The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism
Abstract
:1. Introduction
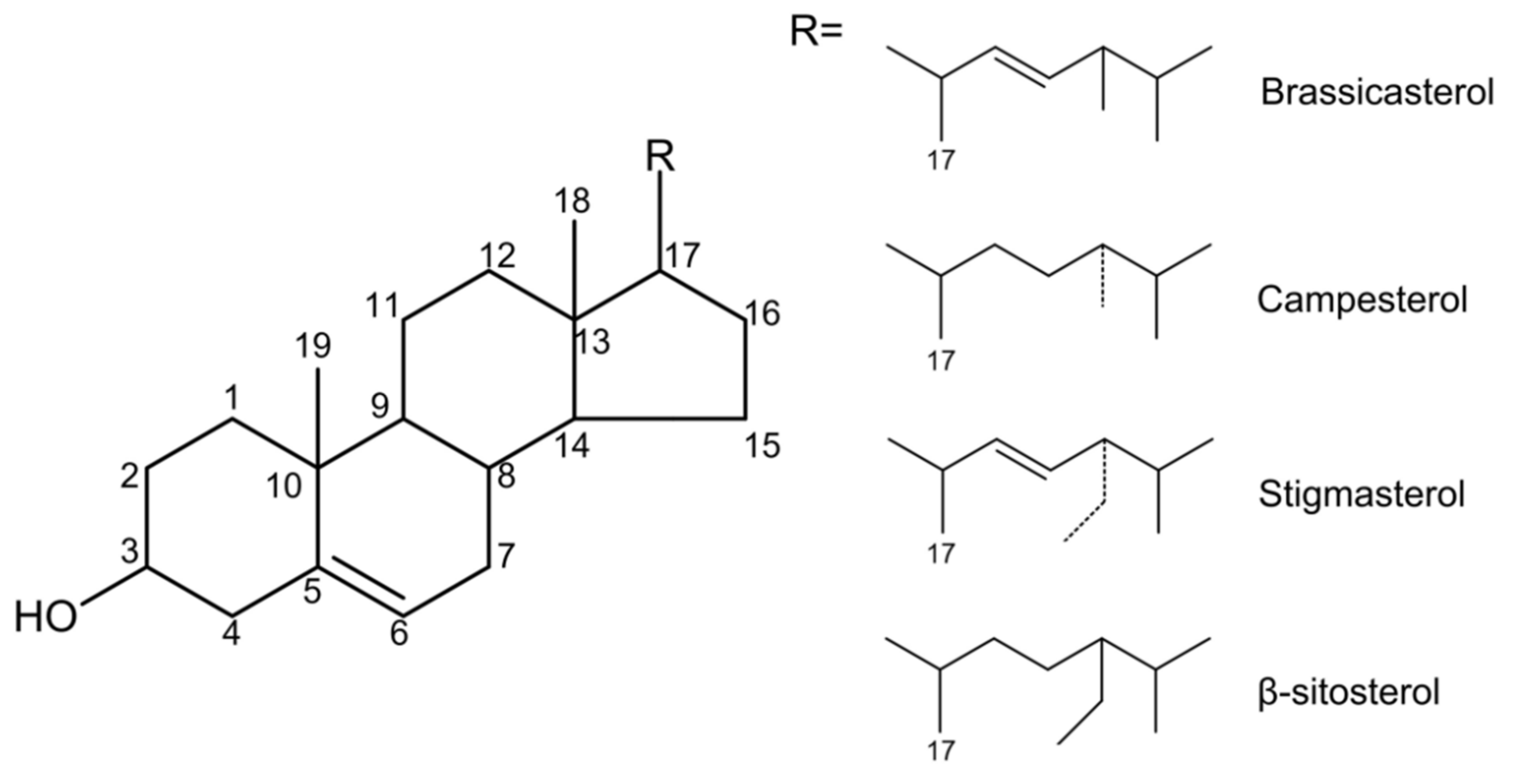
2. Phytosterols Chemistry and Dietary Sources
3. Bioavailability of Phytosterols
4. Cholesterol-Lowering Effect of Phytosterols in Patients with Hypercholesterolemia-Related Diseases
5. The Underlying Mechanism of Phytosterols in Regulating Cholesterol Homeostasis
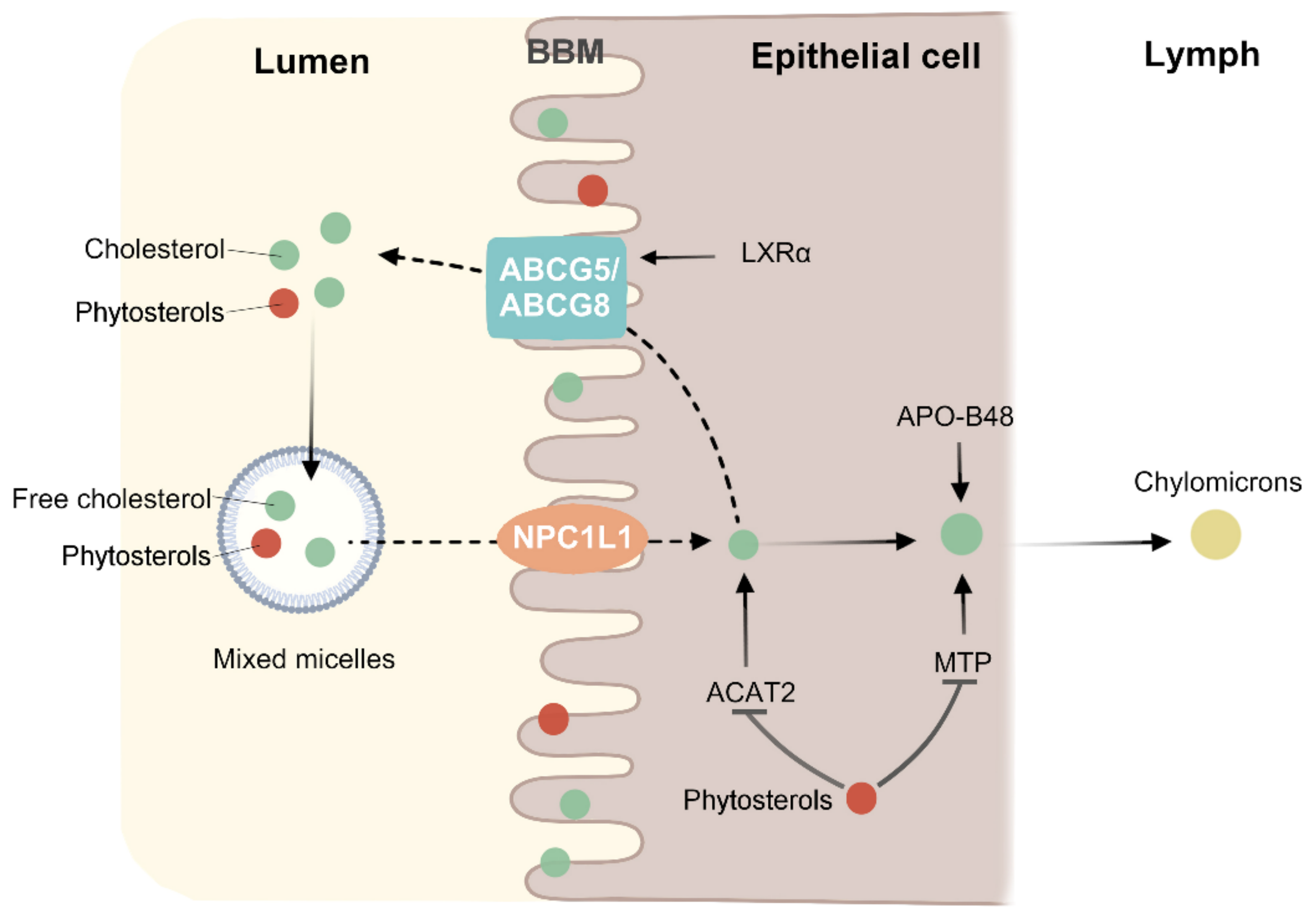
5.1. Phytosterols Regulate the Absorption of Cholesterol in the Gut
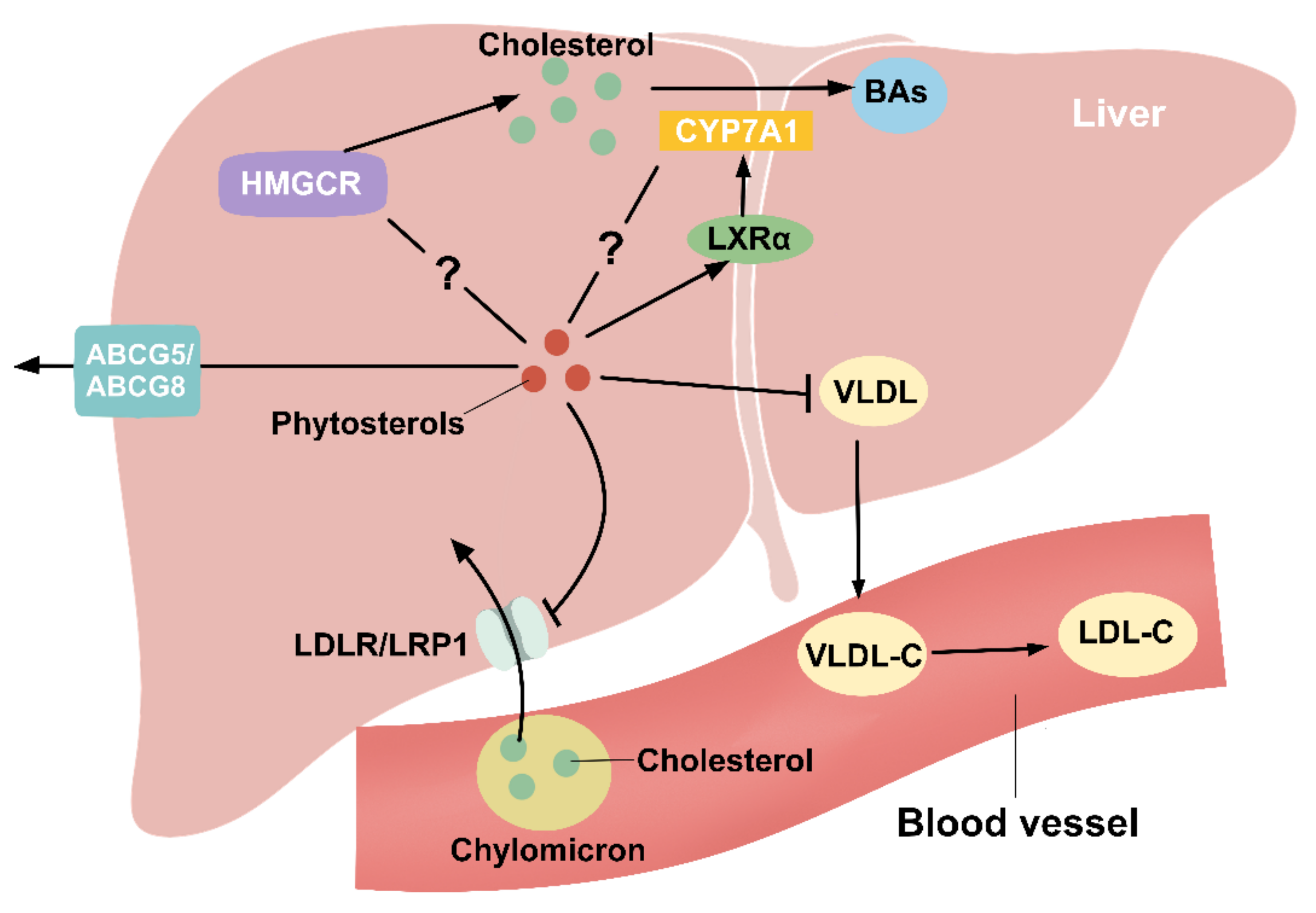
5.2. Phytosterols Regulate Liver Cholesterol Metabolism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Santos-Hidalgo, A.B.; Saura-Calixto, F. Common Sources and Estimated Intake of Plant Sterols in the Spanish Diet. J. Agric. Food Chem. 2006, 54, 3462–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martianto, D.; Bararah, A.; Andarwulan, N.; Średnicka-Tober, D. Cross-Sectional Study of Plant Sterols Intake as a Basis for Designing Appropriate Plant Sterol-Enriched Food in Indonesia. Nutrients 2021, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Mirończuk-Chodakowska, I.; Cicha-Mikołajczyk, A.; Drygas, W. Assessment of Plant Sterols in the Diet of Adult Polish Population with the Use of a Newly Developed Database. Nutrients 2021, 13, 2722. [Google Scholar] [CrossRef]
- Lesma, G.; Luraghi, A.; Bavaro, T.; Bortolozzi, R.; Rainoldi, G.; Roda, G.; Viola, G.; Ubiali, D.; Silvani, A. Phytosterol and γ-Oryzanol Conjugates: Synthesis and Evaluation of their Antioxidant, Antiproliferative, and Anticholesterol Activities. J. Nat. Prod. 2018, 81, 2212–2221. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and Inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Qasimi, M.I.; Nagaoka, K.; Watanabe, G. The Effects of Phytosterols on the Sexual Behavior and Reproductive Function in the Japanese Quail (Coturnix Coturnix Japonica). Poult. Sci. 2017, 96, 3436–3444. [Google Scholar] [CrossRef]
- Ostlund, R.E.; Racette, S.B.; Okeke, A.; Stenson, W.F. Phytosterols that Are Naturally Present in Commercial Corn Oil Significantly Reduce Cholesterol Absorption in Humans. Am. J. Clin. Nutr. 2002, 75, 1000–1004. [Google Scholar] [CrossRef]
- Normén, L.; Dutta, P.; Lia, A.; Andersson, H. Soy Sterol Esters and Beta-Sitostanol Ester as Inhibitors of Cholesterol Absorption in Human Small Bowel. Am. J. Clin. Nutr. 2000, 71, 908–913. [Google Scholar] [CrossRef] [Green Version]
- Agren, J.J.; Tvrzicka, E.; Nenonen, M.T.; Helve, T.; Hänninen, O. Divergent Changes in Serum Sterols during a Strict Uncooked Vegan Diet in Patients with Rheumatoid Arthritis. Br. J. Nutr. 2001, 85, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Xing, B. A Phytosterol-Enriched Spread Improves Lipid Profile and Insulin Resistance of Women with Gestational Diabetes Mellitus: A Randomized, Placebo-Controlle.ed Double-Blind Clinical Trial. Diabetes Technol. Ther. 2016, 18, 499–504. [Google Scholar] [CrossRef]
- Guo, X.-X.; Zeng, Z.; Qian, Y.-Z.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.-P.; Zhou, F. Wheat Flour, Enriched with γ-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats. Nutrients 2019, 11, 1697. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, M.; Fang, Y.-J.; Lu, M.-S.; Pan, Z.-Z.; Huang, W.-Q.; Chen, Y.-M.; Zhang, C.-X. Association between Phytosterol Intake and Colorectal Cancer Risk: A Case-Control Study. Br. J. Nutr. 2017, 117, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-L.; Luo, Z.-L.; Shi, H.-W.; Zhang, L.-X.; Ma, X.-J. Research Advance of Functional Plant Pharmaceutical Cycloartenol about Pharmacological and Physiological Activity. Zhongguo Zhong Yao Za Zhi 2017, 42, 433–437. [Google Scholar]
- Hansel, B.; Courie, R.; Bayet, Y.; Delestre, F.; Bruckert, E. Phytosterols and Atherosclerosis. Rev. Med. Interne 2011, 32, 124–129. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, Phytostanols, and their Conjugates in Foods: Structural Diversity, Quantitative Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Inês, C.; Corbacho, J.; Paredes, M.A.; Labrador, J.; Cordeiro, A.M.; Gomez-Jimenez, M.C. Regulation of Sterol Content and Biosynthetic Gene Expression during Flower Opening and Early Fruit Development in Olive. Physiol. Plant. 2019, 167, 526–539. [Google Scholar] [CrossRef]
- Zhou, W.; Branch, W.D.; Gilliam, L.; Marshall, J.A. Phytosterol Composition of Seeds from Different Maturity Classes. Molecules 2018, 24, 106. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.-B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and Perspectives in Plant Sterol and Plant Stanol Research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef] [Green Version]
- Sioen, I.; Matthys, C.; Huybrechts, I.; Van Camp, J.; De Henauw, S. Consumption of Plant Sterols in Belgium: Estimated Intakes and Sources of Naturally Occurring Plant Sterols and β-carotene. Br. J. Nutr. 2011, 105, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.M.; Fonseca, F.A.; Ballus, C.A.; Figueiredo-Neto, A.M.; Meinhart, A.D.; de Godoy, H.T.; Izar, M.C. Common Sources and Composition of Phytosterols and their Estimated Intake by the Population in the City of São Paulo, Brazil. Nutrition 2013, 29, 865–871. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A Review on Chemical and Physical Modifications of Phytosterols and their Influence on Bioavailability and Safety. Crit. Rev. Food Sci. Nutr. 2021, 1–20. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2020, 11, 599959. [Google Scholar] [CrossRef]
- Ticho, A.L.; Calzadilla, N.; Malhotra, P.; Lee, H.; Anbazhagan, A.N.; Saksena, S.; Dudeja, P.K.; Lee, D.; Gill, R.K.; Alrefai, W.A. NPC1L1-dependent transport of 27-alkyne cholesterol in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2021, 320, C916–C925. [Google Scholar] [CrossRef]
- Scolaro, B.; Andrade, L.F.S.d.; Castro, I.A. Cardiovascular Disease Prevention: The Earlier the Better? A Review of Plant Sterol Metabolism and Implications of Childhood Supplementation. Int. J. Mol. Sci. 2019, 21, 128. [Google Scholar] [CrossRef] [Green Version]
- Tammi, A.; Rönnemaa, T.; Rask-Nissilä, L.; Miettinen, T.A.; Gylling, H.; Valsta, L.; Viikari, J.; Välimäki, I.; Simell, O. Apolipoprotein E Phenotype Regulates Cholesterol Absorption in Healthy 13-Month-Old Children–The STRIP Study. Pediatr. Res. 2001, 50, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Lupattelli, G.; Pisciotta, L.; De Vuono, S.; Siepi, D.; Bellocchio, A.; Melis, F.; Bertolini, S.; Pirro, M.; Mannarino, E. A Silent Mutation of Niemann-Pick C1-like 1 and Apolipoprotein E4 Modulate Cholesterol Absorption in Primary Hyperlipidemias. J. Clin. Lipidol. 2013, 7, 147–152. [Google Scholar] [CrossRef]
- Chan, Y.-M.; Varady, K.A.; Lin, Y.; Trautwein, E.; Mensink, R.P.; Plat, J.; Jones, P.J.H. Plasma Concentrations of Plant Sterols: Physiology and Relationship with Coronary Heart Disease. Nutr. Rev. 2006, 64, 385–402. [Google Scholar] [CrossRef]
- Von Bergmann, K.; Lütjohann, D.; Lindenthal, B.; Steinmetz, A. Efficiency of Intestinal Cholesterol Absorption in Humans Is not Related to ApoE Phenotype. J. Lipid Res. 2003, 44, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borel, P. Factors Affecting Intestinal Absorption of Highly Lipophilic Food Microconstituents (Fat-Soluble Vitamins, Carotenoids and Phytosterols). Clin. Chem. Lab. Med. 2003, 41, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C. Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation. Annu. Rev. Nutr. 2018, 38, 69–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Honda, A.; Ishikawa, T.; Kinoshita, M.; Mashimo, Y.; Takeoka, Y.; Yasuda, D.; Kusano, J.; Tsukamoto, K.; Matsuzaki, Y.; et al. A SNP of NPC1L1 Affects Cholesterol Absorption in Japanese. J. Atheroscler. Thromb. 2010, 17, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Bioavailability of Nanotechnology-Based Bioactives and Nutraceuticals. Adv. Food Nutr. Res. 2019, 88, 235–273. [Google Scholar]
- de Graaf, J.; Stalenhoef, A.F. Use of Margarine Fortified with Phytosterols as a Therapeutic Food. Ned. Tijdschr. Geneeskd. 2000, 144, 918–921. [Google Scholar]
- De Graaf, J.; De Sauvage Nolting, P.R.W.; Van Dam, M.; Belsey, E.M.; Kastelein, J.J.P.; Haydn Pritchard, P.; Stalenhoef, A.F.H. Consumption of Tall Oil-Derived Phytosterols in a Chocolate Matrix Significantly Decreases Plasma Total and Low-Density Lipoprotein-Cholesterol levels. Br. J. Nutr. 2002, 88, 479–488. [Google Scholar] [CrossRef]
- Bao, L.; Li, Y.; Deng, S.-X.; Landry, D.; Tabas, I. Sitosterol-Containing Lipoproteins Trigger Free Sterol-Induced Caspase-Independent Death in ACAT-Competent Macrophages. J. Biol. Chem. 2006, 281, 33635–33649. [Google Scholar] [CrossRef] [Green Version]
- Makhmudova, U.; Schulze, P.C.; Lütjohann, D.; Weingärtner, O. Phytosterols and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 68. [Google Scholar] [CrossRef]
- Genser, B.; Silbernagel, G.; De Backer, G.; Bruckert, E.; Carmena, R.; Chapman, M.J.; Deanfield, J.; Descamps, O.S.; Rietzschel, E.R.; Dias, K.C.; et al. Plant Sterols and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. 2012, 33, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Reaver, A.; Hewlings, S.; Westerman, K.; Blander, G.; Schmeller, T.; Heer, M.; Rein, D. A Randomized, Placebo-Controlled, Double-Blind Crossover Study to Assess a Unique Phytosterol Ester Formulation in Lowering LDL Cholesterol Utilizing a Novel Virtual Tracking Tool. Nutrients 2019, 11, 2108. [Google Scholar] [CrossRef] [Green Version]
- Chau, Y.-P.; Cheng, Y.-C.; Sing, C.-W.; Tsoi, M.-F.; Cheng, V.K.-F.; Lee, G.K.-Y.; Cheung, C.-L.; Cheung, B.M.Y. The Lipid-Lowering Effect of Once-Daily Soya Drink Fortified with Phytosterols in Normocholesterolaemic Chinese: A Double-Blind Randomized Controlled Trial. Eur. J. Nutr. 2020, 59, 2739–2746. [Google Scholar] [CrossRef]
- Silbernagel, G.; Genser, B.; Nestel, P.; März, W. Plant Sterols and Atherosclerosis. Curr. Opin. Lipidol. 2013, 24, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegard, L.; Jessup, W.; Jones, P.J.; Lutjohann, D.; Maerz, W.; Masana, L.; et al. Plant Sterols and Plant Stanols in the Management of Dyslipidaemia and Prevention of Cardiovascular Disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Lomonaco, R.; Sunny, N.E.; Bril, F.; Cusi, K. Nonalcoholic Fatty Liver Disease: Current Issues and Novel Treatment Approaches. Drugs 2013, 73, 1–14. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N. Inflammation and Fibrogenesis in Steatohepatitis. J. Gastroenterol. 2012, 47, 215–225. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the Gut-Liver Axis in NASH Pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Leu, W.-J.; Zhong, N.-S.; Guh, J.-H. Autophagic Activation and Decrease of Plasma Membrane Cholesterol Contribute to Anticancer Activities in Non-Small Cell Lung Cancer. Molecules 2021, 26, 5967. [Google Scholar] [CrossRef]
- Martin, L.A.; Kennedy, B.E.; Karten, B. Mitochondrial Cholesterol: Mechanisms of Import and Effects on Mitochondrial Function. J. Bioenerg. Biomembr. 2016, 48, 137–151. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Guo, J.; Chen, J.; Yang, P.; Tian, J.; Sun, J.; Zong, Y.; Qu, S. Cholesterol Overloading Leads to Hepatic L02 Cell Damage through Activation of the Unfolded Protein Response. Int. J. Mol. Med. 2009, 24, 459–464. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol Metabolism and the Pathogenesis of Non-Alcoholic Steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent Insights on the Role of Cholesterol in Non-Alcoholic Fatty Liver Disease. Biochim. Biophys. Acta 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Charlton, M. FGF-19 Agonism for NASH: A Short Study of a Long Disease. Lancet 2018, 391, 1124–1126. [Google Scholar] [CrossRef]
- Horn, C.L.; Morales, A.L.; Savard, C.; Farrell, G.C.; Ioannou, G.N. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol. Commun. 2022, 6, 12–35. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of Stigmasterol and β-sitosterol Alters Lipid Metabolism and Alleviates NAFLD in Mice Fed a High-Fat Western-Style Diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Laos, S.; Caimari, A.; Crescenti, A.; Lakkis, J.; Puiggròs, F.; Arola, L.; del Bas, J.M. Long-Term Intake of Soyabean Phytosterols Lowers Serum TAG and NEFA Concentrations, Increases Bile Acid Synthesis and Protects against Fatty Liver Development in Dyslipidaemic Hamsters. Br. J. Nutr. 2014, 112, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Javanmardi, M.A.; Mohammad Shahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. Effects of Phytosterol Supplementation on Serum Levels of Lipid Profiles, Liver Enzymes, Inflammatory Markers, Adiponectin, and Leptin in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 651–658. [Google Scholar]
- Gumede, N.M.; Lembede, B.W.; Nkomozepi, P.; Brooksbank, R.L.; Erlwanger, K.H.; Chivandi, E. β-Sitosterol Mitigates the Development of High-Fructose Diet-Induced Nonalcoholic Fatty Liver Disease in Growing Male Sprague-Dawley Rats. Can. J. Physiol. Pharmacol. 2020, 98, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Y.; Qu, D.; Ouyang, P.; Ding, X.; Wu, P.; Guan, Q.; Yang, L. The Regulatory Effects of Phytosterol Esters (PSEs) on Gut Flora and Faecal Metabolites in Rats with NAFLD. Food Funct. 2020, 11, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, X.G.; Ouyang, P.L.; Guan, Q.; Yang, L.; Peng, F.; Du, H.; Yin, F.; Yan, W.; Yu, W.J.; et al. Combined Effect of -3 Fatty Acids and Phytosterol Esters on Alleviating Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease Subjects: A Double-Blind Placebo-Controlled Clinical Trial. Br. J. Nutr. 2020, 123, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of Cardiovascular Disease in China: Current Features and Implications. Nat. Rev. Cardiol. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Matvienko, O.A.; Lewis, D.S.; Swanson, M.; Arndt, B.; Rainwater, D.L.; Stewart, J.; Alekel, D.L. A Single Daily Dose of Soybean Phytosterols in Ground Beef Decreases Serum Total Cholesterol and LDL Cholesterol in Young, Mildly Hypercholesterolemic Men. Am. J. Clin. Nutr. 2002, 76, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Halonen, J.; Lindholm, H.; Konttinen, J.; Simonen, P.; Nissinen, M.J.; Savolainen, A.; Talvi, A.; Hallikainen, M. The Effects of Plant Stanol Ester Consumption on Arterial Stiffness and Endothelial Function in Adults: A Randomised Controlled Clinical Trial. BMC Cardiovasc. Disord. 2013, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Rideout, T.C.; Harding, S.V.; Mackay, D.; Abumweis, S.S.; Jones, P.J. High Basal Fractional Cholesterol Synthesis Is Associated with Nonresponse of Plasma LDL Cholesterol to Plant Sterol Therapy. Am. J. Clin. Nutr. 2010, 92, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Blom, W.A.M.; Koppenol, W.P.; Hiemstra, H.; Stojakovic, T.; Scharnagl, H.; Trautwein, E.A. A Low-Fat Spread with Added Plant Sterols and Fish Omega-3 Fatty Acids Lowers Serum Triglyceride and LDL-Cholesterol Concentrations in Individuals with Modest Hypercholesterolaemia and Hypertriglyceridaemia. Eur. J. Nutr. 2019, 58, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Sialvera, T.E.; Pounis, G.D.; Koutelidakis, A.E.; Richter, D.J.; Yfanti, G.; Kapsokefalou, M.; Goumas, G.; Chiotinis, N.; Diamantopoulos, E.; Zampelas, A. Phytosterols Supplementation Decreases Plasma Small and Dense LDL Levels in Metabolic Syndrome Patients on a Westernized Type Diet. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 843–848. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin Potentiates Cholesterol-Lowering Effects of Phytosterols in Hypercholesterolaemic Individuals. A Randomised Controlled Trial. Metabolism 2018, 82, 22–35. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Koppenol, W.P.; de Jong, A.; Hiemstra, H.; Vermeer, M.A.; Noakes, M.; Luscombe-Marsh, N.D. Plant Sterols Lower LDL-Cholesterol and Triglycerides in Dyslipidemic Individuals with or at Risk of Developing Type 2 Diabetes; a Randomized, Double-Blind, Placebo-Controlled Study. Nutr. Diabetes 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Silvestre, R.Á.; Hernández-Álvarez, E.; Granado-Lorencio, F.; Barberá, R.; Garcia-Llatas, G. A Positive Impact on the Serum Lipid Profile and Cytokines after the Consumption of a Plant Sterol-Enriched Beverage with a Milk Fat Globule Membrane: A Clinical Study. Food Funct. 2018, 9, 5209–5219. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Ho, D.K.-C.; Sing, C.-W.; Tsoi, M.-F.; Cheng, V.K.-F.; Lee, G.K.-Y.; Ho, Y.-N.; Cheung, B.M.Y. Randomized Controlled Trial of the Effect of Phytosterols-Enriched Low-Fat Milk on Lipid Profile in Chinese. Sci. Rep. 2017, 7, 41084. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.-P.; Wang, H.H.; Wang, D.Q.H. Cholesterol Absorption Is Mainly Regulated by the Jejunal and Ileal ATP-Binding Cassette Sterol Efflux Transporters Abcg5 and Abcg8 in Mice. J. Lipid Res. 2004, 45, 1312–1323. [Google Scholar] [CrossRef] [Green Version]
- Reeskamp, L.F.; Volta, A.; Zuurbier, L.; Defesche, J.C.; Hovingh, G.K.; Grefhorst, A. ABCG5 and ABCG8 Genetic Variants in Familial Hypercholesterolemia. J. Clin. Lipidol. 2020, 14, 207–217. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nystrom, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) Is the Intestinal Phytosterol and Cholesterol Transporter and a Key Modulator of Whole-Body Cholesterol Homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [Green Version]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [Green Version]
- Mattson, F.H.; Grundy, S.M.; Crouse, J.R. Optimizing the Effect of Plant Sterols on Cholesterol Absorption in Man. Am. J. Clin. Nutr. 1982, 35, 697–700. [Google Scholar] [CrossRef]
- Plat, J.; Mensink, R.P. Plant Stanol and Sterol Esters in the Control of Blood Cholesterol Levels: Mechanism and Safety Aspects. Am. J. Cardiol. 2005, 96, 15D–22D. [Google Scholar] [CrossRef]
- Cedó, L.; Farràs, M.; Lee-Rueckert, M.; Escolà-Gil, J.C. Molecular Insights into the Mechanisms Underlying the Cholesterol- Lowering Effects of Phytosterols. Curr. Med. Chem. 2019, 26, 6704–6723. [Google Scholar] [CrossRef]
- Doornbos, A.M.E.; Meynen, E.M.; Duchateau, G.S.M.J.E.; van der Knaap, H.C.M.; Trautwein, E.A. Intake Occasion Affects the Serum Cholesterol Lowering of a Plant Sterol-Enriched Single-Dose Yoghurt Drink in Mildly Hypercholesterolaemic Subjects. Eur. J. Clin. Nutr. 2006, 60, 325–333. [Google Scholar] [CrossRef]
- Yang, J.-W.; Ji, H.-F. Phytosterols as Bioactive Food Components against Nonalcoholic Fatty Liver Disease. Crit. Rev. Food Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Meessen, E.C.E.; Groen, A.K. Transintestinal Cholesterol Excretion in Humans. Curr. Opin. Lipidol. 2018, 29, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ikegami, Y.; Kotani, N.; Shimada, A.; Noda, M.; Murakoshi, T. Luminal Plant Sterol Promotes Brush Border Membrane-to-Lumen Cholesterol Efflux in the Small Intestine. J. Clin. Biochem. Nutr. 2018, 63, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Lifsey, H.C.; Kaur, R.; Thompson, B.H.; Bennett, L.; Temel, R.E.; Graf, G.A. Stigmasterol Stimulates Transintestinal Cholesterol Excretion Independent of Liver X Receptor Activation in the Small Intestine. J. Nutr. Biochem. 2020, 76, 108263. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Murakoshi, T. A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels. Nutrients 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Field, F.J.; Born, E.; Mathur, S.N. Stanol Esters Decrease Plasma Cholesterol Independently of Intestinal ABC Sterol Transporters and Niemann-Pick C1-like 1 Protein Gene Expression. J. Lipid Res. 2004, 45, 2252–2259. [Google Scholar] [CrossRef] [Green Version]
- Juritsch, A.; Tsai, Y.-T.; Patel, M.S.; Rideout, T.C. Transcriptional Control of Enterohepatic Lipid Regulatory Targets in Response to Early Cholesterol and Phytosterol Exposure in ApoE Mice. BMC Res. Notes 2017, 10, 529. [Google Scholar] [CrossRef] [Green Version]
- Temel, R.E.; Gebre, A.K.; Parks, J.S.; Rudel, L.L. Compared with Acyl-CoA:Cholesterol O-Acyltransferase (ACAT) 1 and Lecithin:cholesterol Acyltransferase, ACAT2 Displays the Greatest Capacity to Differentiate Cholesterol from Sitosterol. J. Biol. Chem. 2003, 278, 47594–47601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.T.; Wong, W.T.; Guan, L.; Tian, X.Y.; Ma, K.Y.; Huang, Y.; Chen, Z.-Y. Effect of Phytosterols and their Oxidation Products on Lipoprotein Profiles and Vascular Function in Hamster Fed a High Cholesterol Diet. Atherosclerosis 2011, 219, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, F.; Wei, H.; Liu, L.; Shen, T.; Xu, S.; Wei, J.; Ren, J.; Ni, H. Combination of Berberine and Evodiamine Inhibits Intestinal Cholesterol Absorption in High Fat Diet Induced Hyperlipidemic Rats. Lipids Health Dis. 2017, 16, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, F.J.; Born, E.; Mathur, S.N. Effect of Micellar Beta-Sitosterol on Cholesterol Metabolism in CaCo-2 Cells. J. Lipid Res. 1997, 38, 348–360. [Google Scholar] [CrossRef]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol Reduces Plasma Cholesterol Levels and Inhibits Hepatic Synthesis and Intestinal Absorption in the Rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef]
- Plat, J.; Mensink, R.P. Effects of Plant Stanol Esters on LDL Receptor Protein Expression and on LDL Receptor and HMG-CoA Reductase mRNA Expression in Mononuclear Blood Cells of Healthy Men and Women. FASEB J. 2002, 16, 258–260. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Reduction in Intestinal Cholesterol Absorption by Various Food Components: Mechanisms and Implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.M.; Duchateau, G.S.M.J.E.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous Dose-Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Ottestad, I.; Ose, L.; Wennersberg, M.H.; Granlund, L.; Kirkhus, B.; Retterstøl, K. Phytosterol Capsules and Serum Cholesterol in Hypercholesterolemia: A Randomized Controlled Trial. Atherosclerosis 2013, 228, 421–425. [Google Scholar] [CrossRef]
- He, W.-S.; Wang, M.-G.; Pan, X.-X.; Li, J.-J.; Jia, C.-S.; Zhang, X.-M.; Feng, B. Role of Plant Stanol Derivatives in the Modulation of Cholesterol Metabolism and Liver Gene Expression in Mice. Food Chem. 2013, 140, 9–16. [Google Scholar] [CrossRef]
- Cedó, L.; Santos, D.; Ludwig, I.A.; Silvennoinen, R.; García-León, A.; Kaipiainen, L.; Carbó, J.M.; Valledor, A.F.; Gylling, H.; Motilva, M.-J.; et al. Phytosterol-Mediated Inhibition of Intestinal Cholesterol Absorption in Mice Is Independent of Liver X Receptor. Mol. Nutr. Food Res. 2017, 61, 201700055. [Google Scholar] [CrossRef]
- Méndez-González, J.; Süren-Castillo, S.; Calpe-Berdiel, L.; Rotllan, N.; Vázquez-Carrera, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Disodium Ascorbyl Phytostanol Phosphate (FM-VP4), a Modified Phytostanol, Is a Highly Active Hypocholesterolaemic Agent that Affects the Enterohepatic Circulation of Both Cholesterol and Bile Acids in Mice. Br. J. Nutr. 2010, 103, 153–160. [Google Scholar] [CrossRef] [Green Version]

| Study Population | Length of Intervention (Weeks) | Adjustments Considered | The Main Results of Phytosterol Intervention | References |
|---|---|---|---|---|
| Healthy individuals with slightly higher TG levels (≥1.4 mmol/L) and LDL-C concentrations (≥3.4 mmol/L) (n = 260) | 4 | TG, LDL-C, TC, | Participants in the intervention group had significantly lower concentrations of TC (3.9%), TG (10.6%), and LDL-C (5.2%) | [68] |
| Patients with metabolic syndrome (n = 108) | 8 | TC, LDL-C, sdLDL, TG | Patients in the intervention group had significantly lower concentrations of TC (15.9%), TG (19.1%), LDL-C (20.3%), and sdLDL (p < 0.05) | [69] |
| Normocholesterolemic participants (n = 159) | 3 | LDL-C | The concentration of LDL-C (5.96%, p = 0.028) was significantly lower in patients in the intervention group | [42] |
| The fasting TC concentration of the participants was 6.57 ± 0.13 mmol/L (n = 70) | 4 | TC, LDL-C | Patients in the intervention group had significantly lower concentrations of TC (4.8%, p < 0.05) and LDL-C (8.1%, p < 0.05) | [70] |
| Healthy individuals at increased risk of T2DM and patients with T2DM (n = 161) | 6 | TC, LDL-C, TG | Individuals in the phytosterol intervention group had significantly lower fasting TC (4.2%), TG (8.3%), and LDL-C (4.6%) concentrations | [71] |
| Postmenopausal women (n = 38) | 6 | TC, LDL-C | Serum TC (212.9 ± 25.8 mg/dL) and LDL-C concentrations (121.7 ± 24.4 mg/dL) decreased significantly after phytosterol treatment compared to previous (220.0 ± 27.8 mg/dL) (129.4 ± 28.5 mg/dL) | [72] |
| Individuals not taking cholesterol-lowering drugs or without diabetes (n = 221) | 3 | TC, LDL-C, diastolic blood pressure | Serum LDL-C concentration (9.5 ± 2%), TC (p < 0.01), and diastolic blood pressure (p = 0.01) were significantly reduced after phytosterol intervention | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. https://doi.org/10.3390/molecules27020523
Li X, Xin Y, Mo Y, Marozik P, He T, Guo H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules. 2022; 27(2):523. https://doi.org/10.3390/molecules27020523
Chicago/Turabian StyleLi, Xiang, Yan Xin, Yuqian Mo, Pavel Marozik, Taiping He, and Honghui Guo. 2022. "The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism" Molecules 27, no. 2: 523. https://doi.org/10.3390/molecules27020523







