A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy
Abstract
:1. Introduction
2. Methods
- We included studies that met the following criteria:
- Adult patients with T2D.
- Treatment intervention involved resveratrol supplementation in patients with T2D on metformin-based hypoglycemic therapies.
- Patients with T2D on hypoglycemic therapy who did not receive resveratrol supplementation.
- The primary outcome of this meta-analysis was glucose control, whilst the secondary outcome was renal function.
2.1. Search Strategy and Selection
2.2. Data Extraction
2.3. Risk of Bias and Quality of Evidence assessment
2.4. Statistical Analysis
3. Results
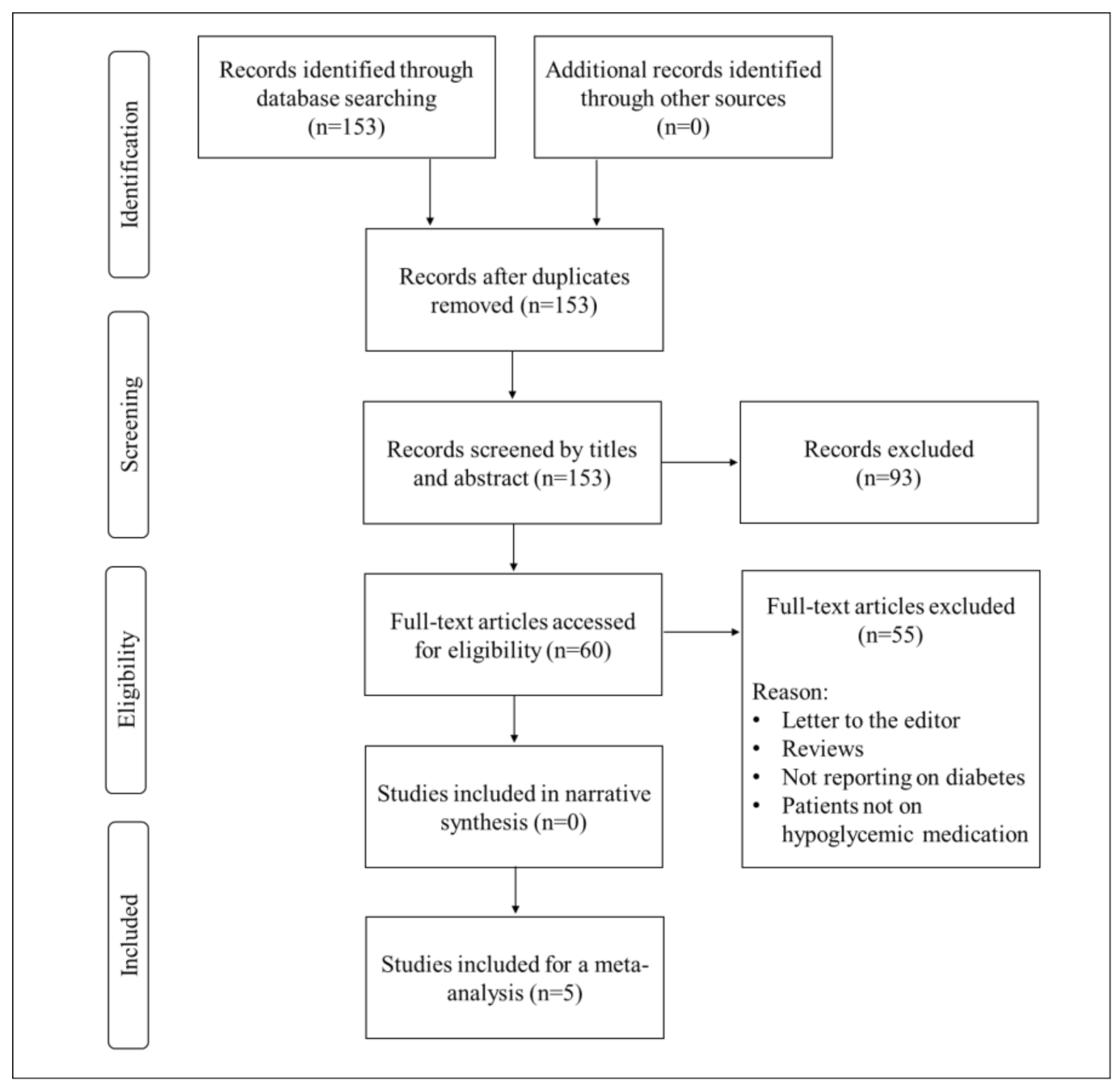
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment and Publication Bias
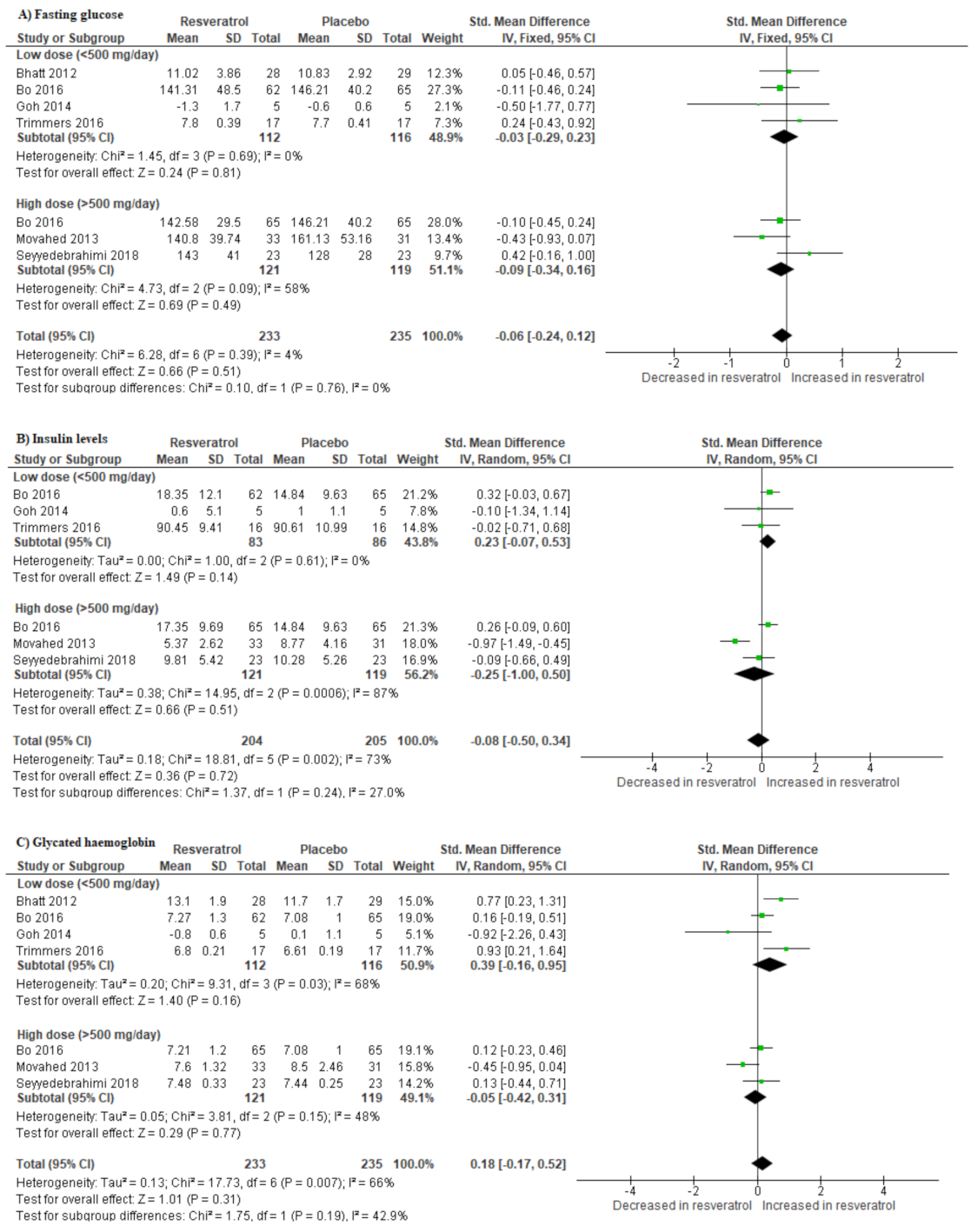
3.4. Impact of Resveratrol Supplementation on Basic Metabolic Parameters in T2D Patients on Hypoglycemic Medication
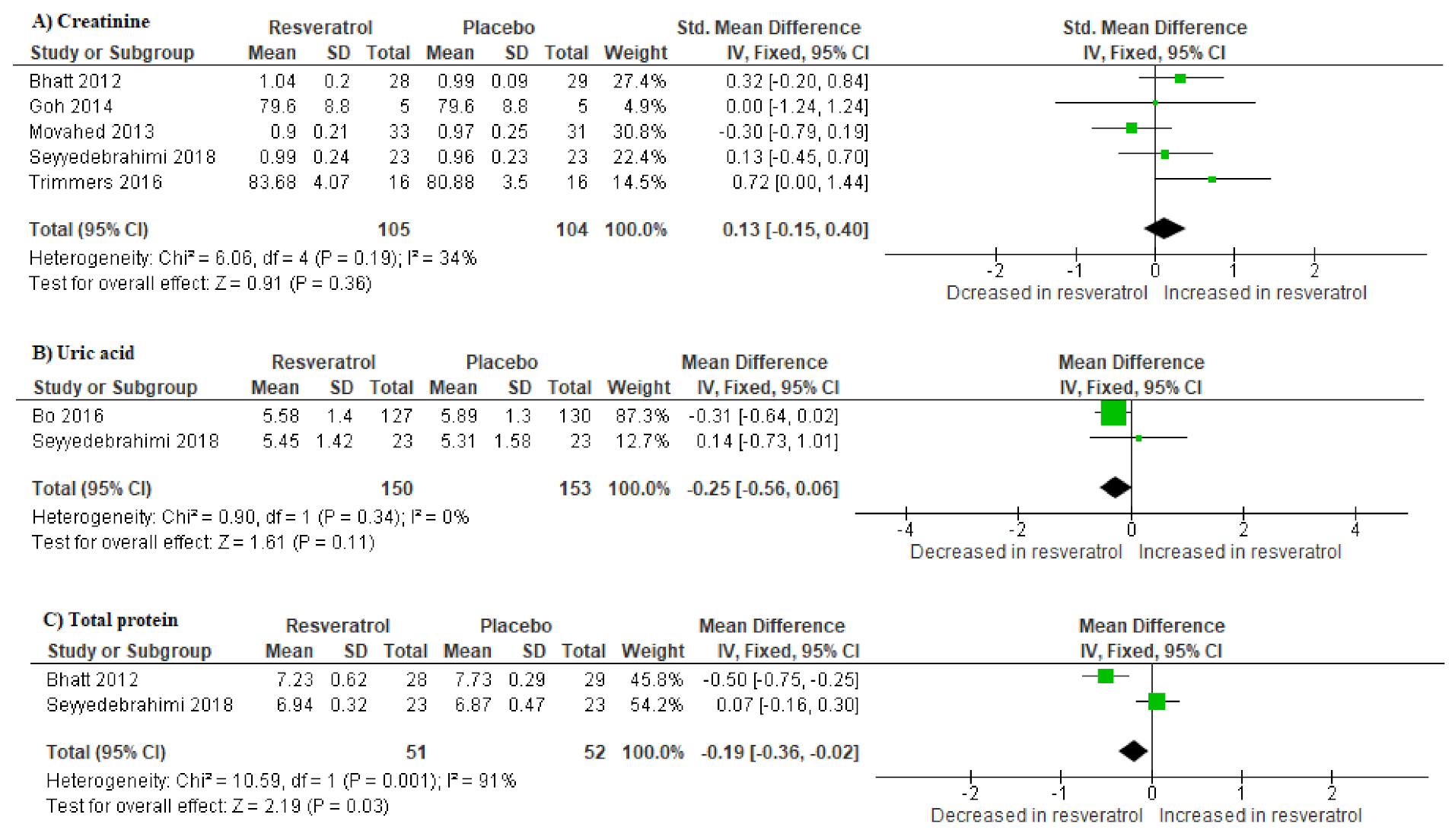
3.5. Impact of Resveratrol Supplementation on Markers of Renal Function in T2D Patients on Hypoglycemic Therapy
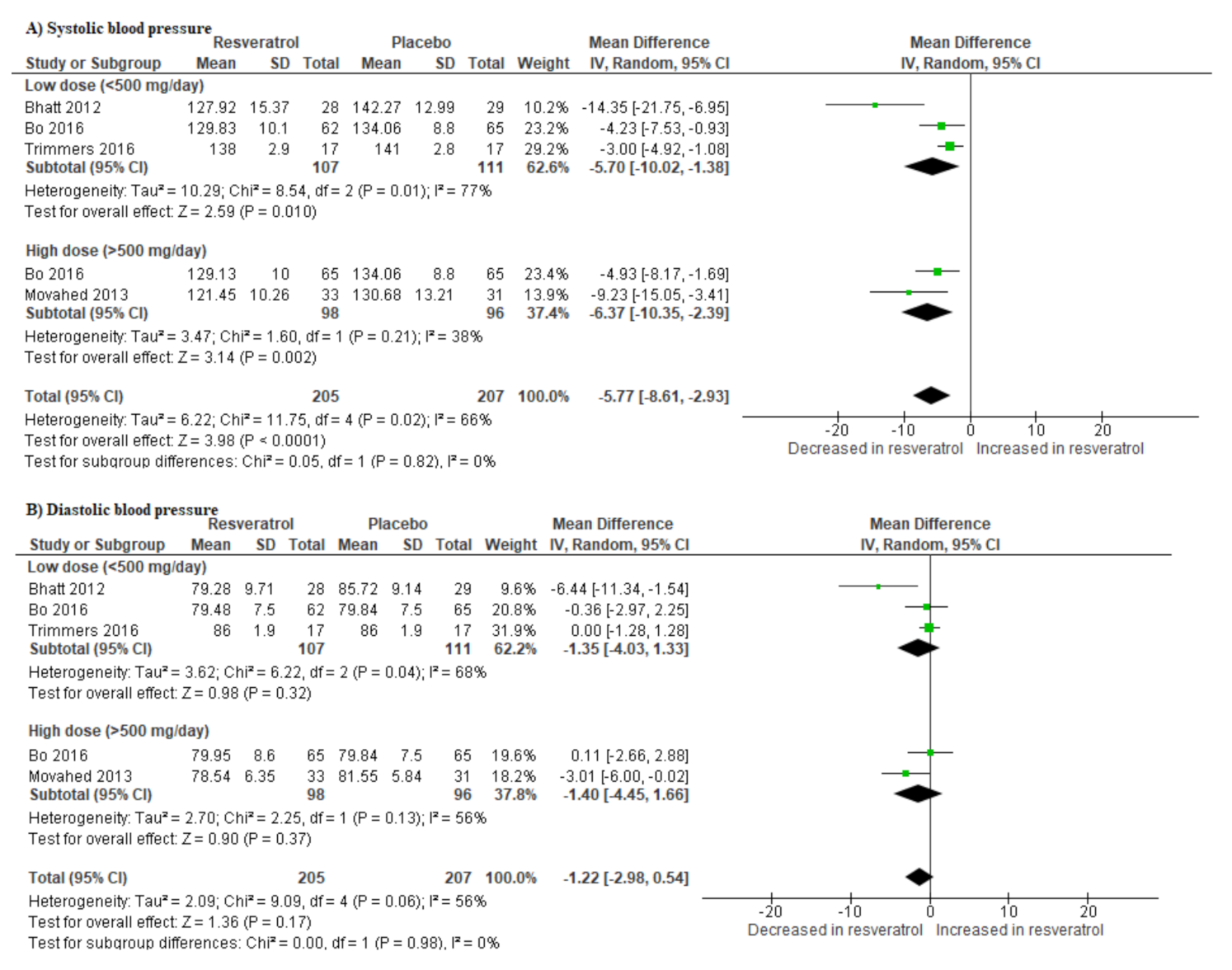
3.6. Resveratrol Supplementation Lowers Blood Pressure in T2D Patients on Hypoglycemic Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| CVDs | Cardiovascular diseases |
| FPG | Fasting plasma glucose |
| GRADE | Grading of Recommendations Assessment Development and Evaluation |
| HbA1c | Glycated hemoglobin |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analysis |
| RCT | Randomized controlled trials |
| SMD | Standardized mean difference |
| T2D | Type 2 diabetes |
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas Eighth Edition 2017. Available online: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html (accessed on 3 August 2020).
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/newsroom/fact-sheets/detail/noncommunicable-diseases (accessed on 2 August 2020).
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberer, F.; Pferschy, P.N.; Tripolt, N.; Sourij, C.; Obermayer, A.M.; Prüller, F.; Novak, E.; Reitbauer, P.; Kojzar, H.; Prietl, B.; et al. Hypoglycaemia leads to a delayed increase in platelet and coagulation activation markers in people with type 2 diabetes treated with metformin only: Results from a stepwise hypoglycaemic clamp study. Diabetes Obes. Metab. 2019, 22, 212–221. [Google Scholar] [CrossRef]
- Grundy, S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016, 64, 1082–1086. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Hoogeveen, R.C.; McNeill, A.M.; Heiss, G.; Schmidt, M.I.; Duncan, B.B.; Pankow, J.S. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int. J. Obes. 2008, 32 (Suppl. 2), S21–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conget, I. Diagnosis, classification and cathogenesis of diabetes mellitus. Rev. Española Cardiol. 2002, 55, 528–535. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.; Orci, L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975, 305, 14–16. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information, Metformin (PubChem CID: 4091). 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/409 (accessed on 5 August 2020).
- Mulherin, A.J.; Oh, A.H.; Kim, H.; Grieco, A.; Lauffer, L.M.; Brubaker, P.L. Mechanisms Underlying Metformin-Induced Secretion of Glucagon-Like Peptide-1 from the Intestinal L Cell. Endocrinology 2011, 152, 4610–4619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Gabuza, K.B.; Muller, C.J.F.; Joubert, E.; Louw, J.; Johnson, R. Aspalathin, a C-glucosyl Dihydrochalcone From Rooibos Improves the Hypoglycemic Potential of Metformin in Type 2 Diabetic (db/db) Mice. Physiol. Res. 2018, 67, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, G.; Limas, C.P.; Macías, P.C.; Estrella, A.; Maffioli, P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med. Sci. 2014, 10, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.L.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sanchez, E.; Uddin, M.S.; Bru, R.; Martinez-Marquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F.; et al. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2020, in press. [Google Scholar]
- Zemel, M.B.; Bruckbauer, A. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.J.; Frendo-Cumbo, S.; MacPherson, R.E.K. Resveratrol and Metformin Recover Prefrontal Cortex AMPK Activation in Diet-Induced Obese Mice but Reduce BDNF and Synaptophysin Protein Content. J. Alzheimer’s Dis. 2019, 71, 945–956. [Google Scholar] [CrossRef]
- Angel, D.-V.M.; Antonieta, G.-S.M.; Rocio, G.-C.; Jorge, R.-E.; Rosado, J.L.; Lourdes, R.-F. Effects of Combined Resveratrol Plus Metformin Therapy in db/db Diabetic Mice. J. Metab. Syndr. 2016, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Nanjan, M.J. Resveratrol supplementation in patients with type 2 diabetes mellitus: A prospective, open label, randomized controlled trial. Int. Res. J. Pharm. 2013, 4, 245–249. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evid. Based Complement. Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.H.X.; Nealon, R.S.; Scholey, A.; Howe, P.R.C. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [Green Version]
- Patney, V.; Whaley-Connell, A.; Bakris, G.L. Hypertension Management in Diabetic Kidney Disease. Diabetes Spectr. 2015, 28, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Steigerwalt, S. Management of Hypertension in Diabetic Patients with Chronic Kidney Disease. Diabetes Spectr. 2008, 21, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Nkambule, B.B. T-cell activation and cardiovascular risk in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin. Immunol. 2020, 210, 108313. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Esfahani, E.N.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef]
- Richardson, M.; Garner, P.; Donegan, S. Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin. Epidemiol. Glob. Health 2019, 7, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrition 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Dludla, P.V.; Silvestri, S.; Orlando, P.; Gabuza, K.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Johnson, R.; Muller, C.J.; et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrition 2020, 12, 739. [Google Scholar] [CrossRef] [Green Version]
- Skeie, G.; Braaten, T.; Hjartåker, A.; Lentjes, M.A.H.; Amiano, P.; Jakszyn, P.; Pala, V.; Palanca, A.; Niekerk, E.M.; Verhagen, H.; et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S226–S238. [Google Scholar] [CrossRef]
- Dickinson, A.; Blatman, J.; El-Dash, N.; Franco, J.C. Consumer Usage and Reasons for Using Dietary Supplements: Report of a Series of Surveys. J. Am. Coll. Nutr. 2014, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, A.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Effect of Resveratrol on Blood Lipid Levels in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Obesity 2019, 27, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. High Blood Pressure and Kidney Disease. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/high-blood-pressure (accessed on 2 September 2020).
- Kitada, M.; Koya, D. Renal Protective Effects of Resveratrol. Oxidative Med. Cell. Longev. 2013, 2013, 568093. [Google Scholar] [CrossRef] [Green Version]
- Hartogh, D.J.D.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrition 2019, 11, 1624. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, J.F.; Leal, V.D.O.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why Is It a Promising Therapy for Chronic Kidney Disease Patients? Oxidative Med. Cell. Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef]
| Author, Year | Country | Sample Size Average Age (% Male) | Experimental Model, Dose Used, and Intervention Period | Experimental Outcome and Proposed Mechanism |
|---|---|---|---|---|
| Bhatt et al., 2012 [24] | India | Total = 57 (n = 29 controls/n = 28 intervention) Average age = 57 (37%) | Type 2 diabetic (T2D) patients, on minimum of 6 months of ongoing oral hypoglycemic treatment (metformin and/or glibenclamide), received resveratrol at 250 mg/daily for 3 months | Supplementation with resveratrol significantly improved the mean glycated hemoglobin (HbA1c), systolic blood pressure, and total protein in T2D. No significant changes in body weight and high-density lipoprotein and low-density lipoprotein cholesterols |
| Movahed et al., 2013 [26] | Iran | Total = 66 (n = 33 controls/n = 33 intervention) Average age = 52 (50%) | T2D patients, on metformin therapy received resveratrol at a dose 1 g/day for 1½ months and a control group which received placebo tablets | Resveratrol treatment significantly decreased systolic blood pressure, fasting blood glucose, hemoglobin A1c, insulin, and insulin resistance, while high-density lipoprotein was significantly increased when compared to their baseline levels. Liver and kidney function markers were unchanged in the intervention group |
| Goh et al., 2014 [39] | Singapore | Total = 10 (n = 5 controls/n = 5 intervention) Average = 56 (100%) | T2D patients, oral hypoglycemic agents, received resveratrol at a dose of 3 g or placebo for 3 months | Resveratrol regulated energy expenditure through increased skeletal muscle NAD-dependent deacetylase sirtuin-1 (SIRT1) and 5′ AMP-activated protein kinase (AMPK) expression |
| Bo et al., 2016 [38] | Italy | Total = 192 (n = 62 control/n = 130 intervention) Average age = 65 (66%) | T2D patients received resveratrol supplementation at two different dosages (500 and 40 mg/day) for 6 months, of which 67.7% were on metformin | Treatment did not affect body weight, body mass index, waist circumference, and values of arterial blood pressure, fasting glucose, glycated hemoglobin, insulin, C-peptide, free fatty acids, liver transaminases, uric acid, adiponectin, and interleukin-6, in both the Resv500 and Resv40 arms vs. placebo. Total cholesterol and triglycerides slightly increased with resveratrol treatment |
| Timmers et al., 2016 [37] | The Netherlands | Total = 17 Average age = 64 (100%) | T2D patients treated with placebo and 150 mg/day resveratrol and average metformin dose of 2188 mg/day in a randomized double-blind crossover study for 1 month | Hepatic and peripheral insulin sensitivity were not affected by resveratrol treatment. Resveratrol also significantly improved ex vivo mitochondrial function |
| Seyyedebrahimi et al., 2018 [40] | Iran | Total = 46 (n = 23 controls/n = 23 intervention) Average age = 57 (86%) | T2D patients received resveratrol supplementation or placebo at a dose of 800 mg/day for 2 months | Resveratrol reduced plasma protein carbonyl content and significantly increased plasma total antioxidant capacity and total thiol content. However, it had no effect on metabolic parameters |
Sample Availability: No samples or compounds were used in the current study. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P.V. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645. https://doi.org/10.3390/molecules25235645
Nyambuya TM, Nkambule BB, Mazibuko-Mbeje SE, Mxinwa V, Mokgalaboni K, Orlando P, Silvestri S, Louw J, Tiano L, Dludla PV. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules. 2020; 25(23):5645. https://doi.org/10.3390/molecules25235645
Chicago/Turabian StyleNyambuya, Tawanda M., Bongani B. Nkambule, Sithandiwe E. Mazibuko-Mbeje, Vuyolwethu Mxinwa, Kabelo Mokgalaboni, Patrick Orlando, Sonia Silvestri, Johan Louw, Luca Tiano, and Phiwayinkosi V. Dludla. 2020. "A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy" Molecules 25, no. 23: 5645. https://doi.org/10.3390/molecules25235645





