Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts
Abstract
:1. Introduction
2. Results
2.1. Content of Phenolic Compounds, Crude Saponin and Ginsenoside
2.2. Enzymatic Conversion of PPD Type Ginsenosides to CK
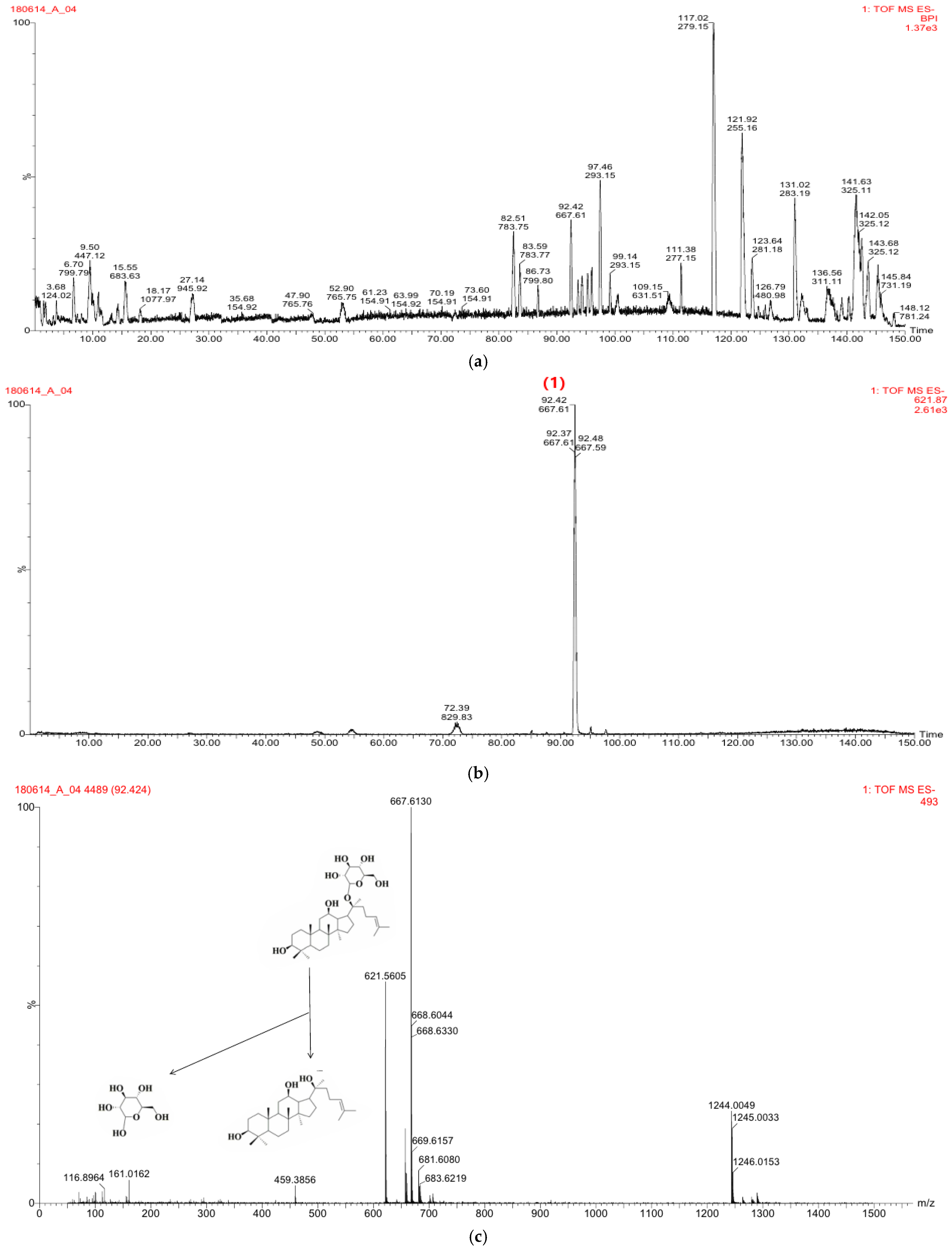
2.2.1. Preparation of CKE
2.2.2. Mass Spectral Fragmentation of CK
2.3. Biological Effects of Compound K Enriched Extract (CKE)
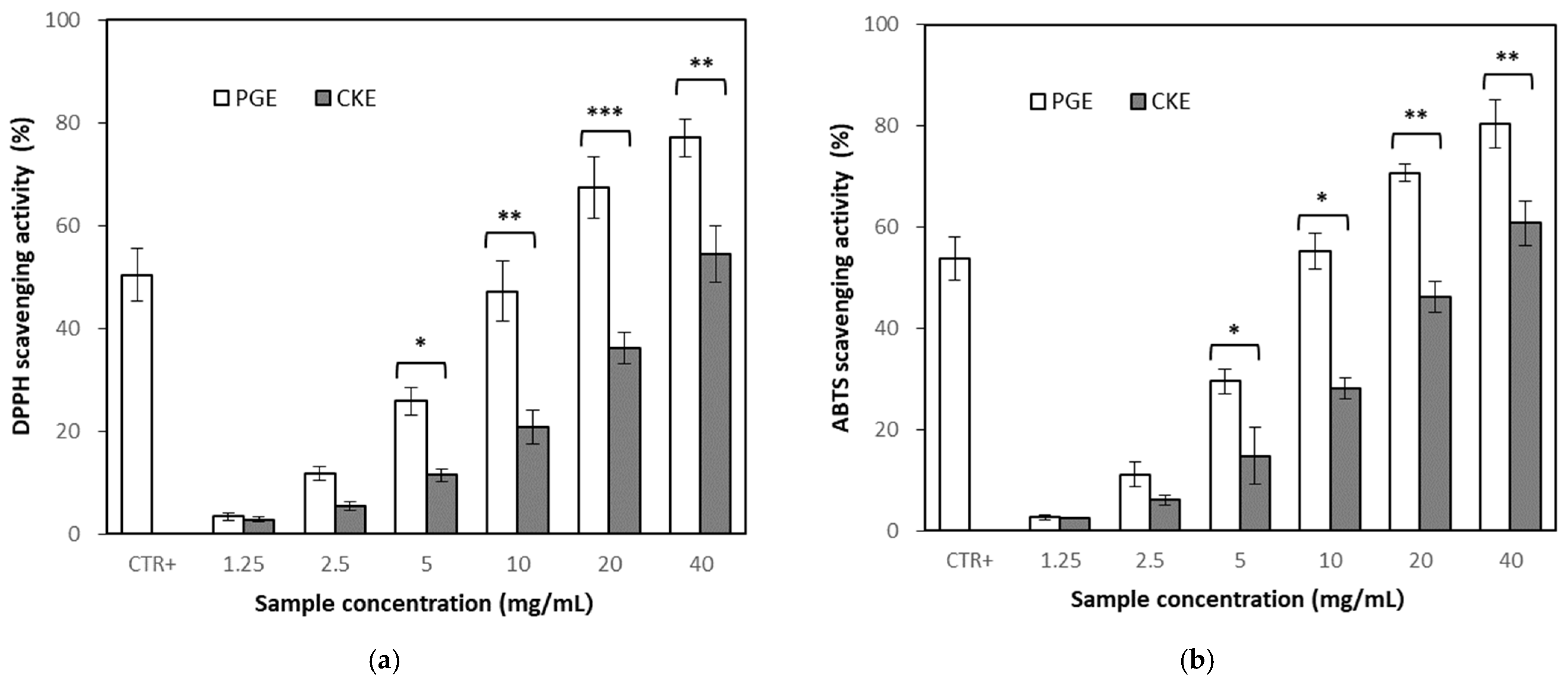
2.3.1. Antioxidant Activity of Partially Purified Ginsenoside Extract (PGE) and CKE
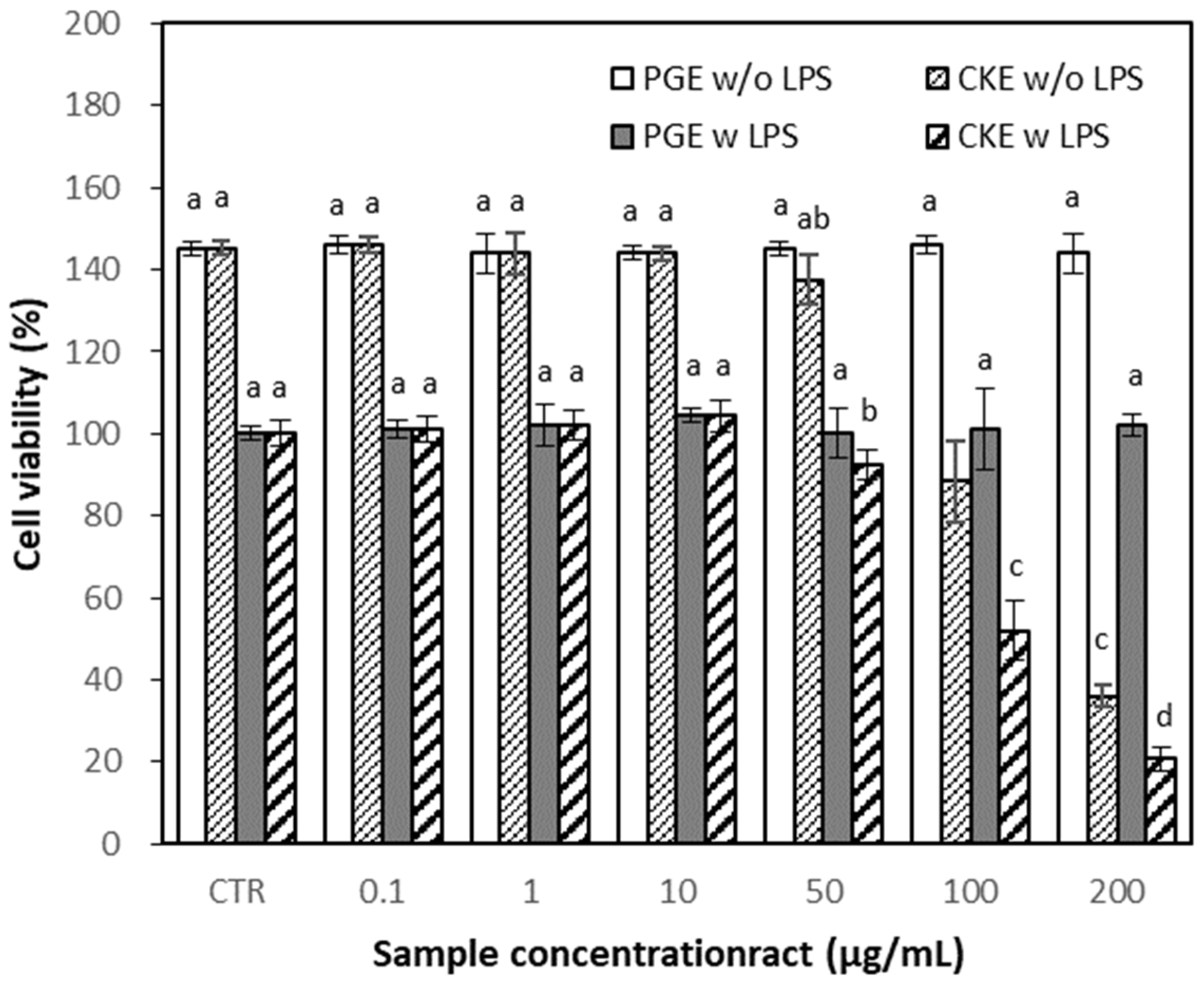
2.3.2. Effects of PGE and CKE on Cell Viability
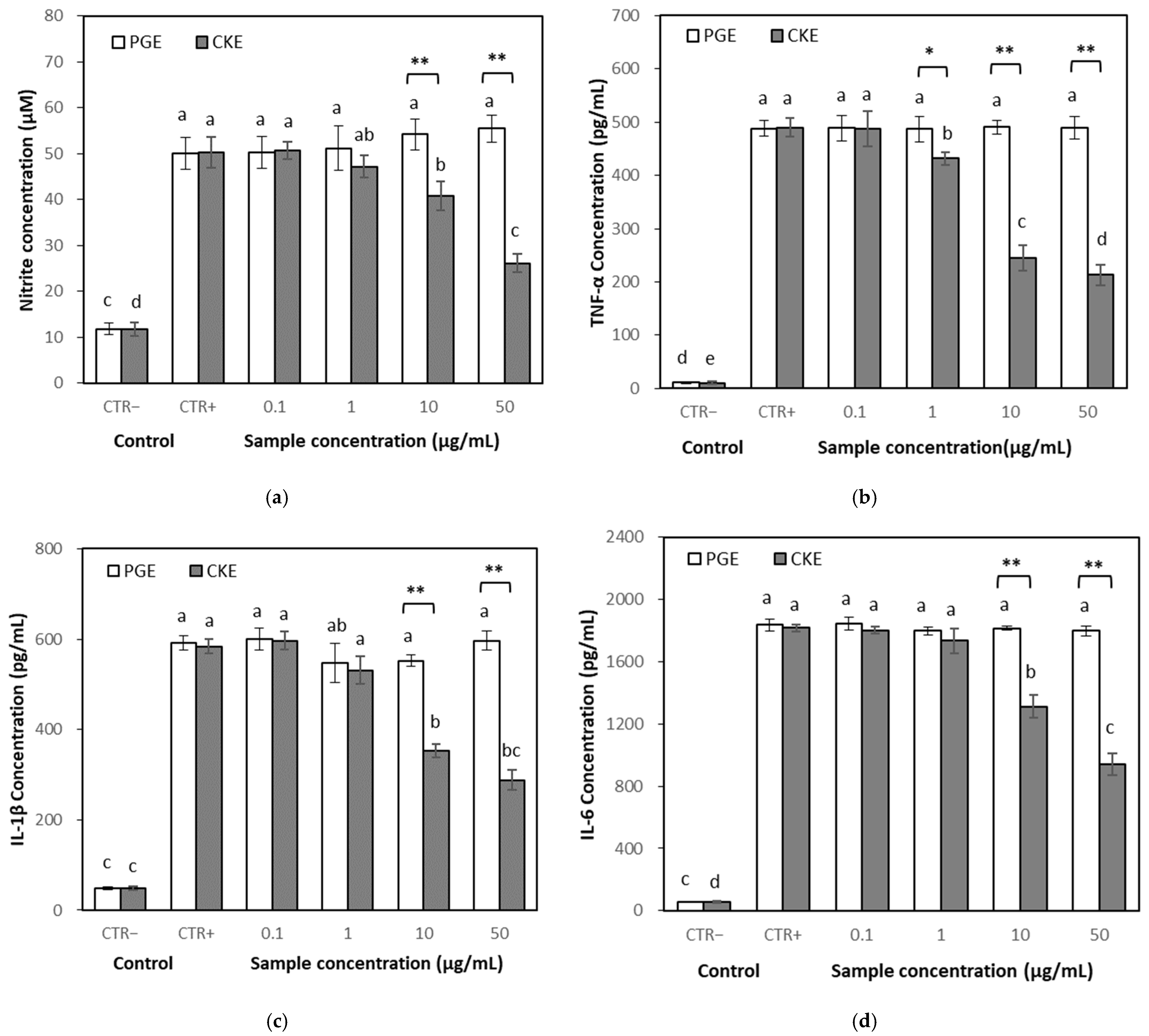
2.3.3. Effects of PGE and CKE on NO Levels
2.3.4. Effects on Inflammatory Cytokines
2.3.5. Bleeding Time and Volume
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extraction
4.2. Chemicals
4.3. Preparation of PGE and CKE
4.4. Analysis of Phenolic Compound, Saponin and Ginsenoside
4.5. Determination of Antioxidant Effects
4.6. Cell Culture and Cytotoxicity Assay
4.7. Determination of Anti-Inflammatory Effects
4.7.1. Determination of NO Levels
4.7.2. Determination of Pro-Inflammatory Cytokines
4.8. Bleeding Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Scaglione, F.; Cattaneo, G.; Alessandria, M.; Cogo, R. Efficacy and safety of the standardised Ginseng extract G115® for potentiating vaccination against common cold and/or influenza syndrome. Drugs Exp. Clin. Res. 1996, 22, 65–72. [Google Scholar] [PubMed]
- Harkey, M.R.; Henderson, G.L.; Gershwin, M.E.; Stern, J.S.; Hackman, R.M. Variability in commercial ginseng products: An analysis of 25 preparations. Am. J. Clin. Nutr. 2001, 73, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.W.; Han, Y.K.; Bae, Y.S.; Lee, S.H. The Disease Severity and Related Pathogens Caused by Root Rot on 6 Years Old Ginseng Cultivation Fields. Korean J. Plant Res. 2019, 32, 144–152. [Google Scholar]
- Kim, G.S.; Lee, S.E.; Noh, H.J.; Kwon, H.; Lee, S.W.; Kim, S.Y.; Kim, Y.B. Effects of Natural Bioactive Products on the Growth and Ginsenoside Contents of Panax ginseng Cultured in an Aeroponic System. J. Ginseng Res. 2012, 36, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Yang, H.; Lee, Y.K.; Lee, C.H.; Seo, J.W.; Kim, J.E.; Kim, S.Y.; Yoon Park, J.H.; Lee, K.W. A short-term, hydroponic-culture of ginseng results in a significant increase in the anti-oxidative activity and bioactive components. Food Sci. Biotechnol. 2020, 29, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Seong, B.J.; Kim, S.I.; Jee, M.G.; Lee, H.C.; Kwon, A.R.; Kim, H.H.; Won, J.Y.; Lee, K.S. Changes in Growth, Active Ingredients, and Rheological Properties of Greenhouse-cultivated Ginseng Sprout during its Growth Period. Korean J. Med. Crop Sci. 2019, 27, 126–135. [Google Scholar] [CrossRef]
- Jang, I.B.; Yu, J.; Suh, S.J.; Jang, I.B.; Kwon, K.B. Growth and Ginsenoside Content in Different Parts of Ginseng Sprouts Depending on Harvest Time. Korean J. Med. Crop Sci. 2018, 26, 205–213. [Google Scholar] [CrossRef]
- Song, J.S.; Jung, S.; Jee, S.; Yoon, J.W.; Byeon, Y.S.; Park, S.; Kim, S.B. Growth and bioactive phytochemicals of Panax ginseng sprouts grown in an aeroponic system using plasma-treated water as the nitrogen source. Sci. Rep. 2021, 11, 2924–2933. [Google Scholar] [CrossRef]
- Kim, S.C.; Kang, Y.M.; Seong, J.A.; Lee, H.Y.; Cho, D.Y.; Joo, O.S.; Lee, J.H.; Cho, K.M. Comprehensive changes of nutritional constituents and antioxidant activities of ginseng sprouts according to the roasting process. Korean J. Food Preserv. 2021, 28, 72–87. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, K.T.; Paik, H.D. Improved Antioxidant, Anti-inflammatory, and Anti-adipogenic Properties of Hydroponic Ginseng Fermented by Leuconostoc mesenteroides. Molecules 2019, 24, 3359. [Google Scholar] [CrossRef] [Green Version]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Kitagawa, I.; Taniyama, T.; Shibuya, H.; Noda, T.; Yoshikawa, M. Chemical studies on crude drug processing. V. on the constituents of ginseng radix rubra: Comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi 1987, 107, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-D.; Yang, Y.-Y.; Ou-Yang, D.-S.; Yang, G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Drug Metabolism: Intestinal Bacterial Hydrolysis is Required for the Appearance of Compound K in Rat Plasma after Oral Administration of Ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.; Kim, D.; Lee, J.; Yoo, J.; Koh, B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J. Ethnopharmacol. 2009, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Lee, H.J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Biswas, T.; Mathur, A.K.; Mathur, A. A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl. Microbiol. Biotechnol. 2017, 101, 4009–4032. [Google Scholar] [CrossRef]
- Cui, C.; Jeon, B.M.; Fu, Y.; Im, W.T.; Kim, S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl. Microbiol. Biotechnol. 2019, 103, 7003–7015. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Park, C.S. Production of Compound K using ginsenosides from ginseng leaf by commercial enzyme. Korean J. Food Preserv. 2019, 26, 703–710. [Google Scholar] [CrossRef]
- Upadhyaya, J.; Kim, M.-J.; Kim, Y.-H.; Ko, S.-R.; Park, H.-W.; Kim, M.-K. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea. J. Ginseng Res. 2015, 40, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Li, T.S.C.; Mazza, G.; Cottrell, A.C.; Gao, L. Ginsenosides in roots and leaves of American ginseng. J. Agric. Food Chem. 1996, 44, 717–720. [Google Scholar] [CrossRef]
- Kang, O.J.; Kim, J.S. Comparison of Ginsenoside Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lyga, Y. Inflammaging in Skin and Other Tissues—The Roles of Complement System and Macrophage. Inflamm. Allergy Drug Targets. 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caterina, R.; D’Ugo, E.; Libby, P. Inflammation and thrombosis—Testing the hypothesis with anti-inflammatory drug trials. J. Thromb. Haemost. 2016, 116, 1012–1021. [Google Scholar]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhang, J.; Zou, L.; Deng, G.; Xu, X.; Wang, F.; Ma, Z.; Zhang, J.; Liu, Y.; et al. C5aR, TNF-α, and FGL2 contribute to coagulation and complement activation in virus-induced fulminant hepatitis. J. Hepatol. 2015, 62, 354–362. [Google Scholar] [CrossRef]
- Page, M.J.; Bester, J.; Pretorius, E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef] [Green Version]
- Grigniani, G.; Maiolo, A. Cytokines and hemostasis. Haematologica 2000, 85, 967–972. [Google Scholar]
- Irfan, M.; Kim, M.; Rhee, M.H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J. Ginseng Res. 2020, 44, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Ana, G.J.; Kim, K.; Ngo, T.; Shin, S.; Bae, O.N.; Lim, K.M.; Chung, J.H. Ginsenoside Rg3, a component of ginseng, induces pro-thrombotic activity of erythrocytes via hemolysis-associated phosphatidylserine exposure. Food Chem. Toxicol. 2019, 131, 110553. [Google Scholar] [CrossRef]
- Day, S.M.; Reeve, J.L.; Myers, D.D.; Fay, W.P. Murine thrombosis models. Thromb. Haemost. 2004, 92, 486–494. [Google Scholar] [CrossRef]
- Liu, Y.; Jennings, N.L.; Dart, A.M.; Du, X.J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J. Exp. Med. 2012, 20, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Antiplatelet strategies. Eur. Heart J. Suppl. 2002, 4, A42–A47. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, E.P.; Burda, B.U.; Williams, S.B.; Guirguis-Blake, J.M.; Evans, C.V. Bleeding risks with aspirin use for primary prevention in adults: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 826–835. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color regents. J. Biol. Chem. 1921, 12, 239–249. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Hamanaka, H.; Odaka, Y. A color reaction of panaxadiol with vanillin and sulfuric acid. Planta Med. 1975, 28, 131–138. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the ues of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ratha, P.; Jhon, D.Y. Increase of Rutin, Quercetin, and Antioxidant Activity during Germinated Buckwheat (Fagopyrum esculentum Moench) Fermentation. Ferment. Technol. 2017, 6, 147. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dejana, E.; Callioni, A.; Quintana, A.; De Gaetano, G. Bleeding time in laboratory animals. II. A comparison of different assay conditions in rats. Thromb. Res. 1979, 15, 191–197. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, M.S. Antiplatelet and antithrombotic activities of methanol extract of Usnea longissimi. Phyto. Res. 2005, 19, 1061–1064. [Google Scholar] [CrossRef]

| Test Group | Part | Phenolic Compound (mg/g dry wt.) | Saponin Content (%, w/w) | Ratio of Dry Weight (%, w/w) |
|---|---|---|---|---|
| Ginseng sprout | Leaf | 26.32 ± 1.78 a | 34.41 ± 0.76 a | 20.44 ± 0.29 b |
| Stem | 5.21 ± 2.02 c | 4.78 ± 0.29 d | 11.81 ± 0.15 c | |
| Root | 6.12 ± 0.93 c | 12.23 ± 0.51 c | 67.84 ± 0.24 a | |
| Total | 10.20 ± 1.51 b | 15.89 ± 0.71 b | - | |
| Six-year-old ginseng root | Root | 6.54 ± 0.35 c | 11.67 ± 0.20 c | - |
| Test Group | Ginsenoside (mg/g Dry wt.) | |||||||
|---|---|---|---|---|---|---|---|---|
| Rb1 | Rb2 | Rc | Rd | F2 | Rg3 | PPD Total | ||
| Ginseng sprout | Leaf | 9.56 ± 0.41 a | 5.54 ± 0.41 a | 9.43 ± 0.12 a | 7.3 ± 90.24 a | 9.45 ± 0.40 a | ND | 41.28 ± 0.22 a |
| Stem | 3.01 ± 0.23 b | 1.34 ± 0.26 b | 1.04 ± 0.25 d | 0.63 ± 0.12 c | 2.92 ± 0.17 b | ND | 8.94 ± 0.21 c | |
| Root | 1.54 ± 0.14 c | 1.04 ± 0.43 b | 3.92 ± 0.33 b | 1.95 ± 0.06 bc | 2.08 ± 0.05 c | ND | 10.53 ± 31 bc | |
| Total | 3.32 ± 0.17 b | 1.98 ± 0.23 b | 4.68 ± 0.18 b | 2.86 ± 0.13 b | 3.66 ± 0.20 ab | ND | 16.64 ± 0.22 ab | |
| Six-year-old ginseng root | 2.52 ± 0.45 b | 1.95 ± 0.14 b | 2.34 ± 0.32 c | 2.58 ± 0.63 b | 2.21 ± 0.10 c | ND | 11.6 ± 0.21 b | |
| Ginsenoside | Ginsenoside Content (μg/mL) | ||
|---|---|---|---|
| Before Reaction | 24 h after Reaction | 48 h after Reaction | |
| Rb1 | 102.51 ± 4.90 | 1.55 ± 0.02 | ND |
| Rc | 125.24 ± 1.92 | 89.85 ± 1.91 | 50.93 ± 0.41 |
| Rb2 | 88.42 ± 5.52 | ND | ND |
| Rd | 95.43 ± 2.23 | 48.31 ± 0.73 | 0.3 ± 0.04 |
| F2 | 97.58 ± 3.54 | 245.60 ± 5.03 | 33.6 ± 0.23 |
| Rg3 | ND | 17.85 ± 0.60 | 53.5 ± 0.91 |
| Compound K (CK) | ND | 79.14 ± 0.95 | 235.71 ± 5.90 |
| Total | 509.01 ± 4.95 | 473.71 ± 1.90 | 373.21 ± 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baik, I.-H.; Kim, K.-H.; Lee, K.-A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules 2021, 26, 4102. https://doi.org/10.3390/molecules26134102
Baik I-H, Kim K-H, Lee K-A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules. 2021; 26(13):4102. https://doi.org/10.3390/molecules26134102
Chicago/Turabian StyleBaik, In-Hee, Kyung-Hee Kim, and Kyung-Ae Lee. 2021. "Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts" Molecules 26, no. 13: 4102. https://doi.org/10.3390/molecules26134102
APA StyleBaik, I.-H., Kim, K.-H., & Lee, K.-A. (2021). Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules, 26(13), 4102. https://doi.org/10.3390/molecules26134102






