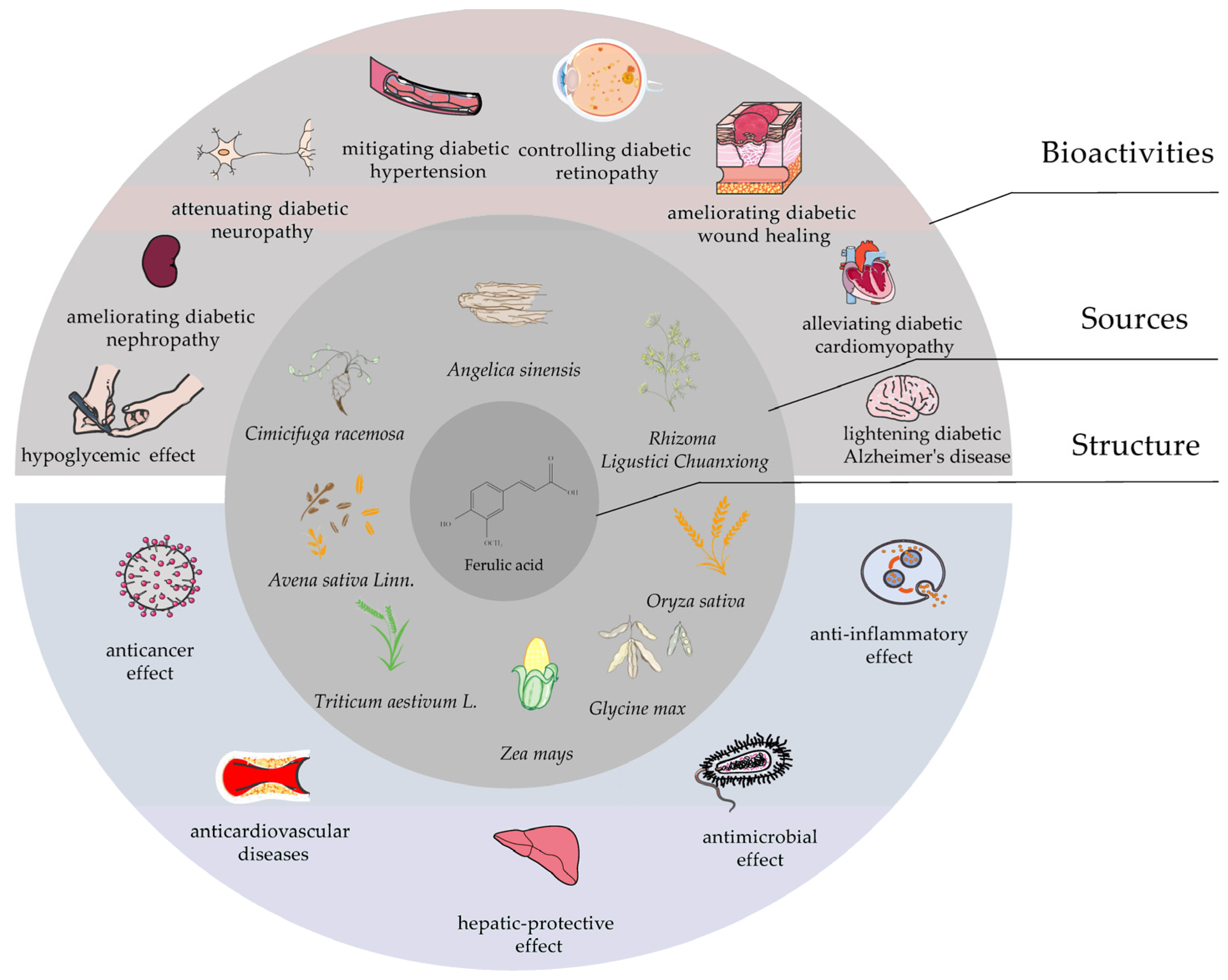
:1. Introduction
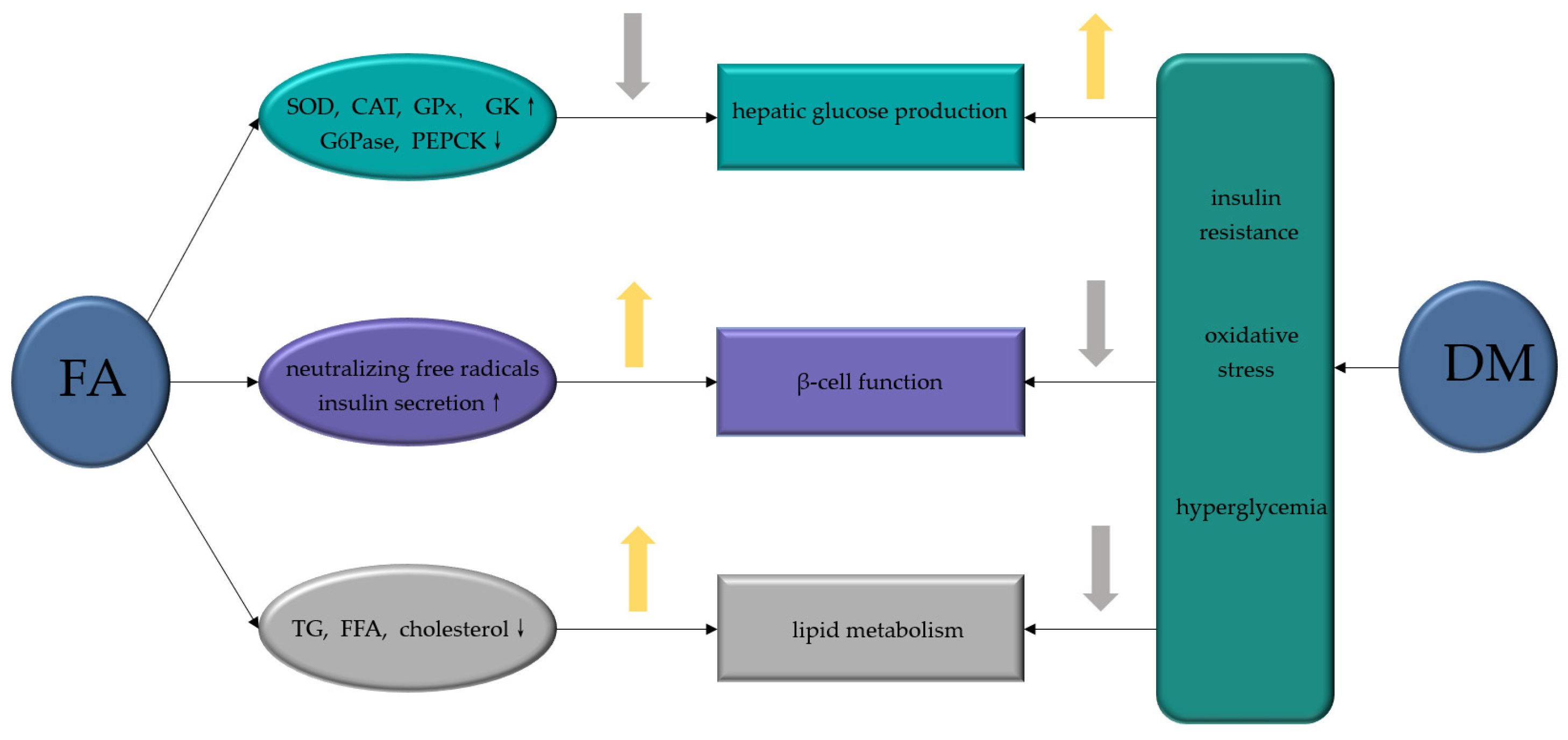
2. Use of FA to Prevent and Treat DM
2.1. The Effects of FA on Hepatic Glucose Production
2.2. The Effects of FA on β-Cell Function
2.3. The Effects of FA on Lipid Metabolism
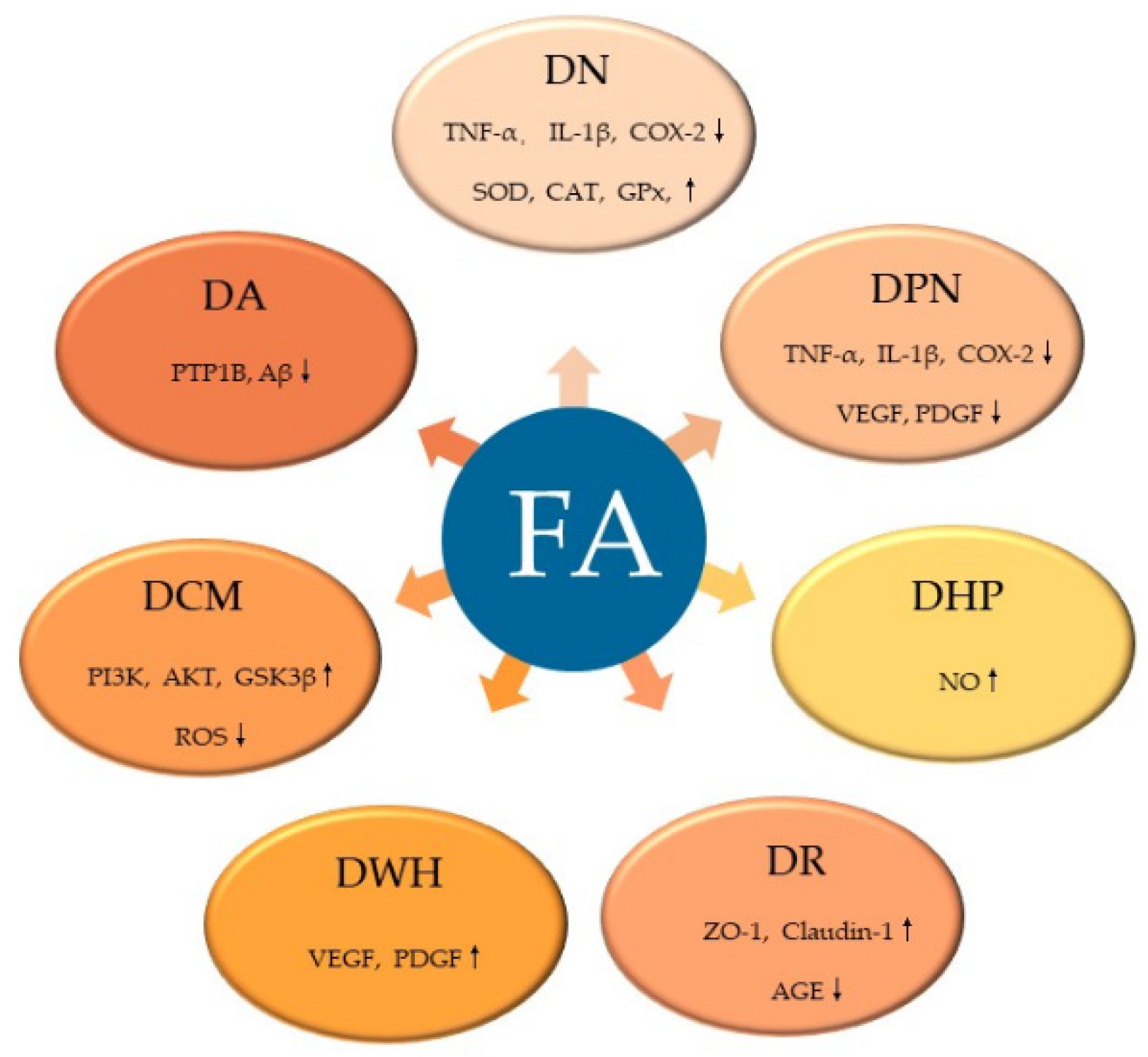
3. Use of FA to Prevent and Treat DM Complications
3.1. The Effects of FA on Diabetic Nephropathy
3.2. The Effects of FA on Diabetic Neuropathy
3.3. The Effects of FA on Diabetic Hypertension
3.4. The Effects of FA on Diabetic Retinopathy
3.5. The Effects of FA on Wound Healing with DM
3.6. The Effects of FA on Diabetic Cardiomyopathy
3.7. The Effects of FA on Diabetic Alzheimer′s Disease
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Amutha, A.; Datta, M.; Unnikrishnan, I.R.; Anjana, R.M.; Rema, M.; Narayan, K.M.V.; Mohan, V.J.P. Clinical profile of diabetes in the young seen between 1992 and 2009 at a specialist diabetes centre in south India. Prim. Care Diabetes 2011, 5, 223–229. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L.J.B. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Paiva, L.B.; Goldbeck, R.; Santos, W.D.; Squina, F.M. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz. J. Pharm. Sci. 2013, 49, 395–411. [Google Scholar] [CrossRef]
- Franke, R.; Humphreys, J.; Hemm, M.; Denault, J.; Ruegger, M.; Cusumano, J.; Chapple, C. The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J. 2002, 30, 33–45. [Google Scholar] [CrossRef]
- Eckardt, N.A. Probing the mysteries of lignin biosynthesis: The crystal structure of caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase provides new insights. Plant Cell 2002, 14, 1185–1189. [Google Scholar] [CrossRef]
- Lam, T.B.T.; Iiyama, K.; Stone, B.A.J.P. Caffeic acid: O-methyltransferases and the biosynthesis of ferulic acid in primary cell walls of wheat seedlings. Phytochemistry 1996, 41, 1507–1510. [Google Scholar]
- Neto-Neves, E.M.; da Silva Maia Bezerra Filho, C.; Dejani, N.N.; de Sousa, D.P. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. Mini Rev. Med. Chem. 2021, 21, 1625–1637. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Zhang, N.; Ji, Z.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Ferulic acid attenuates diabetes-induced cognitive impairment in rats via regulation of PTP1B and insulin signaling pathway. Physiol. Behav. 2017, 182, 93–100. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R.J.F.; Toxicology, C. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B.J.A. Nutrition; metabolism, Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Alam, M.A.J. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Nankar, R.; Prabhakar, P.; Doble, M.J.P. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine 2017, 37, 10–13. [Google Scholar] [CrossRef]
- Saija, A.; Tomaino, A.; Trombetta, D.; De Pasquale, A.; Uccella, N.; Barbuzzi, T.; Paolino, D.; Bonina, F.J.I. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; y González-Rubio, M.G.-L.; Aguilar-Faisal, J.L.; Morales-González, J.A.J.W. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787. [Google Scholar] [CrossRef]
- Su, C.; Yang, C.; Gong, M.; Ke, Y.; Yuan, P.; Wang, X.; Li, M.; Zheng, X.; Feng, W.J.M. Antidiabetic activity and potential mechanism of amentoflavone in diabetic mice. Molecules 2019, 24, 2184. [Google Scholar] [CrossRef]
- Zeng, F.; Luo, J.; Han, H.; Xie, W.; Wang, L.; Han, R.; Chen, H.; Cai, Y.; Huang, H.; Xia, Z. Allopurinol ameliorates liver injury in type 1 diabetic rats through activating Nrf2. Int. J. Immunopathol. Pharm. 2021, 35, 20587384211031417. [Google Scholar] [CrossRef]
- Hamadi, N.; Mansour, A.; Hassan, M.H.; Khalifi-Touhami, F.; Badary, O.J.J. Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2012, 26, 384–392. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y.J.J. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 310–314. [Google Scholar] [CrossRef]
- Friedman, J.E.; Sun, Y.; Ishizuka, T.; Farrell, C.J.; McCormack, S.E.; Herron, L.M.; Hakimi, P.; Lechner, P.; Yun, J.S. Phosphoenolpyruvate Carboxykinase (GTP) Gene transcription and hyperglycemia are regulated by glucocorticoids in genetically obesedb/db transgenic mice. J. Biol. Chem. 1997, 272, 31475–31481. [Google Scholar] [CrossRef] [Green Version]
- Roh, S.S.; Kwon, O.J.; Yang, J.H.; Kim, Y.S.; Lee, S.H.; Jin, J.S.; Jeon, Y.D.; Yokozawa, T.; Kim, H.J. Allium hookeri root protects oxidative stress-induced inflammatory responses and beta-cell damage in pancreas of streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 63. [Google Scholar] [CrossRef]
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin attenuates streptozotocin-induced pancreatic beta cell damage by its protective effects on cellular antioxidant defense. Vitr. Cell Dev. Biol. Anim. 2017, 53, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhang, D.; Kang, J.; Meng, X.; Yang, J.; Yang, L.; Xue, N.; Gao, Q.; Han, S.; Gou, X. Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed. Pharm. 2018, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 381–392. [Google Scholar] [CrossRef]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion in vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free. Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H.J.C.; Biology, M. Ferulic acid confers protection on islet β cells and placental tissues of rats with gestational diabetes mellitus. Cell Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Sannigrahi, S.; Rahaman, N.; Ahmed, F.J.E. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: Effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 2013, 44, 369–379. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M.J.P. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; Volume 960. [Google Scholar]
- Muthukumaran, P.; Thiyagarajan, G.; Babu, R.A.; Lakshmi, B.S. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Biol. Interact. 2018, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F.J.D. obesity; metabolism, Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P.J.C.; Pharmacology, E. Physiology, Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P. Hyperlipidaemia 3Ed: Diagnosis and Management; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.J.A.D. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H.J.B. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-A^{y} mice. Biofactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Qi, M.-y.; Wang, X.-t.; Xu, H.-l.; Yang, Z.-l.; Cheng, Y.; Zhou, B. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats. Food Funct. 2020, 11, 3706–3718. [Google Scholar] [CrossRef]
- Gajjala, P.R.; Sanati, M.; Jankowski, J. Cellular and molecular mechanisms of chronic kidney disease with diabetes mellitus and cardiovascular diseases as its comorbidities. Front. Immunol. 2015, 6, 340. [Google Scholar] [CrossRef]
- Sedeek, M.; Gutsol, A.; Montezano, A.C.; Burger, D.; Nguyen Dinh Cat, A.; Kennedy, C.R.; Burns, K.D.; Cooper, M.E.; Jandeleit-Dahm, K.; Page, P.J.C. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin. Sci. 2013, 124, 191–202. [Google Scholar] [CrossRef]
- Watanabe, N.; Shikata, K.; Shikata, Y.; Sarai, K.; Omori, K.; Kodera, R.; Sato, C.; Wada, J.; Makino, H. Involvement of MAPKs in ICAM-1 expression in glomerular endothelial cells in diabetic nephropathy. Acta Med. Okayama 2011, 65, 247–257. [Google Scholar]
- Chowdhury, S.; Ghosh, S.; Das, A.; Sil, P.C. Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H. Protein kinase C–dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD (P) H oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitada, M.; Koya, D.; Sugimoto, T.; Isono, M.; Araki, S.-i.; Kashiwagi, A.; Haneda, M.J.D. Translocation of glomerular p47phox and p67phox by protein kinase C-β activation is required for oxidative stress in diabetic nephropathy. Diabetes 2003, 52, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.L.; Tojo, A.; Goto, A.; Fujita, T. Radical scavenging effect of gliclazide in diabetic rats fed with a high cholesterol diet. Kidney Int. 2004, 65, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E.J.D. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes 2003, 52, 2570–2577. [Google Scholar] [CrossRef]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef]
- Nasiry, D.; Khalatbary, A.R.; Ahmadvand, H.; Talebpour Amiri, F.; Akbari, E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement. Altern. Med. 2017, 17, 476. [Google Scholar] [CrossRef]
- Makino, E.; Nakamura, N.; Miyabe, M.; Ito, M.; Kanada, S.; Hata, M.; Saiki, T.; Sango, K.; Kamiya, H.; Nakamura, K. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J. Diabetes Investig. 2019, 10, 1199–1208. [Google Scholar] [CrossRef]
- Samii, A.; Unger, J.; Lange, W.J.N. Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats. Neurosci. Lett. 1999, 262, 159–162. [Google Scholar] [CrossRef]
- Kanada, S.; Makino, E.; Nakamura, N.; Miyabe, M.; Ito, M.; Hata, M.; Yamauchi, T.; Sawada, N.; Kondo, S.; Saiki, T.J.I.J. Direct comparison of therapeutic effects on diabetic polyneuropathy between transplantation of dental pulp stem cells and administration of dental pulp stem cell-secreted factors. Int. J. Mol. Sci. 2020, 21, 6064. [Google Scholar] [CrossRef]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.; Dhaliwal, N.; Akhtar, A.; Kuhad, A.; Chopra, K. Beneficial effects of ferulic acid alone and in combination with insulin in streptozotocin induced diabetic neuropathy in Sprague Dawley rats. Life Sci. 2020, 255, 117856. [Google Scholar] [CrossRef] [PubMed]
- Mirzamohammadi, S.; Nematollahi, M.H.; Mehrbani, M.; Mehrabani, M.J.C. Ferulic acid pretreatment could improve prognosis of autologous mesenchymal stromal cell transplantation for diabetic neuropathy. Cytotherapy 2016, 18, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Chiu, J.-H.; Wu, I.-H.; Wang, B.-W.; Pan, C.-M.; Chen, Y.-H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Martinez-Quinones, P.; McCarthy, C.G.; Watts, S.W.; Klee, N.S.; Komic, A.; Calmasini, F.B.; Priviero, F.; Warner, A.; Chenghao, Y.; Wenceslau, C.F. Hypertension Induced Morphological and Physiological Changes in Cells of the Arterial Wall. Am. J. Hypertens 2018, 31, 1067–1078. [Google Scholar] [CrossRef]
- González, R.G.; Barnett, P.; Aguayo, J.; Cheng, H.-M.; Chylack, L.J.D., Jr. Direct measurement of polyol pathway activity in the ocular lens. Diabetes 1984, 33, 196–199. [Google Scholar] [CrossRef]
- Yabe-Nishimura, C.J.P. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–34. [Google Scholar]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2003, 88, 2399–2403. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef]
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625. [Google Scholar] [CrossRef]
- Murad, F. The nitric oxide–cyclic GMP signal transduction system for intracellular and intercellular communication. In Proceedings of the 1992 Laurentian Hormone Conference; Elsevier: Amsterdam, The Netherlands, 1994; pp. 239–248. [Google Scholar]
- Axelsson, K.; Wikberg, J.; Andersson, R.J.L.S. Relationship between nitroglycerin, cyclic GMP and relaxation of vascular smooth muscle. Life Sci. 1979, 24, 1779–1786. [Google Scholar] [CrossRef]
- Lazar, Z.; Meszaros, M.; Bikov, A. The Nitric Oxide Pathway in Pulmonary Arterial Hypertension: Pathomechanism, Biomarkers and Drug Targets. Curr. Med. Chem. 2020, 27, 7168–7188. [Google Scholar] [CrossRef] [PubMed]
- Sellak, H.; Yang, X.; Cao, X.; Cornwell, T.; Soff, G.A.; Lincoln, T.J.C. Sp1 transcription factor as a molecular target for nitric oxide–and cyclic nucleotide–mediated suppression of cGMP-dependent protein kinase-Iα expression in vascular smooth muscle cells. Circul. Res. 2002, 90, 405–412. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L.J.I. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- McIntyre, M.; Bohr, D.F.; Dominiczak, A.F.J.H. Endothelial function in hypertension: The role of superoxide anion. Hypertension 1999, 34, 539–545. [Google Scholar] [CrossRef]
- Jacobsen, J.C.B.; Hornbech, M.S.; Holstein-Rathlou, N.-H. Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus 2011, 1, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Badawy, D.; El-Bassossy, H.M.; Fahmy, A.; Azhar, A. Aldose reductase inhibitors zopolrestat and ferulic acid alleviate hypertension associated with diabetes: Effect on vascular reactivity. Can. J. Physiol. Pharmacol. 2013, 91, 101–107. [Google Scholar] [CrossRef]
- Yin, Y.; Qi, F.; Song, Z.; Zhang, B.; Teng, J.J.B. Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats. Biosci. Trends 2014, 8, 217–226. [Google Scholar] [CrossRef]
- Filla, L.A.; Edwards, J.L.J.M.B. Metabolomics in diabetic complications. Mol. Biosyst. 2016, 12, 1090–1105. [Google Scholar] [CrossRef]
- Heng, L.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P.J. Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet. Med. 2013, 30, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Tan, H.Y.; Zhang, Y.; Feng, Y. Protective effect of a Chinese Medicine formula He-Ying-Qing-Re Formula on diabetic retinopathy. J. Ethnopharmacol. 2015, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zou, W.; Cao, X.; Xu, W.; Lu, Z.; Zhu, Y.; Hu, X.; Hu, J.; Zhu, Q.J.P. Ferulic acid attenuates high glucose-induced apoptosis in retinal pigment epithelium cells and protects retina in db/db mice. PeerJ 2022, 10, e13375. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound. J. 2014, 11, 523–532. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S.J.P. Electrospun biomimetic multifunctional nanofibers loaded with ferulic acid for enhanced antimicrobial and wound-healing activities in STZ-Induced diabetic rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef] [PubMed]
- Springmann, M.; Mason-D’Croz, D.; Robinson, S.; Garnett, T.; Godfray, H.C.J.; Gollin, D.; Rayner, M.; Ballon, P.; Scarborough, P.J.T.L. Global and regional health effects of future food production under climate change: A modelling study. Lancet 2016, 387, 1937–1946. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Abel, E.D.; Kaulbach, H.C.; Tian, R.; Hopkins, J.C.; Duffy, J.; Doetschman, T.; Minnemann, T.; Boers, M.-E.; Hadro, E.; Oberste-Berghaus, C.J.T.J. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J. Clin. Investig. 1999, 104, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Nichols, A.; Thompson, E.; Mallick, A.; Payne, K.; Jones, C.; Manne, N.D.; Sundaram, S.; Shapiro, J.I.; Sodhi, K. Role of serum biomarkers in early detection of diabetic cardiomyopathy in the West Virginian population. Int. J. Med. Sci. 2016, 13, 161. [Google Scholar] [CrossRef]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J.J.C. Altered myocardial calcium cycling and energetics in heart failure—a rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial dysfunction in diabetic cardiomyopathy: The possible therapeutic roles of phenolic acids. Int. J. Med. Sci. 2020, 21, 6043. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.J.J. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional mitochondria in health and disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. BBA-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Rashid, K.; Sil, P.C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol. 2016, 97, 187–198. [Google Scholar] [CrossRef]
- Craft, S.; Watson, G.J.T.L.N. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004, 3, 169–178. [Google Scholar] [CrossRef]
- Chin, S.; Rhee, S.; Chon, S.; Baik, S.; Park, Y.; Nam, M.; Lee, K.; Chun, K.; Woo, J.; Kim, Y.S. Hypoglycemia is associated with dementia in elderly patients with type 2 diabetes mellitus: An analysis based on the Korea National Diabetes Program Cohort. Diabetes Res. Clin. Pract. 2016, 122, 54–61. [Google Scholar] [CrossRef] [PubMed]
| Diabetic Models | Dosage Range | Major Reported Antidiabetic Endpoints | References |
|---|---|---|---|
| STZ (40 mg/kg i.p. injection) in Wistar rats | 10 mg/kg for 45 days, i.v. | Increasing the activities of antioxidant enzymes such as GPx, SOD and CAT, neutralizing STZ-induced free radicals in the pancreas. | [20] |
| High-fat diet + fructose in Wistar rats | 50 mg/kg for 30 days, i.g. | Decreasing hepatic glucose production in the liver tissue, returning blood glucose, serum insulin, glucose tolerance and insulin tolerance to the normal range. | [11] |
| High-fat diet in male C57BL/6N mice | 0.5% supplemented diet ad libitum for 7 weeks | Increasing the activity of hepatic GK enzyme, and reducing the activities of G6Pase and PEPCK. | [19] |
| High-fat diet in gestational SD rat | 20 mg/kg for 12 weeks, i.g. | Inhibiting the apoptosis of β-cells in pancreatic islets. | [28] |
| STZ (60 mg/kg i.p. injection) in Wistar rats | 50 mg/kg for 8 weeks, i.g. | Reducing the lipid peroxidation in pancreatic tissues. | [29] |
| STZ (60 mg/kg i.p. injection) in Wistar rats | 10 mg/kg for 3 weeks (in combination with metformin), i.g. | Improving impaired β-cell regeneration. | [30] |
| High-fat diet in ICR mice | 25 and 50 mg/kg for 8 weeks, i.v. | Reducing the levels of plasma TG, FFA, cholesterol and phospholipids, decreasing expression of SREBP1c, FAS, ACC, CPT1a, and PPARα. | [35] |
| STZ (40 mg/kg i.p. injection) in Wistar rats | 10 mg/kg for 45 days, i.g. | Reducing the levels of TBARS, hydroperoxides and FFA in the liver. | [37] |
| STZ (150 mg/kg i.p. injection) in ICR mice | 0.01% supplemented diet ad libitum for 7 weeks | Reducing TBARS in brown adipose tissue. | [38] |
| Diabetic Models (In Vitro and In Vivo) | Dosage Range | Diabetic Complications | Beneficial Effects and Involved Mechanisms | References |
|---|---|---|---|---|
| STZ (50 mg/kg i.v. injection) in male SD rats | 100 mg/kg for 8 weeks, i.g. | Diabetic nephropathy | Improving the renal organ coefficient, increasing activities of SOD, CAT, and GPx. | [39] |
| STZ (50 mg/kg i.p. injection) in Wistar rats | 50 mg/kg for 8 weeks, i.g. | Diabetic nephropathy | Ameliorating renal cell apoptosis, inflammation and defective autophagy, modulating advanced AGEs, NF-κB, MAPKs, P38, JNK Erk1/2 signaling pathways. | [43] |
| Sucrose (30% in drinking water) in OLETF rats | 10 mg/kg for 20 weeks, i.g. | Diabetic nephropathy | Reducing oxidative stress, inflammatory response, and decreasing the ACR, urinary MDA and MCP-1 levels. | [48] |
| STZ (55 mg/kg i.p. injection) in SD rats | 100 mg/kg for 4 weeks (in combination with insulin), i.g. | Diabetic neuropathy | Downregulating the levels of TNF-α and IL-1β, decreasing COX-2 activity in the sciatic nerve. | [54] |
| STZ (50 mg/kg i.p. injection) in male Wistar rats | 20 mg/kg for 6 weeks, i.g. | Diabetic hypertension | Improving endothelial-dependent relaxation, NO production and vasoconstriction capacity in isolated aorta. | [71] |
| STZ (55 mg/kg i.p. injection) in C57BL/5J mice | 100 mg/kg HF containing FA as a major component for 4 weeks, i.g. | Diabetic retinopathy | Attenuating retinal vascular degeneration through upregulating the level of claudin-1 and inhibiting the activation of AGEs receptors | [78] |
| HG (30 mmol/L) induced ARPE-19 cells | 10 mmol/L | Diabetic retinopathy | Ameliorating the expression p53, Bcl2 and Bax. | [76] |
| STZ (50 mg/kg i.p. injection) in Wistar rats | 10 and 20 mg/kg for 14 days, i.g. | Diabetic wound healing | Improving blood fluidity, inhibiting platelet aggregation, and exhibiting strong antioxidant activity. | [77] |
| STZ (50 mg/kg i.p. injection) in Wistar rats | 50 mg/kg for 8 weeks, i.g. | Diabetic cardiomyopathy | Activating cardiac PI3K, Akt and GSK-3β, and ameliorating the translocation GLUT4. | [89] |
| STZ (35 mg/kg i.p. injection) and high-glucose-fat diet in Wistar rats | 15 and 30 mg/kg for 4 weeks, i.g. | Diabetic Alzheimer′s disease | Regulating the accumulation of PTP1B and Aβ, as well as blocking neuroinflammation and activating the insulin signaling pathway. | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules 2022, 27, 6010. https://doi.org/10.3390/molecules27186010
Li X, Wu J, Xu F, Chu C, Li X, Shi X, Zheng W, Wang Z, Jia Y, Xiao W. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules. 2022; 27(18):6010. https://doi.org/10.3390/molecules27186010
Chicago/Turabian StyleLi, Xu, Jingxian Wu, Fanxing Xu, Chun Chu, Xiang Li, Xinyi Shi, Wen Zheng, Zhenzhong Wang, Ying Jia, and Wei Xiao. 2022. "Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications" Molecules 27, no. 18: 6010. https://doi.org/10.3390/molecules27186010





