Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2
Abstract
1. Introduction
2. Methods
Literature Search
3. Pathogenesis of SARS-CoV-2
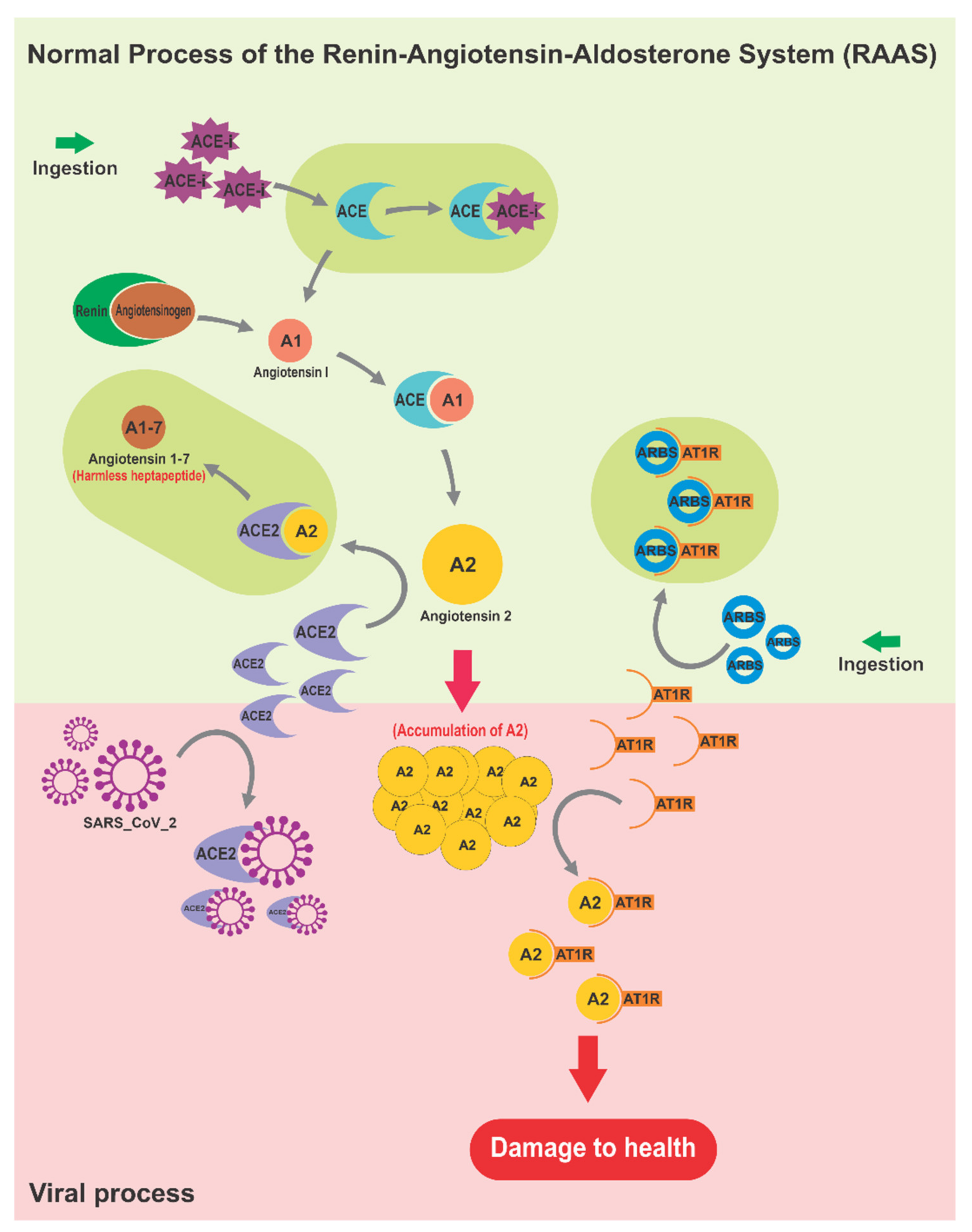
3.1. The Renin–Angiotensin–Aldosterone System as Affected by SARS-CoV-2
3.2. Immune System Boosting Plants and Foods
4. Bioactive Compounds in the Mechanisms of the Virus–Host Interaction
4.1. Entry Inhibitors
4.2. Protease Inhibitors
4.3. Replication Inhibitors
4.4. Virucidal Activity
4.5. Immunomodulatory Agents
4.6. Regulators of RAAS
4.7. Unknown Mechanisms of Action
5. Risks Associated with the Incorrect Use of Natural Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redeploying Plant Defences. Nat. Plants 2020, 6, 177. [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Khalili, S.A.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet Infect. Dis. 2020, 20, 238–244. [Google Scholar] [CrossRef]
- Fani, M.; Teimoori, A.; Ghafari, S. Comparison of the COVID-2019 (SARS-CoV-2) Pathogenesis with SARS-CoV and MERS-CoV Infections. Future Virol. 2020, 15, 317–323. [Google Scholar] [CrossRef]
- Caldaria, A.; Conforti, C.; Di-Meo, N.; Dianzani, C.; Mohammad, J.; Torello, L.; Zalaudek, I.; Giuffrida, R. COVID-19 and SARS: Differences and Similarities. Dermatol. Ther. 2020, e13395. [Google Scholar] [CrossRef]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The Reproductive Number of COVID-19 is Higher Compared to SARS Coronavirus. J. Travel Med. 2020, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S. Another Decade, Another Coronavirus. N. Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are They Closely Related? Clin. Microbiol. Infec. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Lee, P.; Hu, Y.; Chen, P.; Huang, Y.; Hsueh, P. Are Children Less Susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020, 53, 371–372. [Google Scholar] [CrossRef]
- Guerra, E. Recorte a la Educación Superior: Una Medida que Ahondará la Crisis—Opción S. Revista S. 2020. Available online: https://opcions.ec/portal/2020/05/08/la-educacion-publica-y-el-recorte-presupuestario/ (accessed on 8 May 2020).
- EMA. Treatments and Vaccines for COVID-19. European Medicines Agency. 2020. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19 (accessed on 8 November 2020).
- ECDC. Vaccines and Treatment of COVID-19. European Centre for Disease Prevention and Control. 2020. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/treatment (accessed on 5 May 2021).
- Mastroleo, I. Post-trial Obligations in the Declaration of Helsinki 2013: Classification, Reconstruction and Interpretation. Dev. World Bioeth. 2016, 16, 80–90. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.; Khan, R.; Alagawany, M.; Farag, M. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/Extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Tahir, I.; Shah, S.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral Potential of Medicinal Plants against HIV, HSV, Influenza, Hepatitis, and Coxsackievirus: A Systematic Review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Siddiqui, A.; Danciu, C.; Ashraf, S.; Moin, A.; Singh, R.; Alreshidi, M.; Patel, M.; Jahan, S.; Kumar, S.; Alkhinjar, M. Plants-Derived Biomolecules as Potent Antiviral Phytomedicines: New Insights on Ethnobotanical Evidences against Coronaviruses. Plants 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Sahu, U.; Pandey, S.; Samant, M. Current Approaches for Target-Specific Drug Discovery Using Natural Compounds against SARS-CoV-2 Infection. Virus Res. 2020, 290, 198169. [Google Scholar] [CrossRef]
- Tahir, M.; Alqahtani, S.; Alamri, M.; Chen, L. Structural Basis of SARS-CoV-2 3CLpro and Anti-COVID-19 Drug Discovery from Medicinal Plants. J. Pharm. Anal. 2020, 1, 313–319. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, K.; Zhang, X.; Deng, S.; Peng, B. In Silico Screening of Chinese Herbal Medicines with the Potential to Directly Inhibit 2019 Novel Coronavirus. J. Integr. Med. 2020, 18, 152–158. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.; Albrich, W.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann, R.; Lenz, N.; Siegrist, D.; Suter, A. In Vitro Antiviral Activity of Echinaforce®, an Echinacea purpurea Preparation, against Common Cold Coronavirus 229E and Highly Pathogenic MERS-CoV and SARS-CoV. Virol. J. 2020, 10, 2. [Google Scholar]
- Tallei, T.; Tumilaar, S.; Niode, N.; Fatimawali, K.; Johnson, B.; Idroes, R.; Effendi, Y.; Sakib, S.; Emran, T. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Islam, M.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van-Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van-Ranst, M. Plant Lectins are Potent Inhibitors of Coronaviruses by Interfering with Two Targets in the Viral Replication Cycle. Antivir. Res. 2007, 75, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Agrawal, C.; Blunden, G. Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar]
- Olayeriju, O.; Crown, O.; Akinmoladun, A.; Kolawole, A.; Olaleye, M.; Akindahunsi, A. Onions tunic: A Flavonol Rich Competitive Inhibitor of Key Enzyme (Angiotensin-1 Converting Enzyme) Linked Hypertension. Int. J. Sci. Eng. Res. 2017, 8, 2229–5518. [Google Scholar]
- Lin, C.; Tsai, F.; Tsai, C.; Lai, C.; Wan, L.; Ho, T.; Hsieh, C.; Chao, P. Anti-SARS Coronavirus 3C-Like Protease Effects of Isatis indigotica Root and Plant-Derived Phenolic Compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, H.; Kang, S.; Chung, H.; Choi, J. Inhibitory Activities of Cassia tora and its Anthraquinone Constituents on Angiotensin—Converting Enzyme. Phyther. Res. 2009, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K. Coronaviruses and Nature’s Pharmacy for the Relief of Coronavirus Disease 2019. Rev. Bras. Farmacogn. 2020, 30, 603–621. [Google Scholar] [CrossRef]
- Zhuang, M.; Jiang, H.; Suzuki, Y.; Li, X.; Xiao, P.; Tanaka, T.; Ling, H.; Yang, B.; Saitoh, H.; Zhang, L. Procyanidins and Butanol Extract of Cinnamomi Cortex Inhibit SARS-CoV Infection. Antivir. Res. 2009, 82, 73–81. [Google Scholar] [CrossRef]
- Polansky, H.; Lori, G. Coronavirus Disease 2019 (COVID-19): First Indication of Efficacy of Gene-Eden-VIR/Novirin in SARS-CoV-2 Infection. Int. J. Antimicrob. Agents 2020, 55, 105971. [Google Scholar] [CrossRef]
- Temitope, A.; Eleojo, C.; Abiodun, I.; Ayokunnun, A.; Saheed, S. Phytotherapeutic Evidence against Coronaviruses and Prospects for COVID-19. Pharmacogn. J. 2020, 12, 1252–1267. [Google Scholar]
- Shetty, R.; Ghosh, A.; Honavar, S.; Khamar, P.; Sethu, S. Therapeutic Opportunities to Manage COVID-19/SARS-CoV-2 Infection: Present and Future. Indian J. Ophthalmol. 2020, 68, 693–702. [Google Scholar]
- Oladele, J.O.; Ajayi, E.I.; Oyeleke, O.M.; Oladele, O.T.; Olowookere, B.D.; Adeniyi, B.M.; Oyewole, O.I.; Oladiji, A.T. A Systematic Review on COVID-19 Pandemic with Special Emphasis on Curative Potentials of Nigeria Based Medicinal Plants. Heliyon 2020, 6, e04897. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.-M.; Yu, X.-H.; Tang, S.-L.; Tang, C.-K. Coronavirus Disease 2019 (COVID-19): Current Status and Future Perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin is a Powerful Inhibitor of SARS-CoV-2 Infection In vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A Native Nource of Antimicrobial Agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; Head, R.J.; Bennett, L.E. Processed Dietary Plants Demonstrate Broad Capacity for Angiotensin Converting Enzyme and Angiotensin II Receptor Binding Inhibition In Vitro. J. Funct. Foods 2012, 4, 851–863. [Google Scholar] [CrossRef]
- Kim, H.; Shin, H.; Park, H.; Kim, Y.; Yun, Y.; Park, S.; Shin, H.; Kim, K. In Vitro Inhibition of Coronavirus Replications by the Traditionally Used Medicinal Herbal Extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Schapowal, A. Use of Echinaforce to Prevent Coronavirus Infections. Switzerland. 2020. Available online: https://www.who.int/emergencies/ (accessed on 31 December 2019).
- Engler, O.; Strasser, M.; Signer, J.; Schoop, R. Neutralizing Activity of Echinacea purpurea on Coronaviruses Including Highly Pathogenic Middle-East-Respiratory Syndrome Virus (MERS-CoV). Planta Med. Int. Open 2017, 4, 1–202. Available online: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0037-1608557 (accessed on 24 October 2017).
- Banyeres, M. Herbario Virtual de Banyeres de Mariola y Alicante. 2010. Available online: http://herbariovirtualbanyeres.blogspot.com/2010/05/morus-alba-morera-morer.html (accessed on 26 May 2020).
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of SARS-Associated Coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Baltina, L.; Zarubaev, V.; Baltina, L.; Orshanskaya, I.; Fairushina, A.; Kiselev, O.; Yunusov, M. Glycyrrhizic Acid Derivatives as Influenza A/H1N1 Virus Inhibitors. Bioorganic Med. Chem. Lett. 2015, 25, 1742–1746. [Google Scholar] [CrossRef]
- Cheng, P.; Ng, L.; Chiang, L.; Lin, C. Antiviral Effects of Saikosaponins on Human Coronavirus 229E In vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.; Jiang, Y.; Kao, R.; Lu, H.; Fan, K.; Cheng, V.; Tsui, W.; Hung, I.; Lee, T. In Vitro Susceptibility of 10 Clinical Isolates of SARS Coronavirus to Selected Antiviral Compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, Y.; Wandersee, M.; Smith, A.; Zhou, Y.; Simmons, G.; Nelson, N.; Bailey, K.; Vest, Z.; Li, J.; Chan, P. Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication in a Lethal SARS-CoV BALB/c Mouse Model by Stinging Nettle Lectin, Urtica dioica Agglutinin. Antivir. Res. 2011, 90, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Quan, Q.; Zhou, X.; Zhu, Y.; Lan, Y.; Li, S.; Yu, Y.; Cheng, Z. A Comparative Study of Lonicera japonica with Related Species: Morphological Characteristics, ITS Sequences and Active Compounds. Biochem. Syst. Ecol. 2014, 54, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Zhang, H.; Guo, H.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.; Yu, J.; Xiao, P. Identification of Natural Compounds with Antiviral Activities against SARS-Associated Aoronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Cho, J.; Curtis-Long, M.; Lee, K.; Kim, D.; Ryu, H.; Yuk, H.; Park, K. Geranylated Flavonoids Displaying SARS-CoV Papain-Like Protease Inhibition from the Fruits of Paulownia tomentosa. Bioorganic Med. Chem. 2013, 21, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.; Cinatl, J. Investigation of the Influence of EPs® 7630, a Herbal Drug Preparation from Pelargonium sidoides, on Replication of a Broad Panel of Respiratory Viruses. Phytomedicine 2011, 18, 384–386. [Google Scholar] [CrossRef]
- Theisen, L.L.; Muller, C.P. EPs® 7630 (Umckaloabo®), an Extract from Pelargonium sidoides Roots, Exerts Anti-influenza Virus Activity In Vitro and In Vivo. Antivir. Res. 2012, 94, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Van, J. Medicinal Properties and Conservation of Pelargonium sidoides DC. J. Ethnopharmacol. 2014, 152, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hsu, W.; Lin, C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24. [Google Scholar] [CrossRef]
- Yu, M.S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.W.; Jee, J.G.; Keum, Y.S.; Jeong, Y.J. Identification of Myricetin and Scutellarein as Novel Chemical Inhibitors of the SARS Coronavirus Helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.; Saab, A.; Tundis, R.; Statti, G.; Menichimi, F.; Lampronti, D.; Gambari, R.; Cinatl, J.; Doerr, H. Phytochemical Analysis and In Vitro Antiviral Activities of the Essential Oils of Seven Lebanon Species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; Bennett, L.E. Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Crit. Rev. Food Sci. Nutr. 2016, 56, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.; Semwal, R.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Zhao, W.; Wen, R.; Meng, Q.; Zeng, Y. Synthesis of Stilbene Derivatives with Inhibition of SARS Coronavirus Replication. Eur. J. Med. Chem. 2006, 41, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ho, C.; Chuo, W.; Li, S.; Wang, T.; Lin, C. Effective Inhibition of MERS-CoV Infection by Resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, S.; Bhatt, M.L.; Saxena, S.K. Therapeutic Development and Drugs for the Treatment of COVID-19. In Coronavirus Disease 2019 (COVID-19); Nature Publishing Group: Berlin, Germany, 2020; pp. 109–126. [Google Scholar]
- Palit, P.; Chattopadhyay, D.; Thomas, S.; Kundu, A.; Kim, H.; Rezaei, N. Phytopharmaceuticals Mediated Furin and TMPRSS2 Receptor Blocking: Can It Be a Potential Therapeutic Option for Covid-19? Phytomedicine 2020, 85, 153396. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A. Solomon SD. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Christy, M.; Uekusa, Y.; Gerwick, L.; Gerwick, W. Natural Products with Potential to Treat RNA Virus Pathogens Including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Manzano, P.; Peñarreta, J.; Chóez, I.; Barragán, A.; Orellana, A.; Rastrelli, L. Potential Bioactive Compounds of Medicinal Plants against New Coronavirus (SARS-CoV-2): A Review. Bionatura 2020. [Google Scholar] [CrossRef]
- Balslev, H.; Navarrete, H.; Torre, L.; Macía, M. Enciclopedia de Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia, Universidad Católica del Ecuador: Quito, Ecuador, 2008; pp. 1–323. [Google Scholar]
- León, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, H.; Navarrete, C. Libro Rojo de las Plantas Endémicas del Ecuador, 2nd ed.; Pontifica Universidad Católica del Ecuador: Quito, Ecuador, 2011; pp. 1–440. [Google Scholar]
- Sut, S.; Maggi, F.; Dall’Acqua, S. Bioactive Secondary Metabolites from Orchids (Orchidaceae). Chem. Biodivers. 2017, 14, e1700172. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, P.; Escaleras, R. La Medicina Tradicional en el Ecuador: Memorias de las Primeras Jornadas Ecuatorianas de Etnomedicina Andina; Universidad Andina Simón Bolívar: Quito, Ecuador, 2002; pp. 1–192. [Google Scholar]
- Selmi, C.; Ansari, A.; Invernizzi, P.; Podda, M.; Gershwin, E. The Search for a Practical Approach to Emerging Diseases: The Case of Severe Acute Respiratory Syndrome (SARS). Dev. Immunol. 2002, 9, 113–117. [Google Scholar] [CrossRef]
- Zhong, N. Management and Prevention of SARS in China. R. Soc. 2004, 359, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Lau, S.; Woo, P.; Yuen, K. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef]
- Ruiz, M.; Ruperez, M.; Lorenzo, O.; Esteban, V.; Blanco, J.; Mezzano, S.; Egido, J. Angiotensin II Regulates the Synthesis of Proinflammatory Cytokines and Chemokines in the Kidney. Kidney Int. Suppl. 2002, 62, 12–22. [Google Scholar] [CrossRef]
- Carlson, S.H.; Wyss, J.M. Mechanisms Underlying Hypertension and Obesity. Hypertension 2011, 57, 375–376. [Google Scholar] [CrossRef][Green Version]
- Valverde, F.D.M. Plantas Utiles del Litoral Ecuatoriano; Fundación Ecuatoriana de Estudios Ecológicos: Quito, Ecuador, 1998. [Google Scholar]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Sawalha, A.; Zhao, M.; Coit, P.; Lu, Q. Epigenetic Dysregulation of ACE2 and Interferon-regulated Genes Might Suggest Increased COVID-19 Susceptibility and Severity in Lupus Patients. Clin. Immunol. 2020, 215, 108410. [Google Scholar] [CrossRef]
- Remkova, A.; Remko, M. The Renin-Angiotensin-Aldosterone System and Prothrombotic State in Arterial Hypertension. Salud Cienc. 2011, 18, 220–224. [Google Scholar]
- Ayada, C.; Toru, Ü.; Korkut, Y. The Relationship of Stress and Blood Pressure Effectors. Hippokratia 2015, 19, 99. [Google Scholar] [PubMed]
- Gurwitz, D. Angiotensin Receptor Blockers as Tentative SARS-CoV-2 Therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.C.; Sakima, A.; Kasper, S.O.; Vinsant, S.; Garcia, M.A.; Diz, D.I. The Brain Renin-Angiotensin System and Cardiovascular Responses to Stress: Insights from Transgenic Rats with Low Brain Angiotensinogen. J. Appl. Physiol. 2012, 113, 1929–1936. [Google Scholar] [CrossRef]
- Guo, F.; Chen, X.L.; Wang, F.; Liang, X.; Sun, Y.X.; Wang, Y.J. Role of Angiotensin II Type 1 Receptor in Angiotensin II-Induced Cytokine Production in Macrophages. J. Interferon Cytokine Res. 2011, 31, 351–361. [Google Scholar] [CrossRef]
- Engeli, S.; Schling, P.; Gorzelniak, K.; Boschmann, M.; Janke, J.; Ailhaud, G.; Teboul, M.; Massiéra, F.; Sharma, A. The Adipose-Tissue Renin-Angiotensin-Aldosterone System: Role in the Metabolic Syndrome? Int. J. Biochem. Cell Biol. 2003, 35, 807–825. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.; Kaur, R.; Bhardwaj, A.; Bhardwaj, V.; Ohri, P.; Sharma, A.; Ahmad, A.; Bhardwaj, R.; Ahmad, P. Herbal Immune-Boosters: Substantial Warriors of Pandemic Covid-19 Battle. Phytomedicine 2021, 85, 153361. [Google Scholar] [CrossRef]
- Malinowska, M.; Sikora, E.; Ogonowski, J. Production of Triterpenoids with Cell and Tissue Cultures. Acta. Biochim. Pol. 2013, 60, 731–735. [Google Scholar] [CrossRef]
- Teissier, E.; Penin, F.; Pécheur, E. Targeting Cell Entry of Enveloped Viruses as an Antiviral Strategy. Molecules 2011, 16, 221–250. [Google Scholar] [CrossRef]
- Fedoung, E.F.; Biwole, A.B.; Biyegue, C.F.N.; Tounkam, M.N.; Ntonga, P.A.; Nguiamba, V.P.; Essono, D.M.; Funwi, P.F.; Tonga, C.; Nguenang, G.M.; et al. A Review of Cameroonian Medicinal Plants with Potentials for the Management of the COVID-19 Pandemic. Adv. Tradit. Med. 2021, 1–26. [Google Scholar] [CrossRef]
- Walter, T.; Justinraj, S.; Justinraj, C.; Nandini, V. Effect of Nilavembu Kudineer in the Prevention and Management of COVID-19 by Inhibiting ACE2 Receptor. Siddha Pap. 2020. Available online: www.siddhapapers.org (accessed on 21 December 2020).
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis Extract and Baicalein Inhibit Replication of SARS-CoV-2 and its 3C-like Protease In Vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Maiti, S.; Banerjee, A. Epigallocatechin Gallate and Theaflavin Gallate Interaction in SARS-CoV-2 Spike-protein Central Channel with Reference to the Hydroxychloroquine Interaction: Bioinformatics and Molecular Docking Study. Drug Dev. Res. 2021, 82, 86–96. [Google Scholar] [CrossRef]
- Ngwa, W.; Kumar, R.; Thompson, D.; Lyerly, W.; Moore, R.; Reid, T.E.; Lowe, H.; Toyang, N. Potential of Flavonoid-Inspired Phytomedicines against COVID-19. Molecules 2020, 25, 2707. [Google Scholar] [CrossRef]
- Chen, C.; Lin, C.; Huang, K.; Chen, W.; Hsieh, H.; Liang, P.; Hsu, J. Inhibition of SARS-CoV 3C-Like Protease Activity by Theaflavin-3,3′- Digallate (TF3). Evid. Based Complementary Altern. Med. 2005, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Musarra, M.; Pennisi, R.; Ben, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Schötz, K.; Nöldner, M. Mass Spectroscopic Characterisation of Oligomeric Proanthocyanidins Derived from an Extract of Pelargonium sidoides Roots (EPs® 7630) and Pharmacological Screening in CNS Models. Phytomedicine 2007, 14, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Twilley, D.; Esmear, T.; Oosthuizen, C.; Reid, A.M.; Nel, M.; Lall, N. Anti-SARS-CoV Natural Products with the Potential to Inhibit SARS-CoV-2 (COVID-19). Front. Pharmacol. 2020, 11, 1514. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S. Natural Products as Potential Leads Against Coronaviruses: Could They be Encouraging Structural Models Against SARS-CoV-2? Nat. Prod. Bioprospecting 2020, 10, 171–186. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann, R.; Lenz, N.; Siegrist, D.; Suter, A. In Vitro Virucidal Activity of Echinaforce®, an Echinacea purpurea Preparation, against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef]
- Meeran, M.N.; Javed, H.; Sharma, C.; Goyal, S.N.; Kumar, S.; Jha, N.K.; Ojha, S. Can Echinacea be a Potential Candidate to Target Immunity, Inflammation, and Infection—The Trinity of Coronavirus Disease 2019. Heliyon 2021, 7, e05990. [Google Scholar] [CrossRef]
- Grant, W.; Lahore, H.; McDonnell, S.; Baggerly, C.; French, C.; Aliano, J.; Bhattoa, H. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved During an Episode of Common Colds—Practical Advice on Dosages. Evid. Based Complementary Altern. Med. 2018, 2018, 5813095. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Yuen, K.Y. The Management of Coronavirus Infections with Particular Reference to SARS. J. Antimicrob. Chemother. 2008, 62, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, Antimicrobial, and Molecular Characterization of Mortiño (Vaccinium floribundum Kunth) Fruits and Leaves. Food Sci. Nutr. 2018, 6, 934–942. [Google Scholar] [CrossRef]
- Hidalgo, M.; Martin, S.; Recio, I.; Sanchez, C.; De Pascual, B.; Rimbach, G.; De Pascual, S. Potential Anti-inflammatory, Anti-adhesive, Anti/Estrogenic, and Angiotensin-Converting Enzyme Inhibitory Activities of Anthocyanins and their Gut Metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Fauzi, M.; Khairul Ikram, N.K.; Mohd Gazzali, A.; Wahab, H.A. Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef]
- Thabti, I.; Albert, Q.; Philippot, S.; Dupire, F.; Westerhuis, B.; Fontanay, S.; Risler, A.; Kassab, T.; Elfalleh, W.; Aferchichi, A.; et al. Advances on Antiviral Activity of Morus spp. Plant Extracts: Human Coronavirus and Virus-related Respiratory Tract Infections in the Spotlight. Molecules 2020, 25, 1876. [Google Scholar] [CrossRef]
- Xian, Y.; Zhang, J.; Bian, Z.; Zhou, H.; Zhang, Z.; Lin, Z.; Xu, H. Bioactive Natural Compounds against Human Coronaviruses: A Review and Perspective. Acta Pharm. Sin. B 2020, 10, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.; Wong, A.; Kaewpreedee, P.; Sia, S.; Chen, D.; Hui, K.; Chu, D.; Chan, M.; Cheung, P.; Huang, X. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication In vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S. Shedding Light on the Effect of Natural Anti-Herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19. Viruses 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Valadão, A.L.; Abreu, C.M.; Días, J.Z.; Arantes, P.; Verli, H.; Tanuri, A.; De Aguiar, R.S. Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules 2015, 20, 11474–11489. [Google Scholar] [CrossRef]
- Bian, Y.; An, G.J.; Kim, K.; Ngo, T.; Shin, S.; Bae, O.N.; Lim, K.M.; Chung, J.H. Ginsenoside Rg3, a Component of Ginseng, Induces Pro-thrombotic Activity of Erythrocytes Via Hemolysis-associated Phosphatidylserine Exposure. Food Chem. Toxicol. 2019, 131, 110553. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S. Herbal Drugs and Phytopharmaceuticals, 3rd ed.; American Chemical Society: London, UK, 2004; pp. 1774–1775. [Google Scholar]
- Efferth, T.; Banerjee, M.; Paul, N.; Abdelfatah, S.; Arend, J.; Elhassan, G.; Hamdoun, S.; Hamm, R.; Hong, C.; Kadioglu, O. Biopiracy of Natural Products and Good Bioprospecting Practice. Phytomedicine 2016, 23, 166–173. [Google Scholar] [CrossRef]
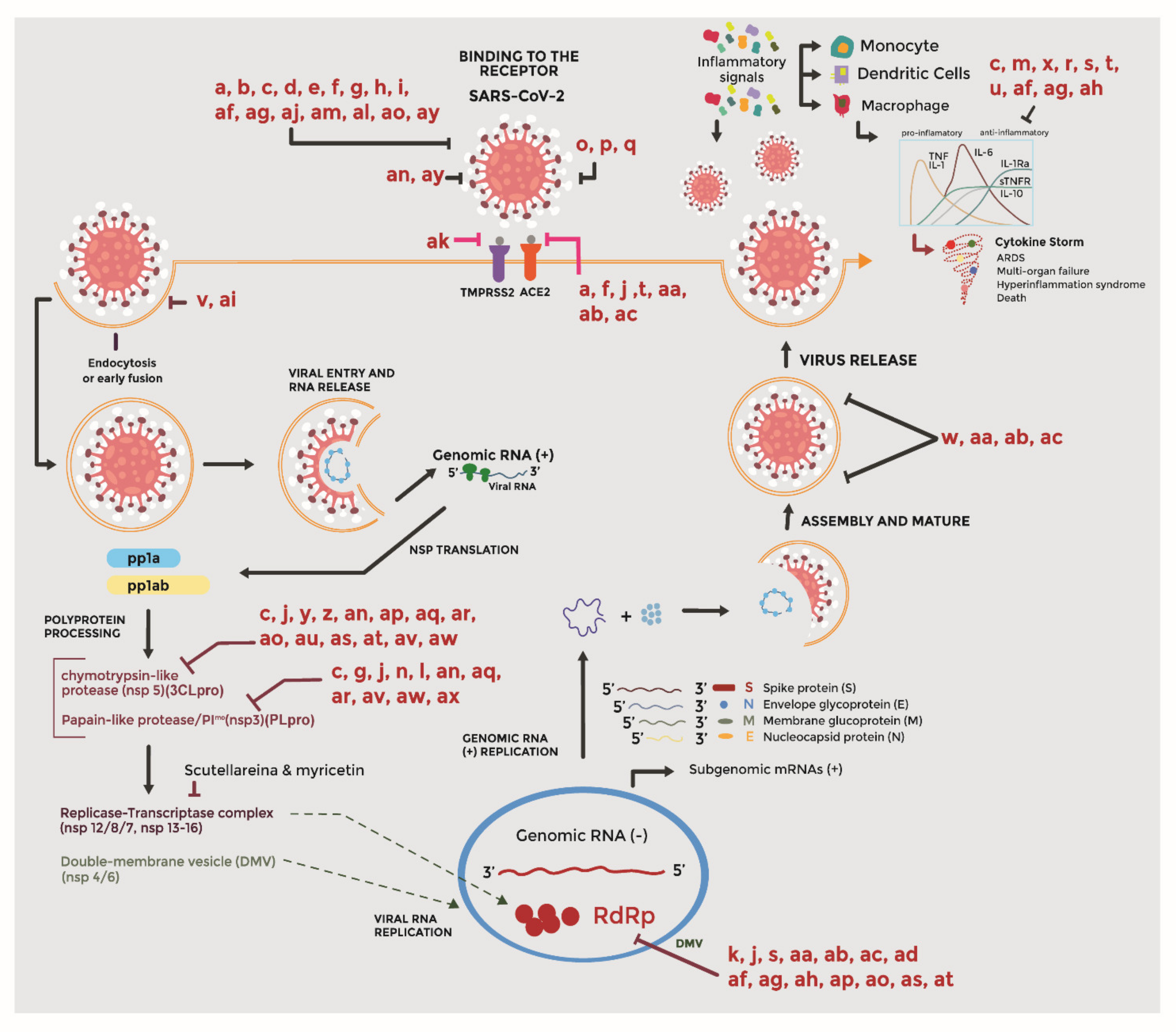
| Species | Receptor | Incubation Period | RO | Case Fatality Rate | References |
|---|---|---|---|---|---|
| SARS-CoV-2 | ACE2 | 1 to 14 days | 3.28 | 3.4 | [3,5,7] |
| SARS-CoV | ACE2 | 2 to 10 days | 1.7–1.9 | 9.6 | [4,8] |
| MERS-CoV | DPP4 | 0.9 | 35 | [2] |
| Scientific/Common Name | Active Principle | Virus/ Antiviral Activity | Reference |
|---|---|---|---|
| Aesculus hippocastanum CN: Horse-chestnut | Aescin (k) | SARS-CoV/Inhibits viral replication | [22] |
| Allium ampeloprasum Var. porrum J. Gay CN: Leek | Mannose-binding specific lectin (b) | SARS-CoV/Ability to bind to the glycosylated molecules found on the surface of viruses, including the spike glycoprotein | [23] [24] |
| Allium cepa L. CN: Onion | Flavonols: quercetin, quercetinglycosides (isoquercitrin, quercitrin and rutin) (c) and kaempferol (j) | SARS-CoV2/Interfere with various stages of the coronavirus entry and replication cycle such as PLpro, 3CLpro, and NTPase/helicase; Inhibits ACE by competing with the substrate, N-[3-(2-furyl) acryloyl]-L-phenylalanylglycylglycine | [25] [26] |
| Brassica oleracea L. CN: Broccoli | Glucosinolate type sinigrin (z) | SARS-CoV/blocks the cleavage process of 3CLpro | [27] [28] |
| Bupleurum spp. CN: Bupleurum | Oleanane-type saikosaponins (aj) | SARS-CoV/Inhibit human coronavirus entry into cells, general replication, and specific 3CLpro mediated replication | [29] |
| Cassia tora L. CN: | Anthraquinone derived emodin (a) | Inhibitory activities on angiotensin-converting enzyme. | [28] |
| Cinnamomumverum J. Presl CN: Cinnamon (cortex) | Butanol (v), procyanidins (ai) | SARS-CoV/Possibly blocks the entry of cells through endocytosis | [30] [31] [32] |
| Curcuma spp. CN: Turmeric | Curcumin (y), Eugenol (an) | SARS-CoV/Inhibits 3CLpro (y); Good binding affinity with Mpro and S protein (an) | [33] [34] |
| Citrus spp. CN: Three main species in the country: Citrus maxima (Rumph. ex Burm.) Merr; Citrus medica L.; Citrus reticulata Blanco. | Hesperetin (f) and naringenin (e) | SARS-CoV-2/(f) Inhibits ACE2 and inhibit the entry of virus into cells host by binding to S protein, helicase, and protease sites on the ACE receptor HCoV229E/(e) Partial inhibition of 229E replication in cells silenced for TPC2 by siRNA | [35] [36] |
| Camellia sinensis Kuntze CN: Green tea | Phenolic compounds: Tannic acid (aa), 3-isotheaflavin-3-galalate (ab) and theaflavin-3,3′-digallate (ac) | Coronavirus in general/Possibly inhibition of RNA polymerase or RNA-dependent proteases; They can also affect the release or assembly of the virus; inhibits ECA and blocking AII receptor binding in vitro, avoiding symptoms of various diseases, especially those of a respiratory nature | [37] [38] |
| Melia azedarach L. CN: Cinamomo | [39] [37] | ||
| Echinacea purpurea Moench CN: Echinaceae® | Caphtharic acid (o), cichoric acid (p) and echinacoside (p) | MERS-CoV, 229E/The extract non-specifically and irreversibly interferes with viral docking receptors (eg, influenza) to block infectivity of pathogens | [40] [41] |
| Ginkgo biloba L. CN: Ginkgo | Ginkgolide, terpenic lactones, flavonoids, polyphenols, oleic acid, among others. | SARS-CoV/Antiviral mechanism is unclear | [19] [42] |
| Glycyrrhiza glabra L. CN: Licorice (root) | Licorice (am) y glycyrrhizin (al) | SARS-CoV/Modulate some virus-host fusion functions through the envelope of the repetition domain 2 of the predominant heptad in viral envelopes; Improvement of the function of upper respiratory mucosal immune system; Inhibit viral adsorption and penetration | [29] [22] [43] [44] |
| Heteromorpha arborescens Cham. CN: Parsley tree | Oleanane-type saikosaponins (aj) | SARS-CoV/Prevent the entry of SARS-CoV into the cell | [45] [46] |
| Hippeastrum striatum Lam CN: Lily | Lectin agglutinin (w) | SARS-CoV/Inhibits the end of the virus cycle infection | [29] [47] |
| Lonicera japonica Thunb CN: Madreselva Eriobotrya japonica Thunb CN: Níspero | Quercetin (c), luteoloside (m), chlorogenic acid (x) | SARS-CoV, RSV, HIV, HSV, PRV and NDV/This mechanism possibly is due to diminishing the inflammation mediators and TNF-β, IL-1β expression. Anti-inflammatory, antiviral, antibacterial, antioxidant activity. Enhances the immune response. | [48] [49] |
| Lycoris spp. CN: hurricane lilies or cluster amaryllis | Lycorine | SARS-CoV/Compound with extensive antiviral activities. However, the antiviral mechanism of this molecule is unclear | [50] |
| Morus alba L. CN: Tree mulberry | Aliphatic, aromatic phenolic, heterocyclic and aliphatic cyclic compounds | SARS-CoV and MERS-CoV/Antiviral mechanism is unclear | [19] [42] |
| Nicotiana tabacum L. CN: Tobacco | N-acetylglucosamine specific lectins (b) | SARS-CoV/Ability to bind to the glycosylated molecules found on the surface of viruses, including the spike glycoprotein. | [23] [29] |
| Paulownia tomentosa Steud CN: Kiri | Flavonoids: (quercetin (c), catechin (d) and naringenin (e) and geranilated flavonoids (tomentin A, tomentin B, tomentin C, tomentin D, tomentin E) (r) | SARS-CoV/Inhibits SARS-CoV (PLpro) by reducing the concentration of pro-inflammatory cytokines (IL-1β) and TNFα | [51] |
| Pelargonium sidoides D.C. CN: Geranium | Prodelphinidin (af), gallocatechin (ag) and their epigallocatechin stereoisomer (ah) | H1N1, H3N2, HCoV-229E/inhibits the entry and replication of 229E; Also is immunomodulatory and cytoprotective effects, inhibition of the interaction between bacteria and host cells; Inhibits viral hemagglutination and Neuraminidase (NA) activity | [52] [53] [54] [55] |
| Psidium guajava CN: Guava | Eugenol (an) | SARS-CoV/Good binding affinity with Mpro and S protein | [34] [21] |
| Scutellaria baicalensis Georgi. CN: Skullcap | Baicalin (g) and scutellarein (l) | SARS-CoV/Inhibits nsP13 in vitro by affecting ATPase activity | [56] [57] [46] |
| Thuja orientalis L. CN: Tree of life | Essential oils: b-ocimene, 1,8-cineole, a-pinene and b-pinene mainly (ad) | SARS-CoV, HSV-1/Inhibitory activity against viral replication in vitro by visually scoring of the virus-induced cytopathogenic effect post-infection | [58] [29] |
| Laurus nobilis L. CN: Laurel | |||
| Salvia officinalis L. CN: Sage | |||
| Urtica dioica L. CN: Nettle | Lectin agglutinin (w) | SARS-CoV/Inhibits the end of the virus cycle infection | [29] [47] |
| Polygonum cuspidatum L. CN: Japanese knotty grass | Anthraquinone derived emodin (a) | SARS-CoV, HCoV-OC43/inhibits by blocking viral entry by binding to the S protein and interfering with the 3CLpro activity of the SARS-CoV and prevented the formation of the Nsp required for viral replication; Blocked the interaction between SARS-CoV S protein and ACE2, inhibited ion channel 3a and interrupted the release of new coronaviruses | [59] [28] |
| Senna obtusifolia L. CN: Abejorra | Emodin (a) | ||
| Rheum spp. CN: Rhubarb | |||
| Aloe spp. CN: Aloe | Aloe emodin (a) | [27] | |
| Vaccinium spp. CN: Blueberry, mortiño, Agráz, among others. | Anthocyanins (t), myricetin (n), gallic acid (u), stilbenoid resveratrol (s) and procyanidins (ai) | SARS-CoV, MERS-CoV/(t) inhibits the production of NO and the secretion of TNF-α in macrophages induced by LPS-INF-γ caused by protocatechic acid, also show ACE inhibitory activity; (n) inhibits the coronavirus helicase protein by affecting the ATPase activity in vitro; Gallic acid decreases the secretion of MCP-1, ICAM-1, and VCAM-1 in endothelial cells; (s) partially mitigates induced cell death and reduces infectious viral replication; (v) possibly blocks the entry of cells through endocytosis | [60] [61] [62] [63] |
| Vitis vinifera L. CN: Red grape | |||
| Zingiber officinale Rosc. CN: Ginger | [6]-gingerol (ak) | SARS-CoV-2/TMPRSS2 receptor blocking | [64] [65] |
| Family | Common Name of Plant with ACE and AT1R Inhibition Activities (%, %) |
|---|---|
| Actinidiaceae | Gold kiwi (−0.2; 20.5), green kiwi (16.6; 2.5) |
| Agaricaceae | Button mushroom (12.5; 0.3) |
| Alliaceae | Chives (23.2; 28.4), garlic (6.8; 27.4), leek (2.8; 42.7), onion (−1.2; 34.2), shallot (0.9; 11.5), red onion (−4.0; 31.8), spring onion (6.4; 53.3), white onion (−1.2; 18.8) |
| Amaranthaceae | Spinach (−0.7; 29.6) |
| Apiaceae | Black carrot juice (91.1; 31.0), carrot (0.7; 5.0), coriander leaf (37.4; 56.6), coriander seed (11.7; 16.4), fennel (−2.1; 15.2), parsley (8.2; 41.3) |
| Arecaceae | Coconut (11.8; −18.0) |
| Asparagaceae | Asparagus (35.1; 27.7) |
| Asteraceae | Radicchio (56; 43.5), red coral lettuce (31.5; 15.8), tarragon (32.1; 30.7) |
| Auricularaceae | Wood Ear mushroom (13.1; 33.4) |
| Betulaceae | Hazelnut (−9.8; 25.1) |
| Brassicaceae | Bok choi (7.1; 30.4), broccoli (6.1; 0.2), brussel sprout (10.3; 1.2), Chinese broccoli (21.9; 38.7), Chinese cabbage (6.5; 28.8), choi sum (21.8; 2.6), red cabbage (24.6; 6.0), savoy cabbage (2.2; 52.1), watercress (18.7; 27.9), yellow mustard seed (5.2; −1.8) |
| Chenopodiacea | Silver beet (−1.0; 31.7), rainbow silver beet (−3.2; 10.2), beetroot (0.8; 6.2) |
| Combretaceae | Kakadu plum (48.7; 0.0) |
| Convolvulaceae | Red sweet potato (8.6; 16.5), sweet potato (4.9; 26.0) |
| Cucurbitaceae | Choko (5.2; 3.4), choko skin (53.2; 14.0), cucumber (14.6; 40.8), pumpkin (3.3; 1.1), squash (4.3; 46.0), zucchini (16.0; 11.8) |
| Ericaceae | Blueberry (−0.1; 43.3) |
| Fabaceae | Green bean (10.7; 27.2), green pea (−7.2; 9.3), lupin (−15.4; 12.1), Parafield lupin (−24.3; 7.6), peanut (1.4; −16.7) |
| Fagaceae | Chestnut (61.7; −5.6) |
| Juglandaceae | Pecan nut (0; 7.8), walnut (−10.9; 2.4) |
| Lamiaceae | Green basil (37.9; 26.4), purple basil (46.3; 11.0), Thai basil (69.5; 36.5), oregano (67.5; 55.7), rosemary (91.0; 55.7), sage (89.3; 68.2), thyme (87.4; 42.1) |
| Lauraceae | Avocado (6.2; 43.4), bay leaf (34.9; 37.3), cinnamon (100.0; 54.4), Indian bay leaf (28.7; 0.4) |
| Lythraceae | Pomegranate flesh (−6.2; 10.7) |
| Marasmiaceae | Enoki mushroom (4.8; −3.7), Shiitake mushroom (26.4; 11.8) |
| Meriplaceae | Maitake mushroom (67.0; 32.1) |
| Myrtaceae | Clove (66.1; 30.8), cedar Bay cherry (63.8; 2.1), riberry (11.3; −12.1) |
| Poaceae | Corn (0; 27.8), lemongrass (5.0; 7.2) |
| Podacarpaceae | Illawarra plum (100; 7.0) |
| Polygonaceae | Rhubarb (16.3; 8.5) |
| Rosaceae | Quince (12.3; 11.1), raspberry (6.2; 6.2), strawberry (20.3; 3.5), red delicious apple (6.8; 1.5) |
| Rubiaceae | Columbian dark coffee bean (63.416.0), Mocha coffee bean (56.7; 21.5) |
| Rutaceae | Desert lemon (6.1; −0.6), green finger lime (11.5; 15.8), red finger lime (−6.3; 13.7), green citrus (14.8; 21.1), lemon skin (12.4; 7.9), lime (−16.4; 6.2), lime skin (47.1; 33.8), mandarin (0.2; 3.6), navel oranges (6.5; −3.9), orange skin (46.1; 7.8), red citrus (2.9; 40.1), red citrus skin (11.8; 17.4), ruby grapefruit (6.6; 14.9), Valencia orange (1.5; 5.4), yellow citrus (5.1; 18.6), yellow citrus skin (10.5; 7.3) |
| Saccharomycetaceae | Brewer’s yeast (31.8; −19.3) |
| Santalum | Quandong (40.6; 8.5) |
| Solanaceae | Potato (1.6; 16.6) |
| Sterculiaceae | Cocoa bean (81.2; 10.5) |
| Theaceae | English breakfast black tea (88.8; 27.1), green tea (41.1; 12.4), Japanese green tea (100; 41.6), Madura black tea (100; 30.5) |
| Pleurotaceae | Oyster mushroom (35.9; 16.1), Honey Brown mushroom (14.6; 8.6) |
| Vitaceae | Muscat grape (59.0; −2.8), white grape seed (100; 0.0), red grape skin (92.7; 14.4), Chambourcin grape (58.2; 10.6), Muscat Hamburg grape (73.5; -7.9), Cabinet Sauvignon grape (72.3; 0.0), Sun Muscat grape (59.0; −1.0), Concord grape (49.3; −3.3), |
| Zingiberaceae | Cardamom (7.4; 1.2), ginger (9.9; 38.0), tumeric (15.1; −1.4) |
| Family | Potential Species | Origin | Region | Potential Anti-Sars Effect | References |
|---|---|---|---|---|---|
| Betulaceae | Birches (Betula spp.) | Introduced | Sierra region | Anticoagulants and antirheumatic | [69] |
| Burseraceae | Palo santo (Bursera graveolens Triana and Planch) | Native | Coast and Sierra regions | Anti-inflammatory and antioxidant | [70] |
| Ericaceae | Mortiño (Vaccinium floribundum Kunth) | Endemic | Sierra region | Antioxidant | [29] |
| Euphorbiaceae | Croto de monte (Croton rivinifolius Kunth) | Endemic | Coast region | Anticarcinogenic and antiviral | [70] |
| Dog tongue (Euphorbia neriifolia L.) | Introduced | Coast región | Antitussive, antifungal and antitumor | [69] | |
| Fabaceae | Frijolillo (Cassia tora L.) | Native | Coast región | Anticoagulants and anti-inflammatory | [70] |
| White rain (Gliricidia brenningii Harms) | Native | Coast región | Antiherpetic and anticarcinogenic | [29] | |
| Orchidaceae | Orchid (Dendrobium spp.) | Introduced | Coast, Sierra y Amazon regions | Antiviral | [71] |
| Guayaquil Orchid (Encyclia angustiloba Schltr) | Endemic | Coast región | Antiviral | [71] | |
| Polygonaceae | Bloodroot (Polygonum arenastrum Boreau) | Introduced | Coast y Amazon regions | Antiviral | [59] |
| Rubiaceae | Cascarilla (Cinchona pubescens Vahl) | Native | Sierra region | Febrifuge, antiviral | [72] |
| Cat’s claw (Uncaria tomentosa D. C.) | Native | Sierra and Amazon regions | Anti-inflammatory | [72] | |
| Colorado (Simira ecuadoriensis Standl) | Endemic | Coast region | Febrifuge and antiviral | [72] | |
| Crucita (Rosenbergiodendron formosum Fagerl.) | Native | Coast region | Febrifuge and antiviral | [72] | |
| Scrophulariaceae | Escrofularia (Scrophularia spp.) | Introduced | Coast región | Anti-inflammatory and antimicrobial | [69] |
| Urticaceae | Nettle (Urtica urens L.) | Introduced | Sierra region | Antiviral | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llivisaca-Contreras, S.A.; Naranjo-Morán, J.; Pino-Acosta, A.; Pieters, L.; Vanden Berghe, W.; Manzano, P.; Vargas-Pérez, J.; León-Tamariz, F.; Cevallos-Cevallos, J.M. Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules 2021, 26, 4099. https://doi.org/10.3390/molecules26134099
Llivisaca-Contreras SA, Naranjo-Morán J, Pino-Acosta A, Pieters L, Vanden Berghe W, Manzano P, Vargas-Pérez J, León-Tamariz F, Cevallos-Cevallos JM. Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules. 2021; 26(13):4099. https://doi.org/10.3390/molecules26134099
Chicago/Turabian StyleLlivisaca-Contreras, Susana A., Jaime Naranjo-Morán, Andrea Pino-Acosta, Luc Pieters, Wim Vanden Berghe, Patricia Manzano, Jeffrey Vargas-Pérez, Fabian León-Tamariz, and Juan M. Cevallos-Cevallos. 2021. "Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2" Molecules 26, no. 13: 4099. https://doi.org/10.3390/molecules26134099
APA StyleLlivisaca-Contreras, S. A., Naranjo-Morán, J., Pino-Acosta, A., Pieters, L., Vanden Berghe, W., Manzano, P., Vargas-Pérez, J., León-Tamariz, F., & Cevallos-Cevallos, J. M. (2021). Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules, 26(13), 4099. https://doi.org/10.3390/molecules26134099








