Importance of Conjugation of the Bile Salt on the Mechanism of Lipolysis
Abstract
:1. Introduction
2. Results
2.1. Micellization
2.2. Analysis of β Parameter
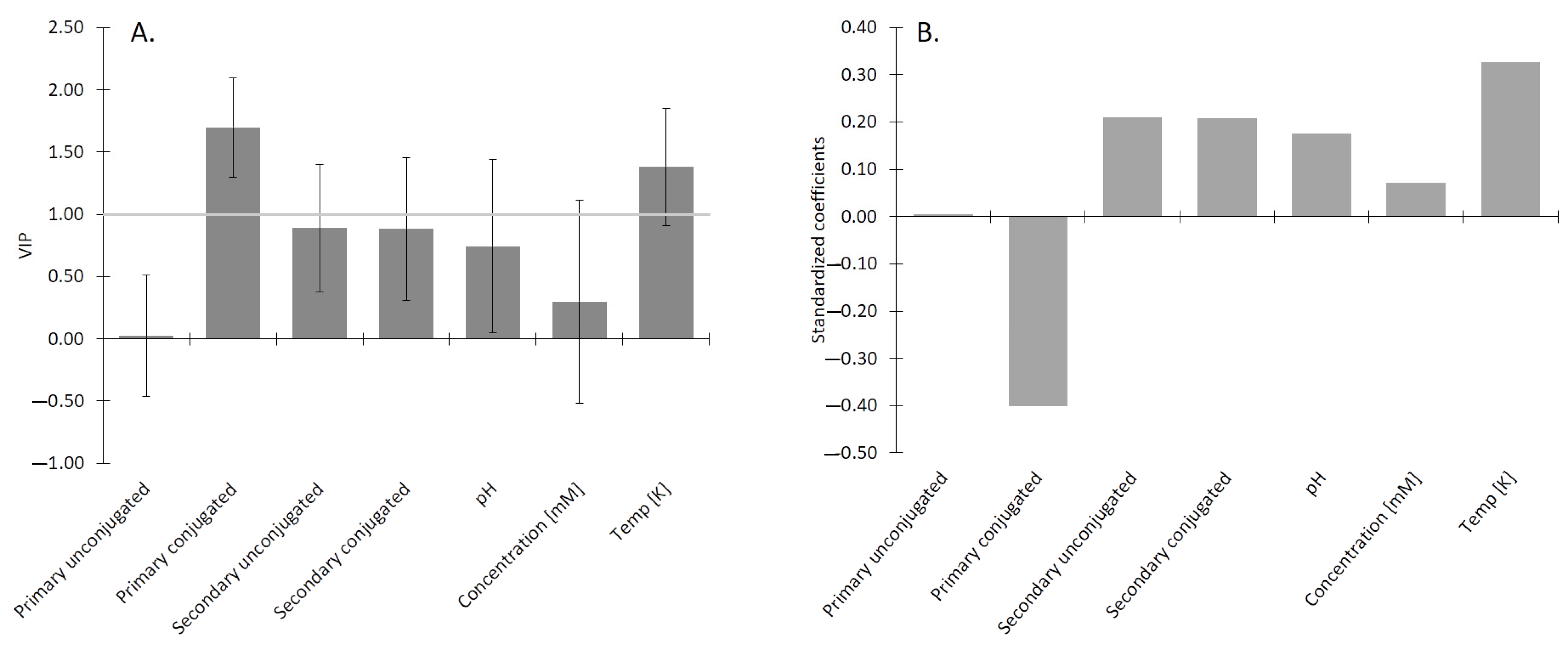
2.3. Aggregation Number
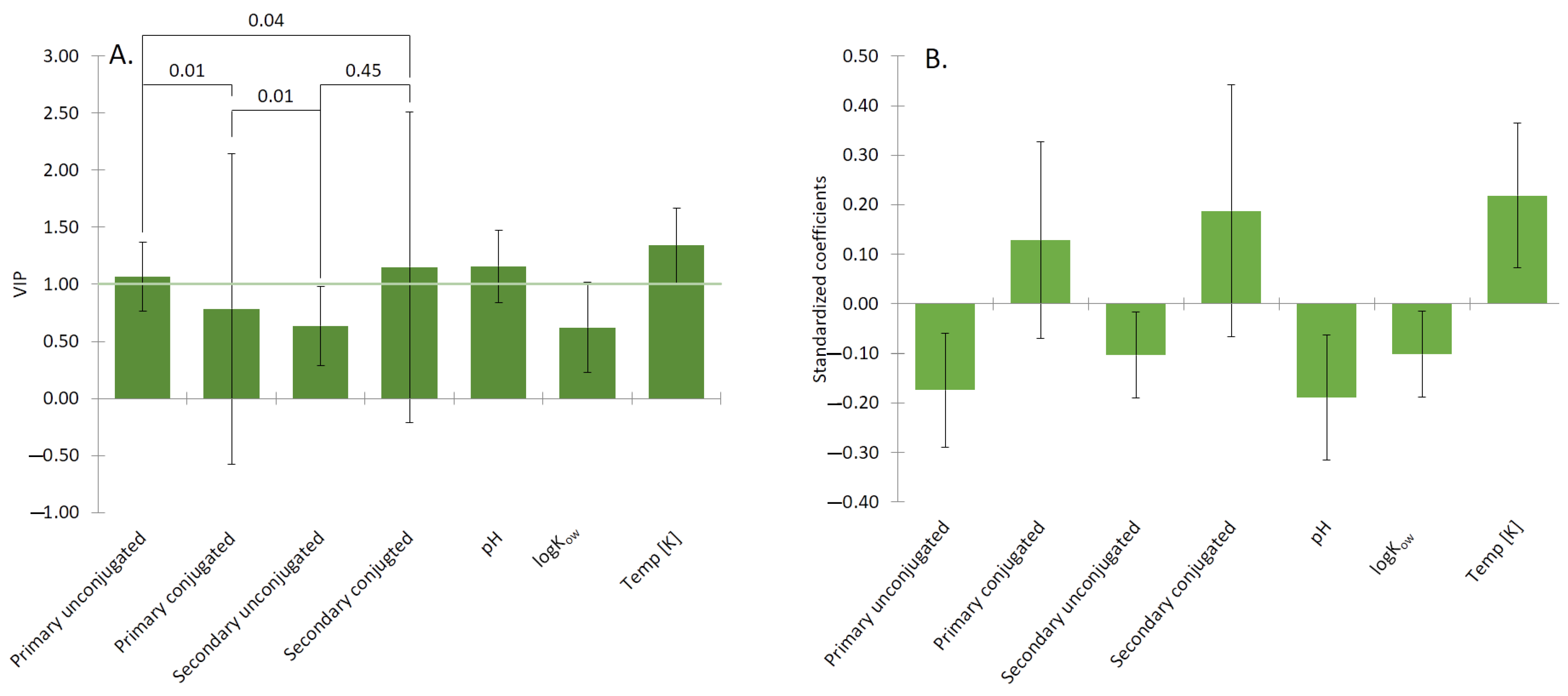
2.4. Molar Solubilization Ratio
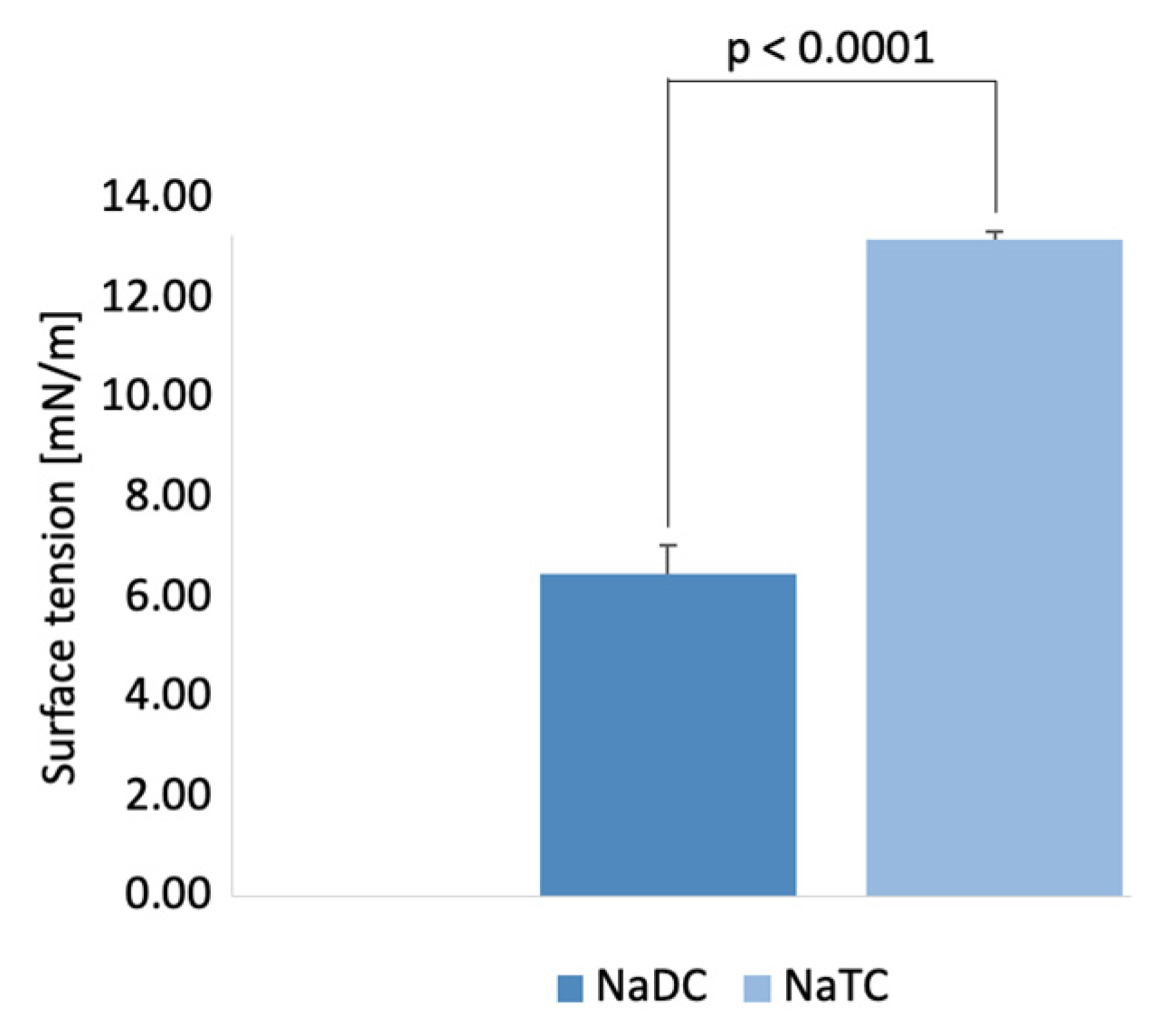
2.5. Measurements of Interfacial Tension at Oil/BS Interface
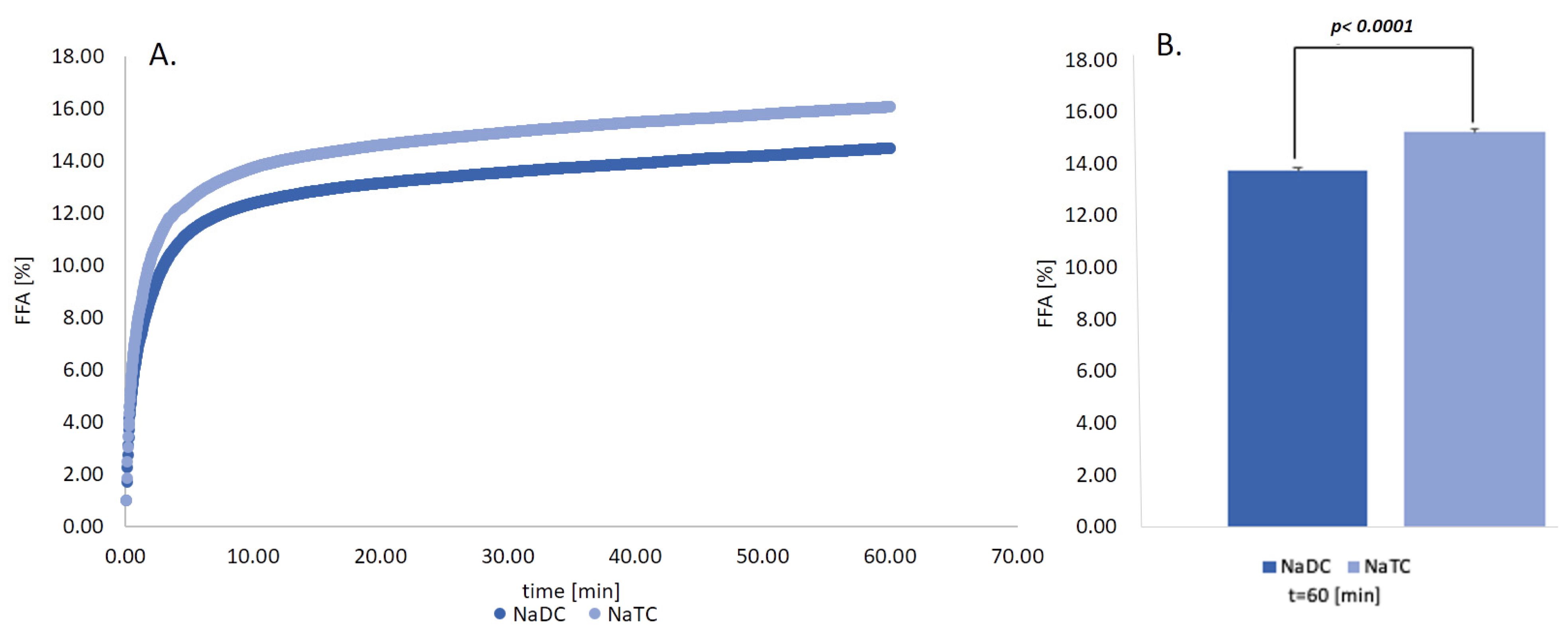
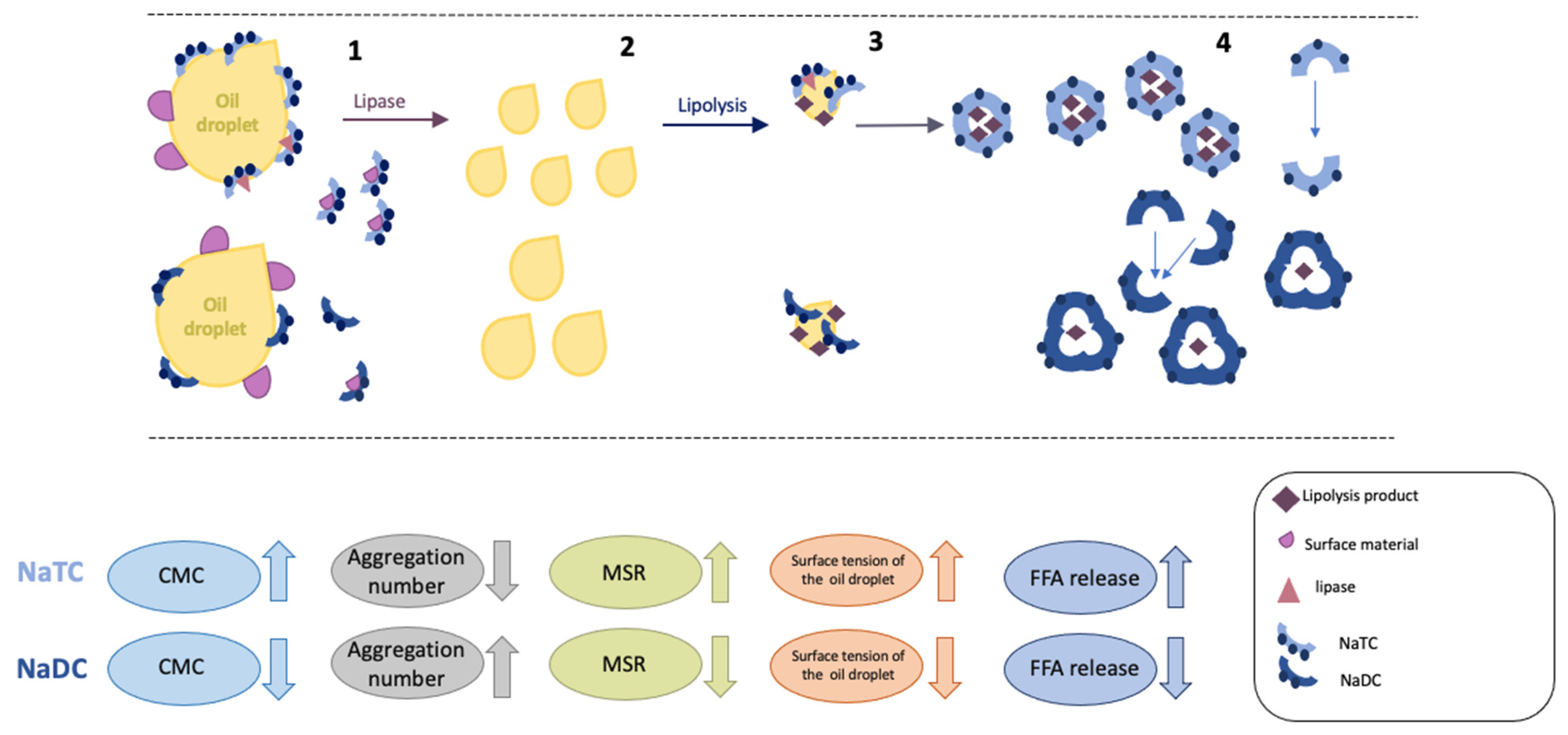
2.6. Impact of Conjugation Form of the BS on the Lipid Digestion
3. Materials and Methods
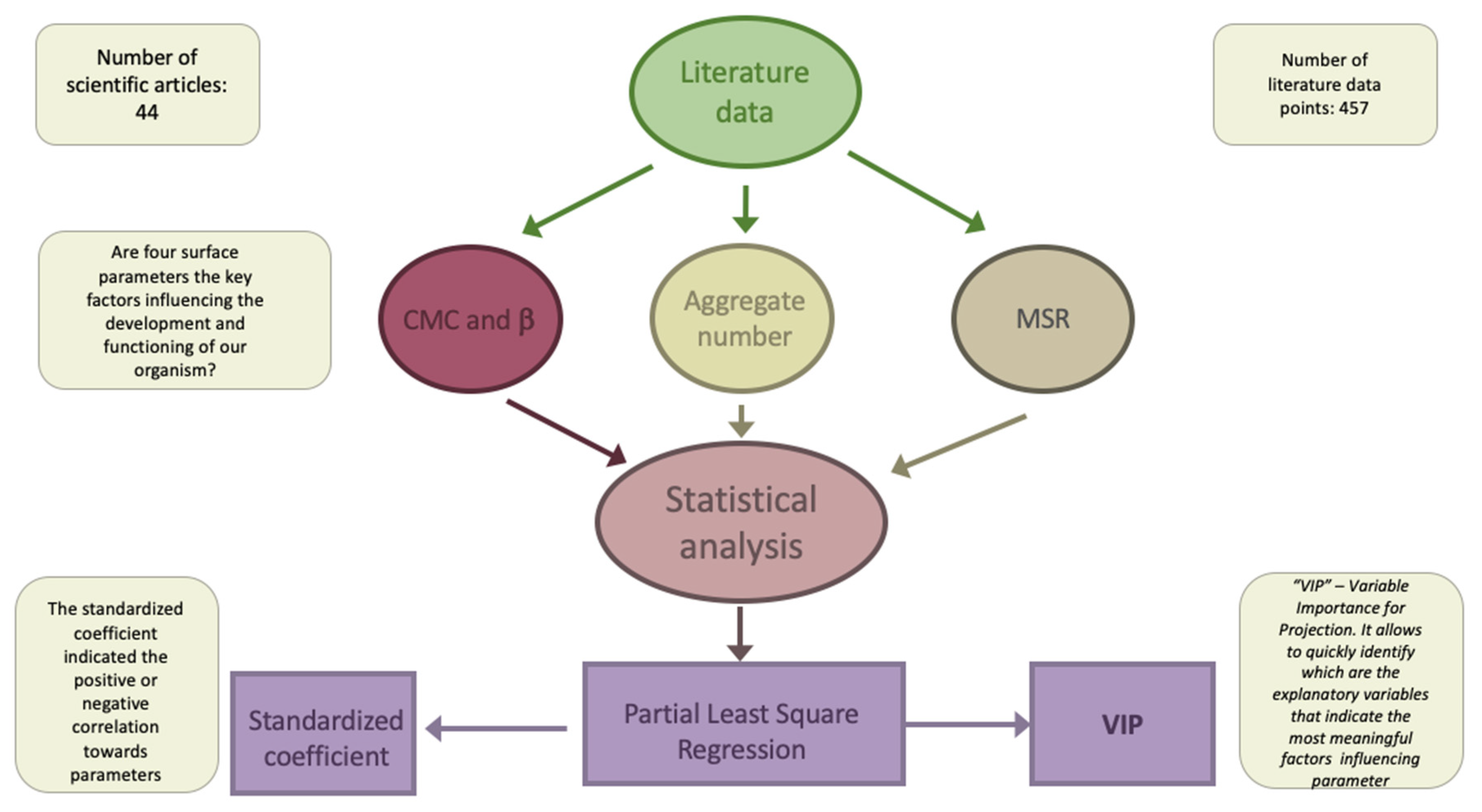
3.1. Meta-Analysis
3.2. Critical Micelle Concentration Determination
3.3. Emulsion
3.4. Droplet Size
3.5. Interfacial Tension Measurements
3.6. In Vitro Duodenal Digestion
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bukiya, A.N.; McMillan, J.; Parrill, A.L.; Dopico, A.M. Structural determinants of monohydroxylated bile acids to activate β1 subunit-containing BK channels. J. Lipid Res. 2008, 49, 2441–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, M.C. Physical-chemical properties of bile acids and their salts. New Compr. Biochem. 1985, 12, 345–403. [Google Scholar] [CrossRef]
- Madenci, D.; Egelhaaf, S.U. Self-assembly in aqueous bile salt solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.L. Bile Formation and Secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, S.J.; Mihalik, S.J.; Kim, D.G.; Cuebas, D.A.; Watkins, P.A. The human liver-specific homolog of very long-chain acyl-CoA synthetase is cholate: CoA ligase. J. Biol. Chem. 2000, 275, 15605–15608. [Google Scholar] [CrossRef] [Green Version]
- Goto, J.; Mano, N.; Goto, T. Development of Highly Selective Analytical Systems for Biological Substances Using Chromatography Combined with Mass Spectrometry—With Special Reference to Bio—Analytical Studies of Bile Acids. Chromatography 2004, 25, 1–8. [Google Scholar]
- Macierzanka, A.; Torcello-Gómez, A.; Jungnickel, C.; Maldonado-Valderrama, J. Bile salts in digestion and transport of lipids. Adv. Colloid Interface Sci. 2019, 274, 102045. [Google Scholar] [CrossRef]
- Mazer, N.A.; Benedek, G.B.; Carey, M.C. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry 1980, 19, 601–615. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Terriza, J.A.H.; Torcello-Gómez, A.; Cabrerizo-Vílchez, M.A. In vitro digestion of interfacial protein structures. Soft Matter 2013, 9, 1043–1053. [Google Scholar] [CrossRef]
- Chu, B.S.; Gunning, A.P.; Rich, G.T.; Ridout, M.J.; Faulks, R.M.; Wickham, M.S.J.; Morris, V.J.; Wilde, P.J. Adsorption of bile salts and pancreatic colipase and lipase onto digalactosyldiacylglycerol and dipalmitoylphosphatidylcholine monolayers. Langmuir 2010, 26, 9782–9793. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M. Mechanisms for the intestinal absorption of bile acids. J. Lipid Res. 1968, 9, 297–309. [Google Scholar] [CrossRef]
- Tso, P.; Nauli, A.; Lo, C.M. Enterocyte fatty acid uptake and intestinal fatty acid-binding protein. Biochem. Soc. Trans. 2004, 32, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahar, R.J.; Stolz, A. Bile acid transport. Gastroenterol. Clin. N. Am. 1999, 28, 27–58. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.K.; Ramesh, S.; Tan, C.Y.; Ching, C.Y.; Lwin, N.; Muchtar, A. Effect of manganese oxide on the densification and properties of ceria-doped scandia stabilized zirconia. J. Ceram. Process. Res. 2016, 17, 443–447. [Google Scholar] [CrossRef]
- Small, D.M.; Dowling, R.H.; Redinger, R.N. The Enterohepatic Circulation of Bile Salts. Arch. Intern. Med. 1972, 130, 552–573. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Jansen, P.L.M.; Faber, K.N.; Geuken, M.; Heegsma, J.; Mol, O.; Plass, J.R.M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 2002, 35, 589–596. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Vaquero, J.; Monte, M.J.; Dominguez, M.; Muntané, J.; Marin, J.J.G. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem. Pharmacol. 2013, 86, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Patti, M.E.; Endocrinologist, A. Bile acids, obesity, and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.; Dao, D.; Lau, T.C.; Henriksbo, B.D.; Cavallari, J.F.; Foley, K.P.; Schertzer, J.D. Bacterial peptidoglycan stimulates adipocyte lipolysis via NOD1. PLoS ONE 2014, 9, e97675. [Google Scholar] [CrossRef]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 2020, 579, 123–129. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Geng, W.; Lin, J. Bacterial bile salt hydrolase: An intestinal microbiome target for enhanced animal health. Anim. Health Res. Rev. 2016, 17, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Marion, S.; Desharnais, L.; Studer, N.; Dong, Y.; Notter, M.D.; Poudel, S.; Menin, L.; Janowczyk, A.; Hettich, R.L.; Hapfelmeier, S.; et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 2020, 61, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dowling, R.H.; Mack, E.; Small, D.M. Effects of controlled interruption of the enterohepatic circulation of bile salts by biliary diversion and by ileal resection on bile salt secretion, synthesis, and pool size in the rhesus monkey. J. Clin. Investig. 1970, 49, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Garewal, H.; Bernstein, H.; Bernstein, C.; Sampliner, R.; Payne, C. Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: A potential biological marker for cancer risk. Cancer Res. 1996, 56, 1480–1483. [Google Scholar]
- Schlottmann, K.; Wachs, P.F.; Krieg, C.R.; Kullmann, F.; Scholmerich, J.; Rogler, G. Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res. 2000, 60, 4270–4276. [Google Scholar]
- Payne, C.M.; Bernstein, H.; Bernstein, C.; Garewal, H. Role of apoptosis in biology and pathology: Resistance to apoptosis in colon carcinogenesis. Ultrastruct. Pathol. 1995, 19, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, M.L.; Sugerman, H.J.; Kellum, J.M.; Moore, E.W. Changes in gallbladder bile composition following gallstone formation and weight reduction. Gastroenterology 1992, 103, 214–221. [Google Scholar] [CrossRef]
- Canny, G.O.; McCormick, B.A. Bacteria in the intestine, helpful residents or enemies from within? Infect. Immun. 2008, 76, 3360–3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, A.; Dressman, J.B. Composition and physicochemical properties of fasted-state human duodenal and jejunal fluid: A critical evaluation of the available data. J. Pharm. Sci. 2014, 103, 3398–3411. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Alaghband-Zadeh, J.; Cross, G.F.; Omar, S.; Le Roux, C.W.; Vincent, R.P. Urine bile acids relate to glucose control in patients with type 2 diabetes mellitus and a body mass index below 30 kg/m2. PLoS ONE 2014, 9, e93540. [Google Scholar] [CrossRef]
- Mallory, A.; Kern, F.; Smith, J.; Savage, D. Patterns of Bile Acids and Microflora in the Human Small Intestine: I. Bile acids. Gastroenterology 1973, 64, 26–33. [Google Scholar] [CrossRef]
- Jungnickel, C.; Łuczak, J.; Ranke, J.; Fernández, J.F.; Müller, A.; Thöming, J. Micelle formation of imidazolium ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 278–284. [Google Scholar] [CrossRef]
- Roda, A.; Hofmann, A.F.; Mysels, K.J. The influence of bile salt structure on self-association in aqueous solutions. J. Biol. Chem. 1983, 258, 6362–6370. [Google Scholar] [CrossRef]
- Pártay, L.B.; Jedlovszky, P.; Sega, M. Molecular aggregates in aqueous solutions of bile acid salts. Molecular dynamics simulation study. J. Phys. Chem. B 2007, 111, 9886–9896. [Google Scholar] [CrossRef]
- Mustan, F.; Ivanova, A.; Madjarova, G.; Tcholakova, S.; Denkov, N. Molecular Dynamics Simulation of the Aggregation Patterns in Aqueous Solutions of Bile Salts at Physiological Conditions. J. Phys. Chem. B 2015, 119, 15631–15643. [Google Scholar] [CrossRef] [PubMed]
- Pártay, L.B.; Sega, M.; Jedlovszky, P. Morphology of bile salt micelles as studied by computer simulation methods. Langmuir 2007, 23, 12322–12328. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. The physical chemistry of cholanic acids. In The Bile Acids: Chemistry, Physiology and Metabolism; Nair, P.P., Kritchevsky, D., Eds.; Plenum Press: New York, NY, USA, 1971; Volume 1, Chapter 8. [Google Scholar]
- Kawamura, H.; Murata, Y.; Yamaguchi, T.; Igimi, H.; Tanaka, M.; Sugihara, G.; Kratohvil, J.P. Spin-label studies of bile salt micelles. J. Phys. Chem. 1989, 93, 3321–3326. [Google Scholar] [CrossRef]
- Heuman, D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 1989, 30, 719–730. [Google Scholar] [CrossRef]
- Joshi, T.; Kadam, Y. Mixed micellization of dodecyldimethylamine oxide with bile salts in aqueous solutions. J. Dispers. Sci. Technol. 2009, 30, 1273–1276. [Google Scholar] [CrossRef]
- Mukherjee, B.; Dar, A.A.; Bhat, P.A.; Moulik, S.P.; Das, A.R. Micellization and adsorption behaviour of bile salt systems. RSC Adv. 2016, 6, 1769–1781. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Bile salt–bile salt interaction in mixed monolayer and mixed micelle formation. J. Chem. Thermodyn. 2019, 128, 406–414. [Google Scholar] [CrossRef]
- Shiloach, A.; Blankschtein, D. Predicting micellar solution properties of binary surfactant mixtures. Langmuir 1998, 14, 1618–1636. [Google Scholar] [CrossRef]
- Dar, A.A.; Rather, G.M.; Ghosh, S.; Das, A.R. Micellization and interfacial behavior of binary and ternary mixtures of model cationic and nonionic surfactants in aqueous NaCl medium. J. Colloid Interface Sci. 2008, 322, 572–581. [Google Scholar] [CrossRef]
- Coret, J.; Shiloach, A.; Berger, P.; Blankschtein, D. Critical micelle concentrations of ternary surfactant mixtures: Theoretical prediction with user-friendly computer programs and experimental design analysis. J. Surfactants Deterg. 1999, 2, 51–58. [Google Scholar] [CrossRef]
- Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devínsky, F. Self-assembly properties of cationic gemini surfactants with biodegradable groups in the spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Dutta, P.; Mukherjee, S.; Bhattacharyya, K. Solvation dynamics in bile salt aggregates. J. Phys. Chem. B 2002, 106, 7745–7750. [Google Scholar] [CrossRef]
- Olesen, N.E.; Westh, P.; Holm, R. Determination of thermodynamic potentials and the aggregation number for micelles with the mass-action model by isothermal titration calorimetry: A case study on bile salts. J. Colloid Interface Sci. 2015, 453, 79–89. [Google Scholar] [CrossRef]
- Poša, M.; Bjedov, S.; Škorić, D.; Sakač, M. Micellization parameters (number average, aggregation number and critical micellar concentration) of bile salt 3 and 7 ethylidene derivatives: Role of the steroidal skeleton II. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.B.; Chalmers, D.K.; Hutchison, K.; Dang, W.; Pouton, C.W. Molecular dynamics simulations of spontaneous bile salt aggregation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 280, 182–193. [Google Scholar] [CrossRef]
- Garidel, P.; Hildebrand, A.; Neubert, R.; Blume, A. Thermodynamic Characterization of Bile Salt Aggregation Isothermal Titration Calorimetry. Langmuir 2000, 16, 5267–5275. [Google Scholar] [CrossRef]
- Pártay, L.B.; Sega, M.; Jedlovszky, P. Size and structure of bile salt micelles: Influence of structure, concentration, counterion concentration, pH, and temperature. J. Colloid Interface Sci. 1968. [Google Scholar] [CrossRef]
- Hofmann, A.F. The function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts. Biochem. J. 1963, 89, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.F. The preparation of chenodeoxycholic acid and its glycine and taurine conjugates. Acta Chem Scand. 1963, 17, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Nagadome, S.; Okazaki, Y.; Lee, S.; Sasaki, Y.; Sugihara, G. Selective solubilization of sterols by bile salt micelles in water: A thermodynamic study. Langmuir 2001, 17, 4405–4412. [Google Scholar] [CrossRef]
- Kolehmainen, E. Solubilization of aromatics in aqueous bile salts. I. benzene and alkylbenzenes in sodium cholate: 1H NMR study. J. Colloid Interface Sci. 1985, 105, 273–277. [Google Scholar] [CrossRef]
- Nagata, M.; Yotsuyanagi, T.; Ikeda, K. Solubilization of Vitamin K1 by Bile Salts and Phosphatidylcholine-Bile Salts Mixed Micelles. J. Pharm. Pharmacol. 1988, 40, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Pabois, O.; Antoine-Michard, A.; Zhao, X.; Omar, J.; Ahmed, F.; Alexis, F.; Harvey, R.D.; Grillo, I.; Gerelli, Y.; Grundy, M.M.L.; et al. Interactions of bile salts with a dietary fibre, methylcellulose, and impact on lipolysis. Carbohydr. Polym. 2020, 231, 115741. [Google Scholar] [CrossRef]
- Wilde, P.J.; Garcia-Llatas, G.; Lagarda, M.J.; Haslam, R.P.; Grundy, M.M.L. Oat and lipolysis: Food matrix effect. Food Chem. 2019, 278, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Shin, J.A.; Hong, S.T.; Lee, K.T. In vitro study for lipolysis of soybean oil, pomegranate oil, and their blended and interesterified oils under a pH-stat model and a simulated model of small intestinal digestion. Nutrients 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizones Ruiz-Henestrosa, V.M.; Bellesi, F.A.; Camino, N.A.; Pilosof, A.M.R. The impact of HPMC structure in the modulation of in vitro lipolysis: The role of bile salts. Food Hydrocoll. 2017, 62, 251–261. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Pilosof, A.M.R. Potential implications of food proteins-bile salts interactions. Food Hydrocoll. 2021, 118, 106766. [Google Scholar] [CrossRef]
- Parker, R.; Rigby, N.M.; Ridout, M.J.; Gunning, A.P.; Wilde, P.J. The adsorption-desorption behaviour and structure function relationships of bile salts. Soft Matter 2014, 10, 6457–6466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.B.; Yi, S.H.; Lee, B.H. Purification and characterization of three different types of bile salt hydrolases from Bifidobacterium strains. J. Dairy Sci. 2004, 87, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Valderrama, J.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.A. Bile salts at the air-water interface: Adsorption and desorption. Colloids Surf. B 2014, 120, 176–183. [Google Scholar] [CrossRef]
- Gallier, S.; Shaw, E.; Laubscher, A.; Gragson, D.; Singh, H.; Jiménez-Flores, R. Adsorption of bile salts to milk phospholipid and phospholipid-protein monolayers. J. Agric. Food Chem. 2014, 62, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martín, A.; Orduna-malea, E.; Thelwall, M. Google Scholar, Web of Science, and Scopus: A systematic comparison of citations in 252 subject categories. J. Informetr. 2018, 12, 1160–1177. [Google Scholar] [CrossRef] [Green Version]
- Łuczak, J.; Markiewicz, M.; Thöming, J.; Hupka, J.; Jungnickel, C. Influence of the Hofmeister anions on self-organization of 1-decyl-3-methylimidazolium chloride in aqueous solutions. J. Colloid Interface Sci. 2011, 362, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Szumała, P.; Pacyna-Kuchta, A.; Wasik, A. Proteolysis of whey protein isolates in nanoemulsion systems: Impact of nanoemulsification and additional synthetic emulsifiers. Food Chem. 2021, 351, 129356. [Google Scholar] [CrossRef] [PubMed]
- Micic, R.D.; Bosnjak Kiralj, M.S.; Panic, S.N.; Tomic, M.D.; Jovic, B.D.; Boskovic, G.C. Activation temperature imposed textural and surface synergism of CaO catalyst for sunflower oil transesterification. Fuel 2015, 159, 638–645. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Sun, X.; Sun, J.; Liu, C.; Liu, C.; Xu, J. Physicochemical properties and storage stability of food protein-stabilized nanoemulsions. Nanomaterials 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Łozińska, N.; Głowacz-Różyńska, A.; Artichowicz, W.; Lu, Y.; Jungnickel, C. Microencapsulation of fish oil—determination of optimal wall material and encapsulation methodology. J. Food Eng. 2020, 268, 109730. [Google Scholar] [CrossRef]
- Addinsoft XLSTAT Statistical And Data Analysis Solution. Available online: https://www.xlstat.com/en/news/xlstat-version-2020-1-3 (accessed on 1 August 2021).
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Commerford, S.R.; Pagliassotti, M.J.; Melby, C.L.; Wei, Y.; Gayles, E.C.; Hill, J.O. Fat oxidation, lipolysis, and free fatty acid cycling in obesity-prone and obesity-resistant rats. Am. J. Physiol.—Endocrinol. Metab. 2000, 279, 875–885. [Google Scholar] [CrossRef]
- Whiting, M.J.; Watts, J.M.K. Supersaturated bile from obese patients without gallstones supports cholesterol crystal growth but not nucleation. Gastroenterology 1984, 86, 243–248. [Google Scholar] [CrossRef]
- Heaton, K.W.; Read, A.E. Gall Stones in Patients with Disorders of the Terminal Ileum and Disturbed Bile Salt Metabolism. Br. Med. J. 1969, 3, 494–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
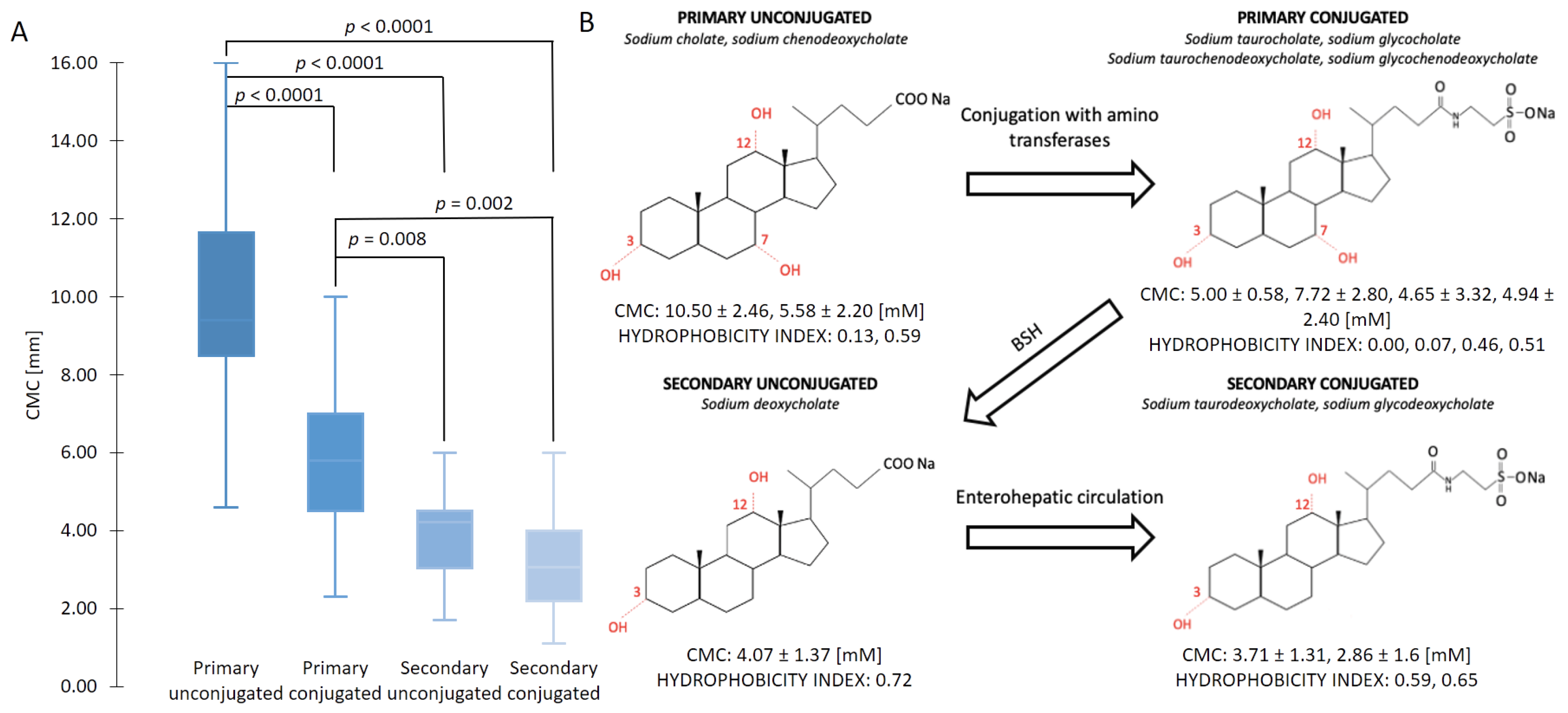
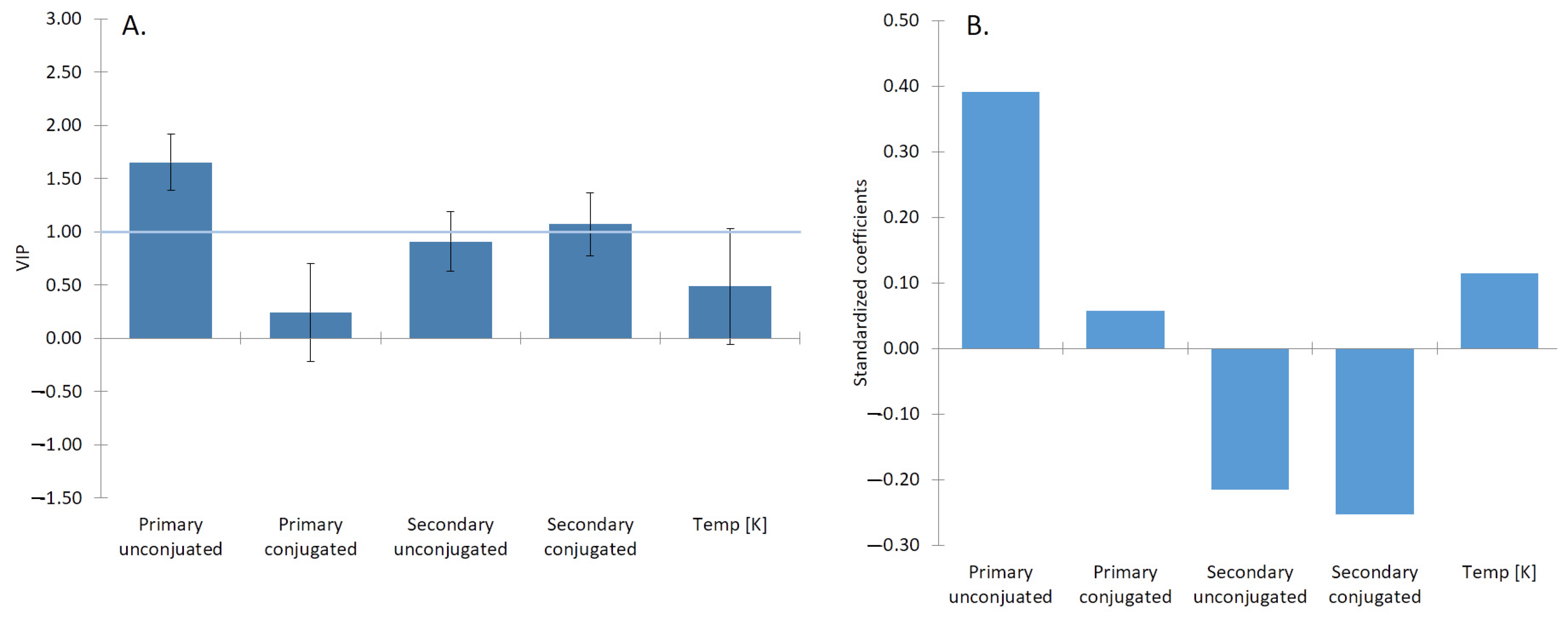
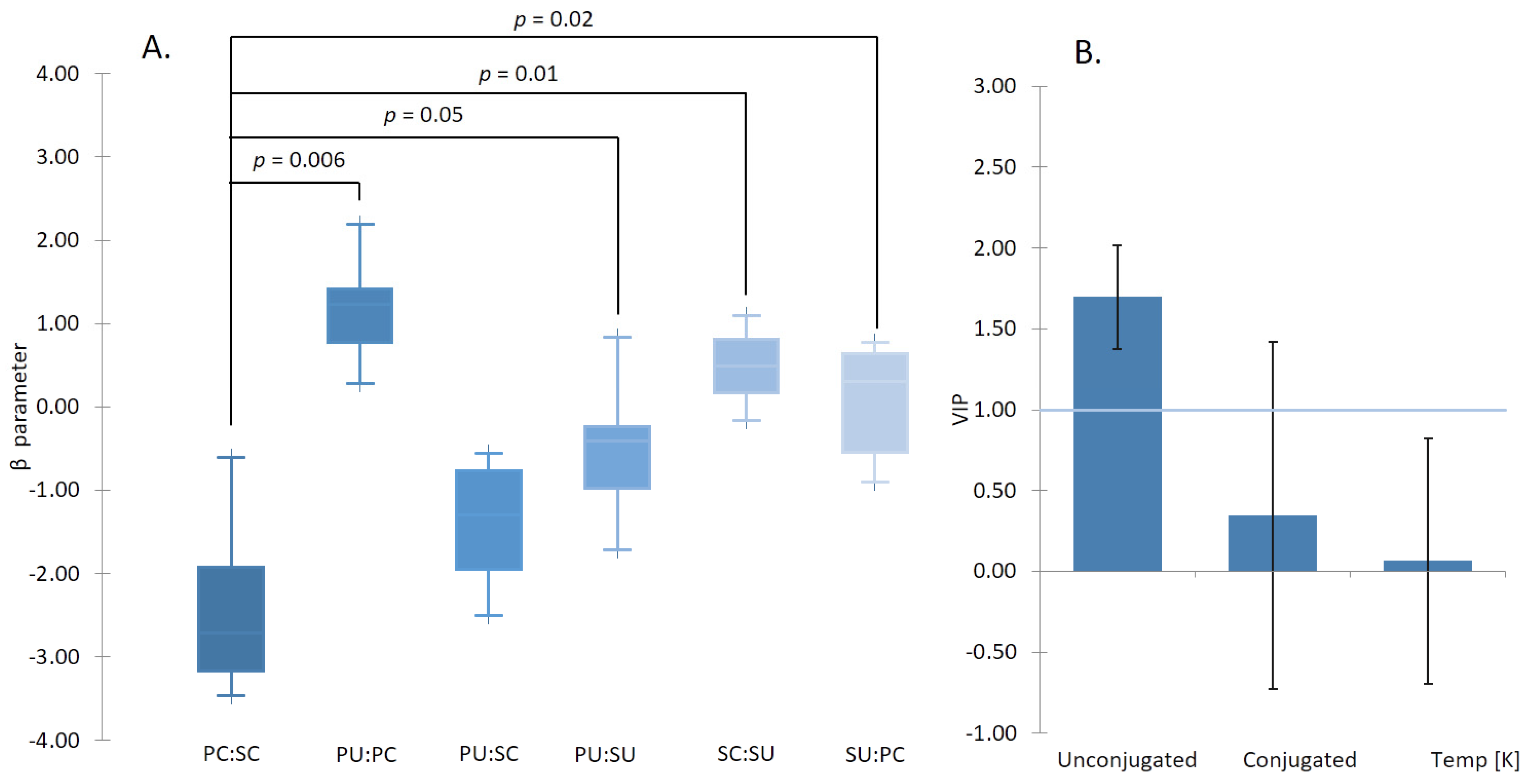
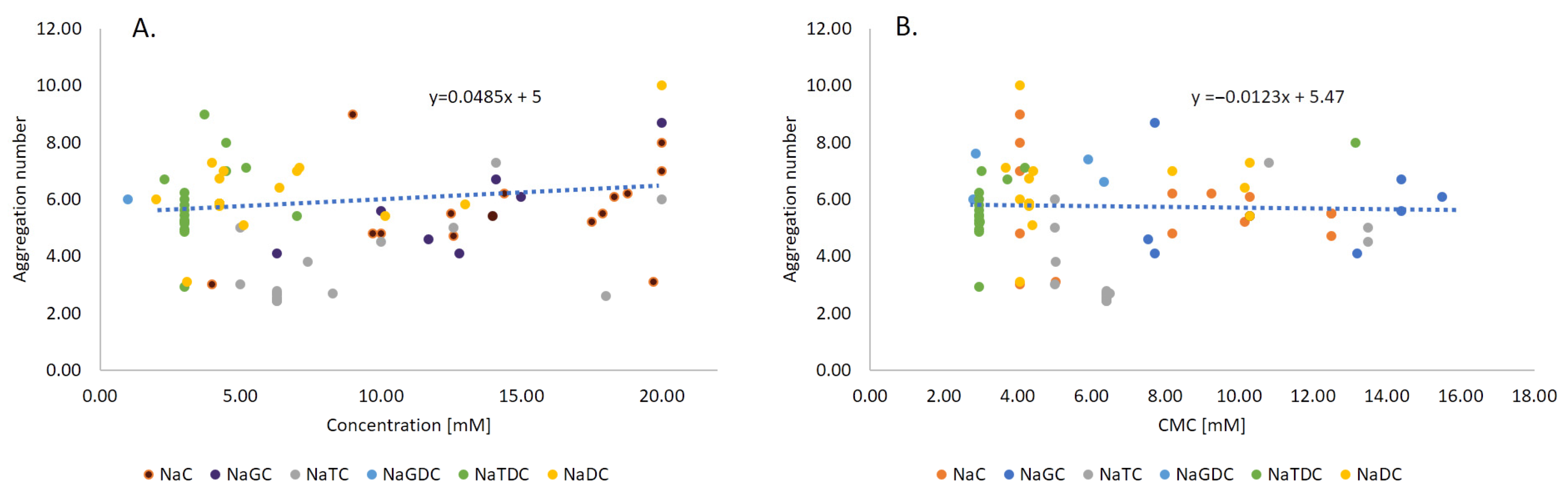
| Duodenum | Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|
| Conjugated [%] | Unconjugated [%] | Ref. | Conjugated [%] | Unconjugated [%] | Ref. | Conjugated [%] | Unconjugated [%] | Ref. |
| 99.70 | 0.30 | [35] | 100.00 | 0.00 | [35] | 88.00 | 11.79 | [36] |
| 94.20 | 5.00 | [35] | 96.50 | 3.50 | [35] | 75.00 | 25.00 | [37] |
| 91.00 | 9.00 | [37] | 84.00 | 15.50 | [37] | |||
| Type of Surfactant | Composition | CMC [mM] | β | References |
|---|---|---|---|---|
| NaC/NaTC | 0.2 | 6.1 | 1.33 | [48] |
| (PU:PC) | 0.4 0.6 0.8 | 8.1 9.18 9.93 | 2.19 1.39 1.48 | [48] |
| NaC/NaDC | 0.2 | 3.6 | –0.40 | [48] |
| (PU:SU) | 0.4 0.6 0.8 | 4.15 4.8 5.41 | −0.31 −0.41 −0.84 | [48] |
| C12TAB/C10TAB | 0.3 | 25.00 | −1.4 | [49] |
| C14TAB/C10TAB | 0.3 | 8.00 | −4.7 | [49] |
| C14TAB/C12TAB | 0.3 | 6.00 | −1.4 | [49] |
| C16TAB/C10TAB | 0.3 | 3.00 | −7.7 | [49] |
| C16TAB/C12TAB | 0.3 | 3.00 | −5.1 | [49] |
| C16TAB/C14TAB | 0.3 | 2.00 | −1.5 | [49] |
| C16Br/C16BzCl | 0.10 0.25 0.5 0.75 0.90 | 13.20 9.33 10.70 22.90 24.10 | −4.24 −4.83 −2.95 −1.27 −1.79 | [50] |
| SOS/SAE2S | −4.98 | [51] |
| Molecule | No. of BS–BS HBs | No. BS–Water HBs |
|---|---|---|
| Primary unconjugated | 0.08 ± 0.13 | 15.00 ± 1.41 |
| Primary conjugated | 0.305 ± 0.35 | 16.50 ± 1.41 |
| Secondary unconjugated | 0.06 ± 0.11 | 13.00 ± 1.41 |
| Secondary conjugated | 0.305 ± 0.30 | 14.00 ± 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łozińska, N.; Jungnickel, C. Importance of Conjugation of the Bile Salt on the Mechanism of Lipolysis. Molecules 2021, 26, 5764. https://doi.org/10.3390/molecules26195764
Łozińska N, Jungnickel C. Importance of Conjugation of the Bile Salt on the Mechanism of Lipolysis. Molecules. 2021; 26(19):5764. https://doi.org/10.3390/molecules26195764
Chicago/Turabian StyleŁozińska, Natalia, and Christian Jungnickel. 2021. "Importance of Conjugation of the Bile Salt on the Mechanism of Lipolysis" Molecules 26, no. 19: 5764. https://doi.org/10.3390/molecules26195764
APA StyleŁozińska, N., & Jungnickel, C. (2021). Importance of Conjugation of the Bile Salt on the Mechanism of Lipolysis. Molecules, 26(19), 5764. https://doi.org/10.3390/molecules26195764








