Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants
Abstract
:1. Introduction
2. AMPs for Infection and Therapy
| Anti-Microbial Peptide | In Vivo/Clinical/Approved | Indication | Administration | Reference |
|---|---|---|---|---|
| Mutacin B-Ny266 (lantibiotic) | In vivo | Multi-drug resistant bacteria infection | - | [61] |
| Actagardine (lantibiotic) | In vivo | Staphylococcal, enterococcal, C. difficile infections | - | [62] |
| Plectasin (defensin) | In vivo | Systemic pneumococcal and streptococcal infections | - | [63] |
| Planosporicin (lantibiotic) | In vivo | Staphylococcal and enterococcal infections | - | [64] |
| Gallidermin/Epidermin (lantibiotic) | In vivo | Acne, eczema, folliculitis, and impetigo | - | [65] |
| Microbisporicin (lantibiotic) | In vivo | Staphylococcal and enterococcal infections; Acne | - | [66] |
| Mersacidin (lantibiotic) | In vivo | Staphylococcal, enterococcal, Clostridioides difficile infections | - | [67] |
| Lacticin 3147 (lantibiotic) | In vivo | Staphylococcal and enterococcal infections; Acne | - | [68] |
| Salivaricin B (lantibiotic) | In vivo | Streptococcal infections (caused mainly by S. pyogenes) and dental caries | - | [69] |
| Duramycin (lantibiotic) | In vivo | Cystic fibrosis, ocular diseases, and disorders | - | [70] |
| Deoxyactagardine/NVB302 (lantibiotic) | In vivo | C. difficile infections | - | [71] |
| Nisin (lantibiotic) | In vivo | Staphylococcal and enterococcal infections | - | [72] |
| Pinensins (lantibiotic) | In vivo | Yeast/fungal infections | - | [73] |
| MX-226 | In vivo | Catheter infections | - | [74] |
| PAC-113 (histatin 3) | Phase II Identifier: NCT00659971 | Oral candidiasis in HIV patients | Oral (Mouthwash) | [75] |
| Omiganan (indolicidin) | Phase III Identifier: NCT00231153 | Prevent local site catheter infection and colonization with central venous catheters | Topical | [46] |
| Iseganan (protegrin-1) | Phase II Identifier: NCT00118781 | Ventilator-associated pneumonia | Oral (Mouthwash) | [76] |
| Phase III Identifier: NCT00022373 | Oral mucositis induced by chemotherapy | Oral (Mouthwash) | [77] | |
| Pexiganan (magainin analog) | Phase III Identifier: NCT00563-394/433 | Diabetic foot ulcer infections | Topical | [44] |
| hLF1-11 (lactoferrin) | Phase I/II Identifier: NCT00509938 | Bacteraemia and fungal infection | Intravenous | [78] |
| CZEN-002 (α-melanocyte-stimulating hormone) | Phase IIb | Vaginal candidiasis | Vaginal gel | [79] |
| Novexatin (defensin) | Phase II Identifier: NCT02343627 | Stubborn fungal nail infection | Topical | [45] |
| LL-37 (cathelicidin) | Phase I/II Identifier: NCT04098562 | Hard-to-heal venous leg ulcers | Topical | [50] |
| PXL01 (lactoferricin) | Phase II Identifier: NCT01022242 | Prevent post-operative adhesion in hands | Hydrogel applied at surgical site | [54] |
| Surotomycin (synthetically modified daptomycin) | Phase III Identifier: NCT01597505 | Diarrhea caused by C. difficile | Oral | [80] |
| LTX-109 (synthetic antimicrobial peptidomimetic) | Phase II Identifier: NCT01803035 | Skin infection, impetigo | Topical | [81] |
| Phase I/II Identifier: NCT01158235 | Nasal infection with S. aureus | Nasal | [81] | |
| SGX942 (indolicidin) | Phase III Identifier: NCT03237325 | Oral mucositis induced by radiation and/or chemotherapy | Intravenous | [82] |
| OP-145 (cathelicidin) | Phase I/II | Chronic otic infection | Eardrops | [83] |
| C16G2 (synthetic specific-directed antimicrobial peptide) | Phase II Identifier: NCT02044081 | Avoid caries caused by S. mutans | Oral (Mouthwash) | [84] |
| Murepavadin (protegrin I) | Phase I Identifier: NCT03409679 | Ventilator-associated pneumonia and bronchiectasis by Pseudomonas aeruginosa | Intravenous | [85] |
| DPK-060 (hybrid peptide from 2 functional domains) | Phase II Identifier: NCT01522391 | Human wound infection caused by S. aureus | Topical | [86] |
| Teicoplanin (Actinoplanes teichomyceticus glycopeptide) | Approved | Bacterial infections | Intravenous and Intramuscular | [87] |
| Daptomycin (anionic peptide) | Approved | Bacterial skin infections | Intravenous | [43] |
| Colistin (Bacillus polymyxa cyclic peptide) | Approved | Multi drug-resistant gram-negative infections | Intravenous | [88] |
| Dalbavancin (Teicoplanin derivative lipoglycopeptide) | Approved | Acute bacterial skin and skin structure infections | Intravenous | [89] |
| Polymyxin (Bacillus polymyxa polypeptide) | Approved | Urinary tract and bloodstream infections | Ophthalmic Topical Intravenous | [38] |
| Enfuvirtide (biomimetic peptide) | Approved | HIV-1 infection | Subcutaneous | [90] |
| Telavancin (vancomycin derivative lipoglycopeptide) | Approved | Bacterial skin infections | Intravenous | [91] |
| Gramicidin D (Bacillus brevis polypeptides) | Approved | Skin and eye infections | Topical Ophthalmic | [40] |
| Oritavancin (vancomycin derivative lipoglycopeptide) | Approved | Bacterial skin infections | Intravenous | [92] |
| Bacitracin (Bacillus licheniformis cyclic peptide) | Approved | Skin and eye infections; wound infections | Topical | [93] |
| Telaprevir (antimicrobial peptidomimetic) | Approved | Hepatitis C infection | Oral | [94] |
| Vancomycin (Amycolatopsis orientalis glycopeptide) | Approved | Bacterial infections | Oral and Intravenous | [95] |
3. Heterologous Production of AMPs
4. Plant Molecular Farming
5. Strategies for Protein Production in Plants
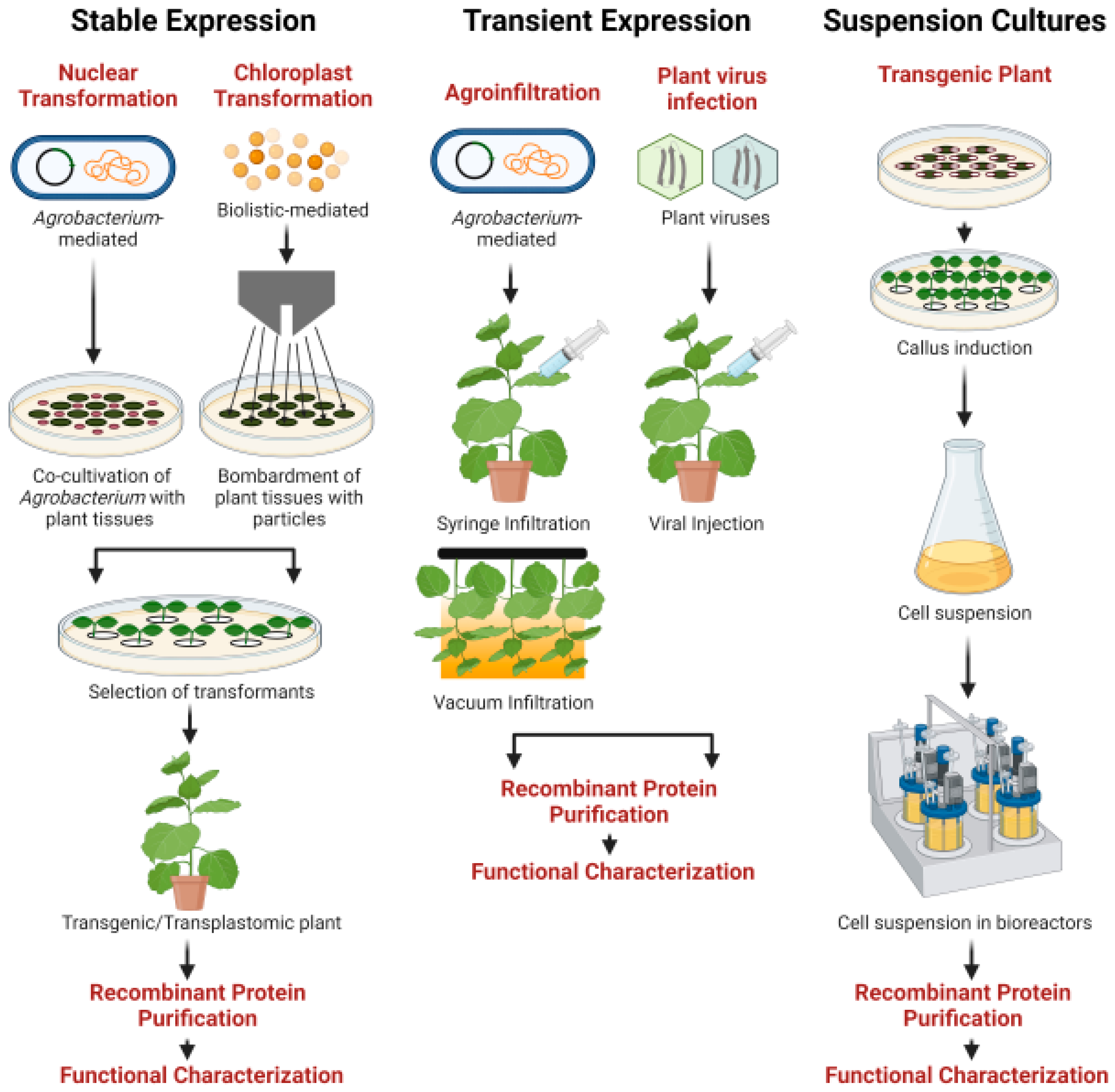
5.1. Stable Nuclear Expression
5.2. Stable Chloroplast Expression
5.3. Transient Expression
5.4. Suspension Cultures
6. AMP Expression in Plants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Li, Y. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta BBA Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Blencke, H.-M.; Paulsen, V.; Haug, T.; Stensvåg, K. Powerful workhorses for antimicrobial peptide expression and characterization. Bioeng. Bugs 2010, 1, 217–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, O.N.; Mulder, K.C.; Barbosa, A.E.; Otero-Gonzalez, A.J.; Lopez-Abarrategui, C.; Rezende, T.M.; Dias, S.C.; Franco, O.L. Exploring the pharmacological potential of promiscuous host-defense peptides: From natural screenings to biotechnological applications. Front. Microbiol. 2011, 2, 232. [Google Scholar] [CrossRef] [Green Version]
- Thevissen, K.; Kristensen, H.H.; Thomma, B.P.; Cammue, B.P.; François, I.E. Therapeutic potential of antifungal plant and insect defensins. Drug Discov. Today 2007, 12, 966–971. [Google Scholar] [CrossRef]
- Lai, Y.; Villaruz, A.E.; Li, M.; Cha, D.J.; Sturdevant, D.E.; Otto, M. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol. Microbiol. 2007, 63, 497–506. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef]
- Martin, M. Interactions of antimicrobial peptides with bacterial membranes and membrane components. Curr. Top. Med. Chem. 2016, 16, 16–24. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gellatly, S.L.; Hancock, R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. CMLS 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial peptides: Their role as infection-selective tracers for molecular imaging. BioMed Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Weibel, D.B. Organization and function of anionic phospholipids in bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 4255–4267. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2016, 96, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Idicula-Thomas, S. Collection of antimicrobial peptides database and its derivatives: Applications and beyond. Protein Sci. 2020, 29, 36–42. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- D’Este, F.; Benincasa, M.; Cannone, G.; Furlan, M.; Scarsini, M.; Volpatti, D.; Gennaro, R.; Tossi, A.; Skerlavaj, B.; Scocchi, M. Antimicrobial and host cell-directed activities of Gly/Ser-rich peptides from salmonid cathelicidins. Fish Shellfish Immunol. 2016, 59, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef] [PubMed]
- Sibel Akalın, A. Dairy-derived antimicrobial peptides: Action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 2014, 36, 79–95. [Google Scholar] [CrossRef]
- Zeth, K.; Sancho-Vaello, E. The human antimicrobial peptides dermcidin and LL-37 show novel distinct pathways in membrane interactions. Front. Chem. 2017, 5, 86. [Google Scholar] [CrossRef]
- Kosikowska, P.; Lesner, A. Antimicrobial peptides (AMPs) as drug candidates: A patent review (2003–2015). Expert Opin. Ther. Pat. 2016, 26, 689–702. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Langen, G.; Imani, J.; Khalifa, W.; Altincicek, B.; von Wettstein, D.; Kogel, K.-H.; Vilcinskas, A. Insect peptide metchnikowin confers on barley a selective capacity for resistance to fungal ascomycetes pathogens. J. Exp. Bot. 2009, 60, 4105–4114. [Google Scholar] [CrossRef] [Green Version]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. The potential of antimicrobial peptides as biocides. Int. J. Mol. Sci. 2011, 12, 6566–6596. [Google Scholar] [CrossRef] [Green Version]
- Maira Galdino da Rocha, P.; Marina Galdino da Rocha, P.; Suely Lins, G. Development of novel therapeutic drugs in humans from plant antimicrobial peptides. Curr. Protein Pept. Sci. 2010, 11, 236–247. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Haug, T.; Styrvold, O.B.; Jørgensen, T.; Stensvåg, K. Strongylocins, novel antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2008, 32, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Barashkova, A.S.; Rogozhin, E.A. Isolation of antimicrobial peptides from different plant sources: Does a general extraction method exist? Plant Methods 2020, 16, 143. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- Hojo, K.; Hara, A.; Kitai, H.; Onishi, M.; Ichikawa, H.; Fukumori, Y.; Kawasaki, K. Development of a method for environmentally friendly chemical peptide synthesis in water using water-dispersible amino acid nanoparticles. Chem. Cent. J. 2011, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, V.; Egelund, P.; Johansson, H.; Quement, S.; Wojcik, F.; Pedersen, D. Greening the synthesis of peptide therapeutics: An industrial perspective. RSC Adv. 2020, 10, 42457–42492. [Google Scholar] [CrossRef]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Rigatto, M.H.; Falci, D.R.; Zavascki, A.P. Clinical use of polymyxin B. Adv. Exp. Med. Biol. 2019, 1145, 197–218. [Google Scholar] [CrossRef]
- Burkhart, B.M.; Gassman, R.M.; Langs, D.A.; Pangborn, W.A.; Duax, W.L.; Pletnev, V. Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers 1999, 51, 129–144. [Google Scholar] [CrossRef]
- Cynthia, L.S. Advances in peptide pharmaceuticals. Curr. Pharm. Biotechnol. 2009, 10, 122–137. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, K.L.; Rybak, M.J. Daptomycin. Pharmacotherapy 2004, 24, 41–57. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Holroyd, K.J.; Zasloff, M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: A randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin. Infect. Dis. 2008, 47, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef]
- Sader, H.; Fedler, K.; Rennie, R.; Stevens, S.; Jones, R. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: Spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob. Agents Chemother. 2004, 48, 3112–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [Green Version]
- Kos, S.; Vanvarenberg, K.; Dolinsek, T.; Cemazar, M.; Jelenc, J.; Préat, V.; Sersa, G.; Vandermeulen, G. Gene electrotransfer into skin using noninvasive multi-electrode array for vaccination and wound healing. Bioelectrochemistry 2017, 114, 33–41. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Hirsch, T.; Schulte, M.; Kueckelhaus, M.; Jacobsen, F.; Mersch, E.A.; Stricker, I.; Afacan, N.; Jenssen, H.; Hancock, R.E.; et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE 2012, 7, e39373. [Google Scholar] [CrossRef] [Green Version]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, S.; Holm, B.; Mahlapuu, M.; Reno, C.; Hart, D.A.; Wiig, M. PXL01 in sodium hyaluronate results in increased PRG4 expression: A potential mechanism for anti-adhesion. Upsala J. Med. Sci. 2016, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Björn, C.; Sjöstrand, V.; Lindgren, K.; Münnich, M.; Mattsby-Baltzer, I.; Ivarsson, M.L.; Olmarker, K.; Mahlapuu, M. A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann. Surg. 2009, 250, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Wiig, M.E.; Dahlin, L.B.; Fridén, J.; Hagberg, L.; Larsen, S.E.; Wiklund, K.; Mahlapuu, M. PXL01 in sodium hyaluronate for improvement of hand recovery after flexor tendon repair surgery: Randomized controlled trial. PLoS ONE 2014, 9, e110735. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [Green Version]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Mota-Meira, M.; LaPointe, G.; Lacroix, C.; Lavoie, M.C. MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob. Agents Chemother. 2000, 44, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, A.; Pag, U.; Wiedemann, I.; Sahl, H.G. Combination of antibiotic mechanisms in lantibiotics. Farmaco 2002, 57, 685–691. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Cavaletti, L.; Losi, D.; Lazzarini, A.; Carrano, L.; Feroggio, M.; Ciciliato, I.; Corti, E.; Candiani, G.; Marinelli, F.; et al. A novel lantibiotic acting on bacterial cell wall synthesis produced by the uncommon actinomycete Planomonospora sp. Biochemistry 2007, 46, 5884–5895. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.R.; Schneider, T.; Sahl, H.G.; Wiedemann, I. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 2006, 50, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglione, F.; Lazzarini, A.; Carrano, L.; Corti, E.; Ciciliato, I.; Gastaldo, L.; Candiani, P.; Losi, D.; Marinelli, F.; Selva, E.; et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem. Biol. 2008, 15, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Appleyard, A.N.; Choi, S.; Read, D.M.; Lightfoot, A.; Boakes, S.; Hoffmann, A.; Chopra, I.; Bierbaum, G.; Rudd, B.A.; Dawson, M.J.; et al. Dissecting structural and functional diversity of the lantibiotic mersacidin. Chem. Biol. 2009, 16, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Piper, C.; Draper, L.A.; Cotter, P.D.; Ross, R.P.; Hill, C. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J. Antimicrob. Chemother. 2009, 64, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Wescombe, P.A.; Heng, N.C.; Burton, J.P.; Chilcott, C.N.; Tagg, J.R. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 2009, 4, 819–835. [Google Scholar] [CrossRef] [Green Version]
- Oliynyk, I.; Varelogianni, G.; Roomans, G.M.; Johannesson, M. Effect of duramycin on chloride transport and intracellular calcium concentration in cystic fibrosis and non-cystic fibrosis epithelia. APMIS 2010, 118, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.J.; Scott, R.W. New horizons for host defense peptides and lantibiotics. Curr. Opin. Pharmacol. 2012, 12, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Kamarajan, P.; Hayami, T.; Matte, B.; Liu, Y.; Danciu, T.; Ramamoorthy, A.; Worden, F.; Kapila, S.; Kapila, Y. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS ONE 2015, 10, e0131008. [Google Scholar] [CrossRef] [Green Version]
- Mohr, K.I.; Volz, C.; Jansen, R.; Wray, V.; Hoffmann, J.; Bernecker, S.; Wink, J.; Gerth, K.; Stadler, M.; Müller, R. Pinensins: The first antifungal lantibiotics. Angew. Chem. 2015, 54, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Czyzewski, A.M.; Jenssen, H.; Fjell, C.D.; Waldbrook, M.; Chongsiriwatana, N.P.; Yuen, E.; Hancock, R.E.W.; Barron, A.E. In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS ONE 2016, 11, e0135961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickels, N.; McManus, C.; Massaro, J.; Friden, P.; Braman, V.; D’Agostino, R.; Oppenheim, F.; Warbington, M.; Dibart, S.; Van Dyke, T. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis. J. Clin. Periodontol. 2001, 28, 404–410. [Google Scholar] [CrossRef]
- Kollef, M.; Pittet, D.; Sánchez García, M.; Chastre, J.; Fagon, J.Y.; Bonten, M.; Hyzy, R.; Fleming, T.R.; Fuchs, H.; Bellm, L.; et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am. J. Respir. Crit. Med. 2006, 173, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Epstein, J.B.; Raber-Durlacher, J.; Donnelly, P.; Strahilevitz, J. The antimicrobial effect of Iseganan HCl oral solution in patients receiving stomatotoxic chemotherapy: Analysis from a multicenter, double-blind, placebo-controlled, randomized, phase III clinical trial. J. Oral Pathol. Med. 2012, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, W.J.F.M.; van Iersel, T.M.P.; Blijlevens, N.M.A.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Alam, M.Z.; Wu, X.; Mascio, C.; Chesnel, L.; Hurdle, J.G. Mode of action and bactericidal properties of surotomycin against growing and nongrowing Clostridium difficile. Antimicrob. Agents Chemother. 2015, 59, 5165–5170. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Håkangård, C.; Olsson, P.; Olsen, W.M. LTX-109 Is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 145. [Google Scholar] [CrossRef] [Green Version]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Elder, J.; Sonis, S.T.; Straube, R.; et al. Dusquetide: A novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled phase 2a clinical study. J. Biotechnol. 2016, 239, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ming, L.; Huang, J.A. The antibacterial effects of antimicrobial peptides OP-145 against clinically isolated multi-resistant strains. Jpn. J. Infect. Dis. 2017, 70, 601–603. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Edlund, A. Targeted antimicrobial peptides: A novel technology to eradicate harmful Streptococcus mutans. J. Calif. Dent. Assoc. 2017, 45, 557–564. [Google Scholar]
- Dale, G.E.; Halabi, A.; Petersen-Sylla, M.; Wach, A.; Zwingelstein, C. Pharmacokinetics, tolerability, and safety of murepavadin, a novel antipseudomonal antibiotic, in subjects with mild, moderate, or severe renal function impairment. Antimicrob. Agents Chemother. 2018, 62, e00490-18. [Google Scholar] [CrossRef] [Green Version]
- Håkansson, J.; Ringstad, L.; Umerska, A.; Johansson, J.; Andersson, T.; Boge, L.; Rozenbaum, R.T.; Sharma, P.K.; Tollbäck, P.; Björn, C.; et al. Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide DPK-060 used for topical treatment. Front. Cell. Infect. Microbiol. 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.P. Clinical pharmacokinetics of teicoplanin. Clin. Pharmacokinet. 2000, 39, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Chen, A.Y.; Zervos, M.J.; Vazquez, J.A. Dalbavancin: A novel antimicrobial. Int. J. Clin. Pract. 2007, 61, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, M. Enfuvirtide: From basic science to FDA approval. In Entry Inhibitors in HIV Therapy; Birkhäuser: Basel, Switzerland, 2007; pp. 161–177. [Google Scholar]
- Saravolatz, L.D.; Stein, G.E.; Johnson, L.B. Telavancin: A novel lipoglycopeptide. Clin. Infect. Dis. 2009, 49, 1908–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouza, E.; Burillo, A. Oritavancin: A novel lipoglycopeptide active against Gram-positive pathogens including multiresistant strains. Int. J. Antimicrob. Agents 2010, 36, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Lublin, A.; Isoda, F.; Patel, H.; Yen, K.; Nguyen, L.; Hajje, D.; Schwartz, M.; Mobbs, C. FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on cbp and protect against proteotoxicity. PLoS ONE 2011, 6, e27762. [Google Scholar] [CrossRef]
- Muir, A.J. Telaprevir for the treatment of chronic hepatitis C infection. Expert Rev. Anti Infect. Ther. 2011, 9, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Meakin, M.; Earl, M.H.; Kummer, T.M.; McAleer, J.P.; Long, T.E. Effects of caspofungin, tolcapone and other FDA-approved medications on MRSA susceptibility to vancomycin. J. Glob. Antimicrob. Resist. 2020, 22, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ingham, A.B.; Moore, R.J. Recombinant production of antimicrobial peptides in heterologous microbial systems. Biotechnol. Appl. Biochem. 2007, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z. RAPD: A database of recombinantly-produced antimicrobial peptides. FEMS Microbiol. Lett. 2008, 289, 126–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Hu, J.; Li, S.; Jin, X.; Zhang, C.; Cong, Y.; Hu, X.; Tan, Y.; Huang, J.; Chen, Z.; et al. Design and expression of peptide antibiotic hPAB-beta as tandem multimers in Escherichia coli. Peptides 2005, 26, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, F.; Yu, X.; Ji, S.; Wang, J.; Cheng, H.; Wang, C.; Zhang, W. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr. Purif. 2007, 53, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shukla, P. Sophisticated cloning, fermentation, and purification technologies for an enhanced therapeutic protein production: A review. Front. Pharmacol. 2017, 8, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parachin, N.S.; Mulder, K.C.; Viana, A.A.; Dias, S.C.; Franco, O.L. Expression systems for heterologous production of antimicrobial peptides. Peptides 2012, 38, 446–456. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Esposito, D. Enhanced soluble protein expression using two new fusion tags. Protein Expr. Purif. 2006, 46, 122–129. [Google Scholar] [CrossRef]
- Xu, Z.; Zhong, Z.; Huang, L.; Peng, L.; Wang, F.; Cen, P. High-level production of bioactive human beta-defensin-4 in Escherichia coli by soluble fusion expression. Appl. Microbiol. Biotechnol. 2006, 72, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, D.; Cong, Y.; Wang, J.; Zhu, J.; Yang, J.; Hu, Z.; Hu, X.; Tan, Y.; Hu, F.; et al. Recombinant antimicrobial peptide hPAB-β expressed in Pichia pastoris, a potential agent active against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2011, 89, 281–291. [Google Scholar] [CrossRef]
- Cregg, J.M.; Tolstorukov, I.; Kusari, A.; Sunga, J.; Madden, K.; Chappell, T. Expression in the yeast Pichia pastoris. Methods Enzymol. 2009, 463, 169–189. [Google Scholar] [CrossRef]
- Holaskova, E.; Galuszka, P.; Frebort, I.; Oz, M.T. Antimicrobial peptide production and plant-based expression systems for medical and agricultural biotechnology. Biotechnol. Adv. 2015, 33, 1005–1023. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, S.T. Engineering plants as platforms for production of vaccines. Am. J. Plant Sci. 2020, 11, 707–735. [Google Scholar] [CrossRef]
- Ma, J.K.C.; Barros, E.; Bock, R.; Christou, P.; Dale, P.J.; Dix, P.J.; Fischer, R.; Irwin, J.; Mahoney, R.; Pezzotti, M.; et al. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005, 6, 593–599. [Google Scholar] [CrossRef]
- Twyman, R.M.; Schillberg, S.; Fischer, R. Transgenic plants in the biopharmaceutical market. Expert Opin. Emerg. Drugs 2005, 10, 185–218. [Google Scholar] [CrossRef]
- Korban, S.S. Targeting and expression of antigenic proteins in transgenic plants for production of edible oral vaccines. In Vitro Cell. Dev. Biol. Plant 2002, 38, 231–236. [Google Scholar] [CrossRef]
- Govea-Alonso, D.O.; Rybicki, E.; Rosales-Mendoza, S. Plant-based vaccines as a global vaccination approach: Current perspectives. In Genetically Engineered Plants as a Source of Vaccines against Wide Spread Diseases; Springer: New York, NY, USA, 2014; pp. 265–280. [Google Scholar] [CrossRef]
- Sinha, R.; Shukla, P. Antimicrobial peptides: Recent insights on biotechnological interventions and future perspectives. Protein Pept. Lett. 2019, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugaraj, B.; Malla, A.; Phoolcharoen, W. Emergence of novel coronavirus 2019-nCoV: Need for rapid vaccine and biologics development. Pathogens 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J.M. The expression of a nopaline synthase—Human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Caffferkey, R.; Bowdish, K. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.-C.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular pharming—VLPs made in plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Stoger, E.; Fischer, R.; Moloney, M.; Ma, J.K.-C. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 2014, 65, 743–768. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Nieto-Gómez, R. Green therapeutic biocapsules: Using plant cells to orally deliver biopharmaceuticals. Trends Biotechnol. 2018, 36, 1054–1067. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-made vaccines and reagents for the One Health initiative. Hum. Vaccin. Immunother. 2017, 13, 2912–2917. [Google Scholar] [CrossRef]
- Chan, H.-T.; Daniell, H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [Green Version]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Ramalingam, S. Plant expression platform for the production of recombinant pharmaceutical proteins. Austin J. Biotechnol. Bioeng. 2014, 1, 4. [Google Scholar]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant molecular farming: A viable platform for recombinant biopharmaceutical production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Xu, J.; Towler, M.; Weathers, P.J. Platforms for plant-based protein production. Bioprocess. Plant In Vitro Syst. 2018, 509–548. [Google Scholar] [CrossRef]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Xu, J.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef]
- Moon, K.-B.; Park, J.-S.; Park, Y.-I.; Song, I.-J.; Lee, H.-J.; Cho, H.S.; Jeon, J.-H.; Kim, H.-S. Development of systems for the production of plant-derived biopharmaceuticals. Plants 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.L.; Sampaio, K.B.; Costa, F.F.; Franco, O.L.; Dias, S.C.; Cunha, N.B. Molecular farming of antimicrobial peptides: Available platforms and strategies for improving protein biosynthesis using modified virus vectors. An. Acad. Bras Cienc. 2019, 91, e20180124. [Google Scholar] [CrossRef]
- Boominathan, A.; Vanhoozer, S.; Basisty, N.; Powers, K.; Crampton, A.L.; Wang, X.; Friedricks, N.; Schilling, B.; Brand, M.D.; O’Connor, M.S. Stable nuclear expression of ATP8 and ATP6 genes rescues a mtDNA Complex V null mutant. Nucleic Acids Res. 2016, 44, 9342–9357. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, A.; Li, X.; Lu, C. The role of chloroplast gene expression in plant responses to environmental stress. Int. J. Mol. Sci. 2020, 21, 6082. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Daniell, H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol. J. 2016, 14, 2079–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.-Y.; Gelvin, S. T-DNA binary vectors and systems. Plant Physiol. 2008, 146, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Gehl, C.; Li, G.; Serek, M. An efficient protocol for Agrobacterium-mediated transformation and regeneration of Campanula medium (Canterbury bells) based on leaf disc explants. Plant Cell Tissue Organ Cult. PCTOC 2020, 140, 635–645. [Google Scholar] [CrossRef]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.S.; Ahmed, M.M.; Liao, Y.C.; Waheed, M.T.; Sameeullah, M.; Darkhshan; Hussain, S.; Saud, S.; et al. Recent developments in therapeutic protein expression technologies in plants. Biotechnol. Lett. 2015, 37, 265–279. [Google Scholar] [CrossRef]
- Chebolu, S.; Daniell, H. Chloroplast-derived vaccine antigens and biopharmaceuticals: Expression, folding, assembly and functionality. Curr. Top. Microbiol. Immunol. 2009, 332, 33–54. [Google Scholar] [CrossRef]
- Gorantala, J.; Grover, S.; Goel, D.; Rahi, A.; Jayadev Magani, S.K.; Chandra, S.; Bhatnagar, R. A plant based protective antigen [PA(dIV)] vaccine expressed in chloroplasts demonstrates protective immunity in mice against anthrax. Vaccine 2011, 29, 4521–4533. [Google Scholar] [CrossRef]
- Song, I.; Kang, Y.J.; Kim, D.H.; Kim, M.K.; Ko, K. Expression and in vitro function of anti-cancer mAbs in transgenic Arabidopsis thaliana. BMB Rep. 2020, 53, 229–233. [Google Scholar] [CrossRef]
- Park, S.H.; Ji, K.-Y.; Kim, H.M.; Ma, S.H.; Park, S.Y.; Do, J.H.; Oh, D.-B.; Kang, H.S.; Shim, J.S.; Joung, Y.H. Optimization of the human colorectal carcinoma antigen GA733-2 production in tobacco plants. Plant Biotechnol. Rep. 2021, 15, 55–67. [Google Scholar] [CrossRef]
- Meneguetti, B.T.; Machado, L.d.S.; Oshiro, K.G.N.; Nogueira, M.L.; Carvalho, C.M.E.; Franco, O.L. Antimicrobial peptides from fruits and their potential use as biotechnological tools—A review and outlook. Front. Microbiol. 2017, 7, 2136. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Samson, N.P.; Koya, V.; Daniell, H. A protocol for expression of foreign genes in chloroplasts. Nat. Protoc. 2008, 3, 739–758. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [Green Version]
- Zoschke, R.; Bock, R. Chloroplast translation: Structural and functional organization, operational control, and regulation. Plant Cell 2018, 30, 745–770. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Daniell, H. The engineered chloroplast genome just got smarter. Trends Plant Sci. 2015, 20, 622–640. [Google Scholar] [CrossRef] [Green Version]
- Daniell, H.; Jin, S.; Zhu, X.-G.; Gitzendanner, M.A.; Soltis, D.E.; Soltis, P.S. Green giant—A tiny chloroplast genome with mighty power to produce high-value proteins: History and phylogeny. Plant Biotechnol. J. 2021, 19, 430–447. [Google Scholar] [CrossRef]
- Zhang, B.; Shanmugaraj, B.; Daniell, H. Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr. Opin. Chem. Biol. 2017, 38, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Daniell, H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007, 145, 1129–1143. [Google Scholar] [CrossRef] [Green Version]
- Cody, W.B.; Scholthof, H.B. Plant virus vectors 3.0: Transitioning into synthetic genomics. Annu. Rev. Phytopathol. 2019, 57, 211–230. [Google Scholar] [CrossRef]
- Salazar-González, J.; Rosales-Mendoza, S.; Bañuelos-Hernandez, B. Viral vector-based expression strategies. In Genetically Engineered Plants Source of Vaccines Against Wide Spread Diseases; Springer: New York, NY, USA, 2014; pp. 43–60. [Google Scholar]
- Ariga, H.; Toki, S.; Ishibashi, K. Potato virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiol. 2020, 61, 1946–1953. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient transient expression of recombinant proteins in plants by the novel pEff vector based on the genome of potato virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef]
- Tiwari, S.; Verma, P.C.; Singh, P.K.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2009, 27, 449–467. [Google Scholar] [CrossRef]
- Shih, S.M.; Doran, P.M. Foreign protein production using plant cell and organ cultures: Advantages and limitations. Biotechnol. Adv. 2009, 27, 1036–1042. [Google Scholar] [CrossRef]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Ghidey, M.; Islam, S.M.A.; Pruett, G.; Kearney, C.M. Making plants into cost-effective bioreactors for highly active antimicrobial peptides. New Biotechnol. 2020, 56, 63–70. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef]
- Plasson, C.; Michel, R.; Lienard, D.; Saint-Jore-Dupas, C.; Sourrouille, C.; de March, G.G.; Gomord, V. Production of recombinant proteins in suspension-cultured plant cells. Methods Mol. Biol. 2009, 483, 145–161. [Google Scholar] [CrossRef]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klöckner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Büchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef]
- Magy, B.; Tollet, J.; Laterre, R.; Boutry, M.; Navarre, C. Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol. J. 2014, 12, 457–467. [Google Scholar] [CrossRef]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472. [Google Scholar] [CrossRef]
- Schillberg, S.; Raven, N.; Fischer, R.; Twyman, R.M.; Schiermeyer, A. Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr. Pharm. Des. 2013, 19, 5531–5542. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Patiño-Rodríguez, O.; Ortega-Berlanga, B.; Llamas-González, Y.Y.; Flores-Valdez, M.A.; Herrera-Díaz, A.; Montes-de-Oca-Luna, R.; Korban, S.S.; Alpuche-Solís, Á.G. Transient expression and characterization of the antimicrobial peptide protegrin-1 in Nicotiana tabacum for control of bacterial and fungal mammalian pathogens. Plant Cell Tissue Organ Cult. PCTOC 2013, 115, 99–106. [Google Scholar] [CrossRef]
- Chahardoli, M.; Fazeli, A.; Ghabooli, M. Recombinant production of bovine Lactoferrin-derived antimicrobial peptide in tobacco hairy roots expression system. Plant Physiol. Biochem. 2018, 123, 414–421. [Google Scholar] [CrossRef]
- Chahardoli, M.; Fazeli, A.; Niazi, A.; Ghabooli, M. Recombinant expression of LFchimera antimicrobial peptide in a plant-based expression system and its antimicrobial activity against clinical and phytopathogenic bacteria. Biotechnol. Biotechnol. Equip. 2018, 32, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Holásková, E.; Galuszka, P.; Micuchova, A.; Šebela, M.; Oz, T.; Frébort, I. Molecular farming in barley: Development of a novel production platform to produce human antimicrobial peptide LL-37. Biotechnol. J. 2018, 13, 1700628. [Google Scholar] [CrossRef]
- Wu, T.; Tang, D.; Chen, W.; Huang, H.; Wang, R.; Chen, Y. Expression of antimicrobial peptides thanatin(S) in transgenic Arabidopsis enhanced resistance to phytopathogenic fungi and bacteria. Gene 2013, 527, 235–242. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, S.Y.; Moon, Y.S.; Kang, K.K. Enhanced resistance to bacterial and fungal pathogens by overexpression of a human cathelicidin antimicrobial peptide (hCAP18/LL-37) in Chinese cabbage. Plant Biotechnol. Rep. 2012, 6, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaji, V.; Smart, C.D. Over-expression of snakin-2 and extensin-like protein genes restricts pathogen invasiveness and enhances tolerance to Clavibacter michiganensis subsp. michiganensis in transgenic tomato (Solanum lycopersicum). Transgenic Res. 2012, 21, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, W.; Qi, L.; Wang, J.; Du, L.; Xu, H.; Wang, A.; Zhang, Z. Expression of a potato antimicrobial peptide SN1 increases resistance to take-all pathogen Gaeumannomyces graminis var. tritici in transgenic wheat. Funct. Integr. Genom. 2013, 13, 403–409. [Google Scholar] [CrossRef]
- DeGray, G.; Rajasekaran, K.; Smith, F.; Sanford, J.; Daniell, H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001, 127, 852–862. [Google Scholar] [CrossRef]
- Osusky, M.; Osuska, L.; Kay, W.; Misra, S. Genetic modification of potato against microbial diseases: In vitro and in planta activity of a dermaseptin B1 derivative, MsrA2. Theor. Appl. Genet. 2005, 111, 711–722. [Google Scholar] [CrossRef]
- Yevtushenko, D.P.; Misra, S. Comparison of pathogen-induced expression and efficacy of two amphibian antimicrobial peptides, MsrA2 and temporin A, for engineering wide-spectrum disease resistance in tobacco. Plant Biotechnol. J. 2007, 5, 720–734. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-L.; Prasad, V.; Sanjaya; Chen, K.H.; Liu, P.C.; Chan, M.-T.; Cheng, C.-P. Transgenic tomato plants expressing an Arabidopsis thionin (Thi2.1) driven by fruit-inactive promoter battle against phytopathogenic attack. Planta 2005, 221, 386–393. [Google Scholar] [CrossRef]
- Prasad, B.D.; Jha, S.; Chattoo, B.B. Transgenic indica rice expressing Mirabilis jalapa antimicrobial protein (Mj-AMP2) shows enhanced resistance to the rice blast fungus Magnaporthe oryzae. Plant Sci. 2008, 175, 364–371. [Google Scholar] [CrossRef]
- Song, L.; Zhao, D.-g.; Wu, Y.-j.; Li, Y. Transient expression of chicken alpha interferon gene in lettuce. J. Zhejiang Univ. Sci. B 2008, 9, 351–355. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, K.D.; Hwang, B.K.; Ok, S.H.; Shin, J.S. Overexpression of Lipid Transfer Protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep. 2009, 28, 419–427. [Google Scholar] [CrossRef]
- Jha, S.; Tank, H.G.; Prasad, B.D.; Chattoo, B.B. Expression of Dm-AMP1 in rice confers resistance to Magnaporthe oryzae and Rhizoctonia solani. Transgenic Res. 2009, 18, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; Chang, C.C.; Juang, R.S.; Chen, R.B.; Yang, H.Y.; Chu, L.W.; Wang, S.R.; Tseng, T.H.; Wang, C.S.; Chen, L.J.; et al. Porcine lactoferrin expression in transgenic rice and its effects as a feed additive on early weaned piglets. J. Agric. Food Chem. 2010, 58, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Chattoo, B.B. Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res. 2010, 19, 373–384. [Google Scholar] [CrossRef]
- Jan, P.-S.; Huang, H.-Y.; Chen, H.-M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. Appl. Environ. Microbiol. 2010, 76, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-B.; Li, B.; Jin, S.; Daniell, H. Expression and characterization of antimicrobial peptides retrocyclin-101 and protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol. J. 2011, 9, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Ghag, S.B.; Shekhawat, U.K.; Ganapathi, T.R. Petunia floral defensins with unique prodomains as novel candidates for development of fusarium wilt resistance in transgenic banana plants. PLoS ONE 2012, 7, e39557. [Google Scholar] [CrossRef]
- Fukuta, S.; Kawamoto, K.-i.; Mizukami, Y.; Yoshimura, Y.; Ueda, J.-i.; Kanbe, M. Transgenic tobacco plants expressing antimicrobial peptide bovine lactoferricin show enhanced resistance to phytopathogens. Plant Biotechnol. 2012, 29, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.S.; Yajima, W.R.; Rahman, M.H.; Shah, S.; Liu, J.-J.; Ekramoddoullah, A.K.M.; Kav, N.N.V. A cysteine-rich antimicrobial peptide from Pinus monticola (PmAMP1) confers resistance to multiple fungal pathogens in canola (Brassica napus). Plant Mol. Biol. 2012, 79, 61–74. [Google Scholar] [CrossRef]
- Cabanos, C.; Ekyo, A.; Amari, Y.; Kato, N.; Kuroda, M.; Nagaoka, S.; Takaiwa, F.; Utsumi, S.; Maruyama, N. High-level production of lactostatin, a hypocholesterolemic peptide, in transgenic rice using soybean A1aB1b as carrier. Transgenic Res. 2013, 22, 621–629. [Google Scholar] [CrossRef]
- Zeitler, B.; Bernhard, A.; Meyer, H.; Sattler, M.; Koop, H.-U.; Lindermayr, C. Production of a de-novo designed antimicrobial peptide in Nicotiana benthamiana. Plant Mol. Biol. 2013, 81, 259–272. [Google Scholar] [CrossRef]
- Jung, Y.-J. Enhanced resistance to bacterial pathogen in transgenic tomato plants expressing cathelicidin antimicrobial peptide. Biotechnol. Bioprocess Eng. 2013, 18, 615–624. [Google Scholar] [CrossRef]
- Company, N.; Nadal, A.; La Paz, J.-L.; Martínez, S.; Rasche, S.; Schillberg, S.; Montesinos, E.; Pla, M. The production of recombinant cationic α-helical antimicrobial peptides in plant cells induces the formation of protein bodies derived from the endoplasmic reticulum. Plant Biotechnol. J. 2014, 12, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Montesinos, L.; Izquierdo, E.; Campo, S.; Mieulet, D.; Guiderdoni, E.; Rossignol, M.; Badosa, E.; Montesinos, E.; San Segundo, B.; et al. Production of cecropin A antimicrobial peptide in rice seed endosperm. BMC Plant Biol. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, S.; Stephan, A.; Hahn, S.; Bortesi, L.; Jarczowski, F.; Bettmann, U.; Paschke, A.-K.; Tusé, D.; Stahl, C.H.; Giritch, A.; et al. Broad and efficient control of major foodborne pathogenic strains of Escherichia coli by mixtures of plant-produced colicins. Proc. Natl. Acad. Sci. USA 2015, 112, E5454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephan, A.; Hahn-Löbmann, S.; Rosche, F.; Buchholz, M.; Giritch, A.; Gleba, Y. Simple purification of Nicotiana benthamiana-produced recombinant colicins: High-yield recovery of purified proteins with minimum alkaloid content supports the suitability of the host for manufacturing food additives. Int. J. Mol. Sci. 2017, 19, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Kamesh, A.C.; Xiao, Y.; Sun, V.; Hayes, M.; Daniell, H.; Koo, H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 2016, 105, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Vetchinkina, E.M.; Komakhina, V.V.; Vysotskii, D.A.; Zaitsev, D.V.; Smirnov, A.N.; Babakov, A.V.; Komakhin, R.A. Expression of plant antimicrobial peptide pro-SmAMP2 gene increases resistance of transgenic potato plants to Alternaria and Fusarium pathogens. Genetika 2016, 52, 1055–1068. [Google Scholar] [CrossRef]
- Hao, G.; Zhang, S.; Stover, E. Transgenic expression of antimicrobial peptide D2A21 confers resistance to diseases incited by Pseudomonas syringae pv. tabaci and Xanthomonas citri, but not Candidatus Liberibacter asiaticus. PLoS ONE 2017, 12, e0186810. [Google Scholar] [CrossRef]
- Paškevičius, Š.; Starkevič, U.; Misiūnas, A.; Vitkauskienė, A.; Gleba, Y.; Ražanskienė, A. Plant-expressed pyocins for control of Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badrhadad, A.; Nazarian-Firouzabadi, F.; Ismaili, A. Fusion of a chitin-binding domain to an antibacterial peptide to enhance resistance to Fusarium solani in tobacco (Nicotiana tabacum). 3 Biotech 2018, 8, 391. [Google Scholar] [CrossRef]
- Shi, X.; Cordero, T.; Garrigues, S.; Marcos, J.F.; Daròs, J.-A.; Coca, M. Efficient production of antifungal proteins in plants using a new transient expression vector derived from tobacco mosaic virus. Plant Biotechnol. J. 2019, 17, 1069–1080. [Google Scholar] [CrossRef]
- Shams, M.V.; Nazarian-Firouzabadi, F.; Ismaili, A.; Shirzadian-Khorramabad, R. Production of a recombinant dermaseptin peptide in Nicotiana tabacum hairy roots with enhanced antimicrobial activity. Mol. Biotechnol. 2019, 61, 241–252. [Google Scholar] [CrossRef]
- Khademi, M.; Varasteh-Shams, M.; Nazarian-Firouzabadi, F.; Ismaili, A. New recombinant antimicrobial peptides confer resistance to fungal pathogens in tobacco plants. Front. Plant Sci. 2020, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, E.; Sakowicz, T.; Kowalczyk, A.; Konieczka, M.; Grzegorczyk, J.; Sitarek, P.; Skała, E.; Czarny, P.; Śliwiński, T.; Kowalczyk, T. Production of recombinant colicin M in Nicotiana tabacum plants and its antimicrobial activity. Plant Biotechnol. Rep. 2020, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, J.; Doshi, K.; Dussault, M.; Hall, J.C.; Holbrook, L.; Jones, G.; Kaldis, A.; Klima, C.L.; Macdonald, P.; McAllister, T.; et al. Bringing plant-based veterinary vaccines to market: Managing regulatory and commercial hurdles. Biotechnol. Adv. 2015, 33, 1572–1581. [Google Scholar] [CrossRef] [Green Version]
| Expression System | Advantages | Disadvantages |
|---|---|---|
| Bacteria |
|
|
| Mammalian Cells |
|
|
| Yeast |
|
|
| Insect cells |
|
|
| Plants |
|
|
| Anti-Microbial Peptide | Plant Species | Stable or Transient | Nucleus or Chloroplast | Expression Level | Application | Reference |
|---|---|---|---|---|---|---|
| MSI-99 (Magainin) | Tobacco (Nicotiana tabacum) | Stable | Chloroplast | Undefined | Enhanced resistance to phytopathogenic bacteria (Pseudomonas syringae) and fungi (Aspergillus flavus; Fusarium moniliforme; Verticillium dahlia) | [177] |
| MsrA2 (Dermaseptin) | Potato (Solanum tuberosum) | Stable | Nucleus | 1–5 μg/g FW | Broad-range and enhanced resistance to virulent phytopathogenic fungi (Alternaria, Cercospora, Fusarium, Phytophthora, Pythium, Rhizoctonia; Verticillium sp.) and bacteria (Erwinia carotovora) | [178] |
| Tobacco (Nicotiana tabacum) | Stable | Nucleus | 6–7 μg/g FW | Resistance to phytopathogenic fungi (Fusarium solani; F. oxysporum; Alternaria alternata; Botrytis cinerea; Sclerotinia sclerotiorum), oomycete (Pythium aphanidermatum) and bacterium (Pectobacterium carotovorum) | [179] | |
| Thi2.1 (Thionin) | Tomato (Lycopersicon esculentum) | Stable | Nucleus | Undefined | Crop protection (F. oxysporum f. sp. lycopersici; R. solanacearum strain Pss4) | [180] |
| Mj-AMP2 (Knottin) | Rice (Oryza sativa) | Stable | Nucleus | 0.32–0.38% total protein | Enhanced resistance to fungal pathogen (Magnaporthe oryzae) | [181] |
| ChIFN-alpha (interferon-α) | Lettuce (Lactuca sativa) | Transient | Nucleus | 0.393 μg/kg FW | Antiviral activity against vesicular stomatitis virus (VSV) | [182] |
| Lipid Transfer Proteins (LTPs) | Tobacco (Nicotiana tabacum) | Stable | Nucleus | Undefined | Enhanced resistance to pathogen (Phytophthora nicotianae; Pseudomonas syringae pv. tabaci) | [183] |
| Dm-AMP1 (Defensin) | Rice (Oryza sativa) | Stable | Nucleus | 0.43–0.57% total soluble protein | Enhanced resistance to pathogen (Magnaporthe oryzae; Rhizoctonia solani) | [184] |
| rLF (Lactoferrin) | Rice (Oryza sativa) | Stable | Nucleus | 0.1% rice bran weight | Functional feed additive on early weaned piglets | [185] |
| Rs-AFP2 (Defensin) | Rice (Oryza sativa) | Stable | Nucleus | 0.45–0.53% total soluble protein | Enhanced resistance to fungal pathogen (Magnaporthe oryzae; Rhizoctonia solani) | [186] |
| CecB (Cecropin) | Tomato (Solanum lycopersicum) | Stable | Nucleus | 0.001 µg/mg FW | Plant protection against bacterial pathogens (Ralstonia solanacearum; Xanthomonas campestris) | [187] |
| Retrocyclin-101 (Defensin) | Tobacco (Nicotiana tabacum) | Stable | Chloroplast | 32–38% total soluble protein | Control viral (tobacco mosaic virus) and bacterial (Erwinia carotovora) infections | [188] |
| Protegrin-1 (Cathelicidin) | Tobacco (Nicotiana tabacum) | Stable | Chloroplast | 17–26% total soluble protein | Control bacterial infections (Erwinia carotovora) | [188] |
| Tobacco (Nicotiana tabacum) | Transient | Nucleus | Undefined | Control mammalian bacteria (Klebsiella pneumoniae; Staphylococcus aureus; Escherichia coli; Mycobacterium bovis) and fungal (Candida albicans) pathogens | [169] | |
| Petunia Floral defensins | Banana (Musa spp.) | Stable | Nucleus | Undefined | Effective resistance against pathogenic fungal Fusarium oxysporum f. sp. cubense (foc) infection | [189] |
| Snakin-2 (Snakin) | Tomato (Solanum lycopersicum) | Stable | Nucleus | Undefined | Enhanced resistance to Clavibacter michiganensis subsp. michiganensis | [175] |
| Lactoferricin B (Lactoferrin) | Tobacco (Nicotiana tabacum) | Stable | Nucleus | Undefined | Enhanced tolerance to pathogenic bacterial (Pseudomonas syringae pv. tabaci) and fungal (Botrytis cinerea) diseases | [190] |
| PmAMP1 (cysteine-rich protein) | Canola (Brassica napus) | Stable | Nucleus | Undefined | Effective resistance against fungal pathogens (Alternaria brassicae; Leptosphaeria maculans; Sclerotinia sclerotiorum) | [191] |
| hCAP18/LL-37 (Fusion of two cathelicidin antimicrobial proteins) | Chinese cabbage (Brassica rapa cv. Osome) | Stable | Nucleus | Undefined | Enhanced resistance to bacteria (P. carotovorum subsp. carotovorum) and fungal (Fusarium oxysporum f. sp. Lycopersici; Colletotrichum higginsianum; Rhizoctonia solani | [174] |
| Lactostatin (anionic peptide) | Rice (Oryza sativa) | Stable | Nucleus | 2 mg/g dry seeds | Anti-hypercholestero lemic drug for potential clinical use | [192] |
| SP1-1 (de-novo designed) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 0.025 mg/g FW | Antimicrobial activity (P. syringae pv. Syringae; P. syringae pv. Tomato; P. corrugate; Pectobacterium carotovorum ssp. carotovorum) | [193] |
| SN-1 (Snakin) | Wheat (Triticum aestivum) | Stable | Nucleus | Undefined | Antifungal activity in vitro and enhanced resistance to fungus (Gaeumannomyces graminis var. tritici) and | [176] |
| Thanatin (S) (synthetic thanatin) | Arabidopsis (Arabidopsis thaliana) | Stable | Nucleus | Undefined | Acquired resistance to bacterial pathogen (Pseudomonas syringae pv. tomato.) and fungal pathogens (Botrytis cinerea; powdery mildew) Antibacterial and antifungal activity in vitro | [173] |
| LL-37 (Cathelicidin) | Tomato (Solanum lycopersicum) | Stable | Nucleus | 16.8–58.2 µg/mL total soluble protein | Enhanced antibacterial activity (Pectobacterium carotovorum ssp. Carotovorum (Pcc); Xanthomonas campestris pv. Vesicatoria (Xcv) | [194] |
| Barley (Hordeum vulgare L.) | Stable | Nucleus | 0.55 mg/kg seeds | Antibacterial activity against E. coli TOP10 in vitro | [172] | |
| BP100.gtag (synthetic peptide) | Rice (Oryza sativa) | Stable | Nucleus | 0.5% total soluble protein | Plant protection against bacterial pathogens (Erwinia amylovora; Pseudomonas syringae; Xanthomonas axonopodis) | [195] |
| CecA (Cecropin) | Rice (Oryza sativa) | Stable | Nucleus | 1–4 μg/g seeds | Resistance to fungal pathogen (Fusarium verticillioides) and bacterial pathogen (Dickeya dadantii) | [196] |
| Recombinant colicins (Colicin) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 0.6–3 mg/g FW | Effective and broad control of foodborne pathogenic Escherichia coli strains | [197] |
| Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 0.58–2.31 mg/g FW | Broad activity, high potency, and purity as food antibacterial | [198] | |
| Retrocyclin | Tobacco | Stable | Chloroplast | 116 μg of RC101/g of lyophilized leaf | Effective against Streptococcus mutans and impaired biofilm formation following a single topical application of tooth-mimetic surface. | [199] |
| Protegrin | Tobacco | Stable | Chloroplast | Undefined | Effective against Streptococcus mutans and impaired biofilm formation following a single topical application of tooth-mimetic surface. | [199] |
| pro-SmAMP2 (Hevein-like peptide) | Potato (Solanum tuberosum) | Stable | Nucleus | Undefined | Crop protection from Alternaria sp. and Fusarium sp. pathogens in resistant potato cultivar | [200] |
| D2A21 (synthetic peptide) | Citrus fruit (Carrizo citrange) | Stable | Nucleus | Undefined | Reduced development of canker disease caused by bacterium (Xanthomonas citri) | [201] |
| PaeM4 (Pyocin) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 800 μg/g FW | Broad spectrum of antimicrobial activity against clinical isolates of Pseudomonas aeruginosa | [202] |
| CBD-alfAFP (Defensin) | Tobacco (Nicotiana tabacum) | Stable | Nucleus | Undefined | Enhanced resistance to plant pathogen (Fusarium solani) | [203] |
| LFchimera (Lactoferrin-derived peptides) | Tobacco (Nicotiana tabacum) | Stable | Nucleus | Undefined | Antimicrobial activity against clinical (Escherichia coli; Staphylococcus aureus) and phytopathogenic bacteria (Ralstonia solanacearum; Erwinia amylovira) | [171] |
| Tobacco (Nicotiana tabacum) | Suspension Cultures | Hairy roots | 4.8 μg/g FW | Effective antimicrobial activity against Escherichia coli | [170] | |
| Penicillium digitatum AfpB (antifungal protein) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 225 ± 37 µg/g FW | Protect tomato plants against Botrytis cinerea causing grey mold disease | [204] |
| DrsB1 (Dermaseptin) | Tobacco (Nicotiana tabacum) | Suspension culture | Nucleus | Undefined | Effective antimicrobial effects of plant bacterial and fungal phytopathogens | [205] |
| Tobacco (Nicotiana tabacum) | Stable | Nucleus | 5.5–6.0 µg/g FW | Enhanced resistance to plant pathogens (Alternaria alternata; Alternaria solani;, Fusarium oxysporum; Fusarium solani fungi) | [206] | |
| Laterosporulin-1 (synthetic anionic AMP/ELP fusion) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 375 µg/g FW | High antibacterial activity against Staphylococcus epidermidis | [161] |
| ADP2-3 (synthetic anionic AMP/ELP fusion) | Tobacco (Nicotiana benthamiana) | Transient | Nucleus | 563 µg/g FW | High antibacterial activity against Staphylococcus epidermidis | [161] |
| Colicin M (Colicin) | Tobacco (Nicotiana tabacum) | Stable | Nucleus | 2 mg/g FW | Antibacterial activity against control and clinical pathogens (Escherichia coli; Klebsiella pneumoniae) | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugaraj, B.; Bulaon, C.J.I.; Malla, A.; Phoolcharoen, W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules 2021, 26, 4032. https://doi.org/10.3390/molecules26134032
Shanmugaraj B, Bulaon CJI, Malla A, Phoolcharoen W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules. 2021; 26(13):4032. https://doi.org/10.3390/molecules26134032
Chicago/Turabian StyleShanmugaraj, Balamurugan, Christine Joy I. Bulaon, Ashwini Malla, and Waranyoo Phoolcharoen. 2021. "Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants" Molecules 26, no. 13: 4032. https://doi.org/10.3390/molecules26134032






