Using Volatile Organic Compounds to Investigate the Effect of Oral Iron Supplementation on the Human Intestinal Metabolome
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient and Stool Sample Selection
4.2. Extraction of VOCs
4.3. Downstream Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017, 31, 225–233. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [Green Version]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [Green Version]
- Gereklioglu, C.; Asma, S.; Korur, A.; Erdogan, F.; Kut, A. Medication adherence to oral iron therapy in patients with iron deficiency anemia. Pak. J. Med. Sci. 2016, 32, 604–607. [Google Scholar] [CrossRef]
- Kortman, G.A.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef] [Green Version]
- Polage, C.R. Good and Bad Bacteria Fight for Iron in the Gut. Sci. Transl. Med. 2013, 5, 199. [Google Scholar] [CrossRef]
- Ahmed, I.; Greenwood, R.; Costello, B.; Ratcliffe, N.M.; Probert, C.S. Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 43, 596–611. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Melkina, O.E.; Khmel, I.A.; Plyuta, V.A.; Koksharova, O.A.; Zavilgelsky, G.B. Ketones 2-heptanone, 2-nonanone, and 2-undecanone inhibit DnaK-dependent refolding of heat-inactivated bacterial luciferases in Escherichia coli cells lacking small chaperon IbpB. Appl. Microbiol. Biotechnol. 2017, 101, 5765–5771. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef]
- Chambers, S.T.; Bhandari, S.; Scott-Thomas, A.; Syhre, M. Novel diagnostics: Progress toward a breath test for invasiveAspergillus fumigatus. Med. Mycol. 2011, 49, S54–S61. [Google Scholar] [CrossRef] [Green Version]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; De Simone, C.; Carbo, A.; Shaykhutdinov, R.; Jobin, C.; Arthur, J.C.; Corl, B.A.; Vogel, H.; et al. Probiotic Bacteria Produce Conjugated Linoleic Acid Locally in the Gut That Targets Macrophage PPAR γ to Suppress Colitis. PLoS ONE 2012, 7, e31238. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Read, S.; Asseman, C.; Malmstrom, V.; Mottet, C.; Stephens, L.A.; Stepankova, R.; Tlaskalova, H.; Powrie, F. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 2001, 182, 190–200. [Google Scholar] [CrossRef]
- Li, B.; Zheng, S.G. How regulatory T cells sense and adapt to inflammation. Cell. Mol. Immunol. 2015, 12, 519–520. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2016, 66, 863–871. [Google Scholar] [CrossRef]
- Mahalhal, A.; Williams, J.M.; Johnson, S.; Ellaby, N.; Duckworth, C.A.; Burkitt, M.D.; Liu, X.; Hold, G.; Campbell, B.J.; Pritchard, D.M.; et al. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS ONE 2018, 13, e0202460. [Google Scholar] [CrossRef] [Green Version]
- Nepelska, M.; Cultrone, A.; Béguet-Crespel, F.; Le Roux, K.; Doré, J.; Arulampalam, V.; Blottière, H.M. Butyrate Produced by Commensal Bacteria Potentiates Phorbol Esters Induced AP-1 Response in Human Intestinal Epithelial Cells. PLoS ONE 2012, 7, e52869. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Protect against Colitis by Regulating Gut Innate and Adaptive Immune Responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef] [Green Version]
- Reade, S.; Mayor, A.; Aggio, R.; Khalid, T.S.; Pritchard, D.M.; Ewer, A.K.; Probert, C.S. Optimisation of Sample Preparation for Direct SPME-GC-MS Analysis of Murine and Human Faecal Volatile Organic Compounds for Metabolomic Studies. J. Anal. Bioanal. Tech. 2014, 5. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry; Royal Society of Chemistry (RSC): Cambridge, UK, 2013. [Google Scholar]
- Aggio, R.B.M.; Villas−Bôas, S.G.; Ruggiero, K. Metab: An R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 2011, 27, 2316–2318. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]


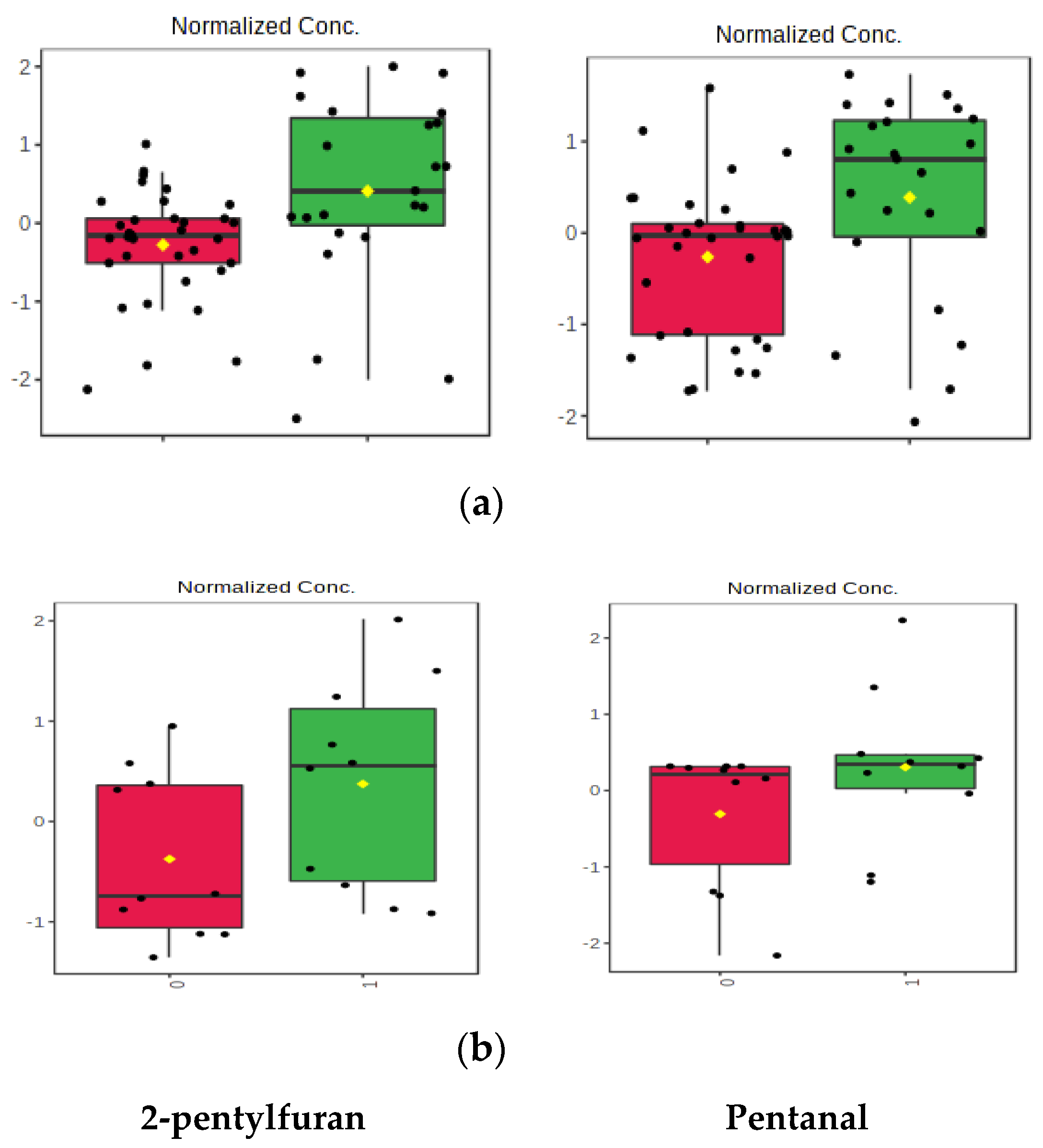
| Unpaired Samples, Group 1 | Paired Samples, Group 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Total Samples | Pre | Post | Total Samples | |
| Male:Female | 21:14 | 8:14 | 57 | 4:6 | 4:6 | 20 |
| Mean age (y) | 71.4 | 71.1 | 69.5 | NA | ||
| p | False Discovery Rate | Trend | |
|---|---|---|---|
| Octanal | 5.2 × 10−4 | 0.004 | Increase |
| Heptanal | 9.8 × 10−4 | 0.019 | Increase |
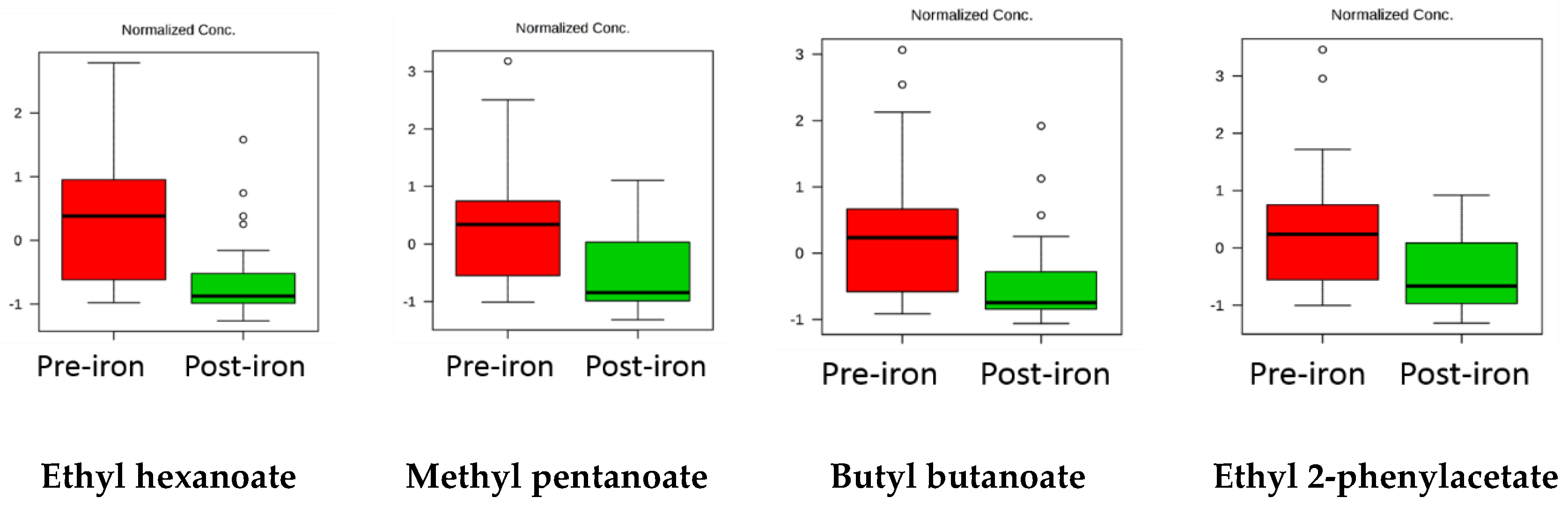
| Ethyl hexanoate | 4 × 10−4 | 0.015 | Decrease |
| 2,4-dimethylpentan-3-ol | 9.5 × 10−4 | 0.019 | Decrease |
| VOC That Decreased | Fold Change | VOC That Increased | Fold Change |
|---|---|---|---|
| 2,3,5-trimethylpyrazine | 15.4 | Octanal | 7.3 |
| Ethyl 2-phenylacetate | 14.9 | Heptanal | 4.2 |
| Ethyl hexanoate | 13.3 | 2-pentylfuran | 4.1 |
| Methyldisulfanylmethane | 12.0 | Pentanal | 3.1 |
| Heptanoic acid | 10.7 | 2-methyltetrazol-5-amine | 2.8 |
| 4-methylpentanoic acid | 8.4 | Heptan-2-one | 2.7 |
| Methyl pentanoate | 8.3 | Oct-1-en-3-ol | 2.6 |
| Butyl butanoate | 7.6 | ||
| Ethyl pentanoate | 5.9 | ||
| Ethyl butanoate | 5.6 | ||
| Methyl butanoate | 4.3 | ||
| 2,4-dimethylpentan-3-ol | 2.9 | ||
| Ethenylbenzene | 2.7 | ||
| 1,3-di-tert-butylbenzene | 2.6 | ||
| Hexanoic acid | 2.5 | ||
| 2-methylbutanoic acid | 2.5 | ||
| Tetradecane | 2.5 | ||
| 2-methylpropanoic acid | 2.4 | ||
| 5-methyloxolan-2-one | 2.4 | ||
| 2-methylpropanal | 2.3 | ||
| Ethenyl acetate | 2.1 | ||
| 6,6-dimethyl-2-methylenebicyclo3.1.1heptane | 2.1 | ||
| Acetic acid | 2.1 | ||
| 1-methyl-3-propan-2-ylbenzene | 2.1 |
| VOC That Decreased | Fold Change | VOC That Increased | Fold Change |
|---|---|---|---|
| (1R,5S,6R,7S,10R) 4,10-dimethyl-7-propan-2-yltricyclo(4.4.0.0,5)dec-3-ene | 20.1 | 2-pentylfuran | 3.5 |
| 3-isopropenyl-1-isopropyl-4-methyl-4-vinylcyclohexene | 14.0 | methyldisulfanylmethane | 3.4 |
| ethyl butanoate | 6.4 | cyclohexanecarboxylic acid | 2.9 |
| butan-1-ol | 6.4 | hexanal | 2.4 |
| 4-hydroxy-4-methylpentan-2-one | 5.0 | pentanal | 2.3 |
| 4Z-4,11,11-trimethyl-8-methylidenebicyclo(7.2.0)undec-4-ene | 4.6 | pentane-2,3-dione | 2.2 |
| 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | 4.5 | ||
| 1-methyl-3-propan-2-ylbenzene | 4.4 | ||
| (5s)- 2-methyl-5-propan-2-ylcyclohexa-1,3-diene | 4.3 | ||
| 5Z-2,6,10-trimethyl-1,5,9-undecatriene | 4.1 | ||
| 4-methyl-1-propan-2-ylbicyclo(3.1.0)hex-3-ene | 4.0 | ||
| 7-methyl-3-methylideneocta-1,6-diene | 4.0 | ||
| 6,6-dimethyl-2-methylenebicyclo(3.1.1)heptane | 3.5 | ||
| 4,7,7-trimethylbicyclo(4.1.0)hept-4-ene | 3.4 | ||
| 5-methylheptan-2-one | 3.3 | ||
| 4,6,6-trimethylbicyclo(3.1.1)hept-3-ene | 3.1 | ||
| 2-phenylethanol | 2.9 | ||
| ethenylbenzene | 2.7 | ||
| ethylbenzene | 2.3 | ||
| ethanol | 2.2 | ||
| 1,2-xylene | 2.2 |
Sample Availability: All faecal samples have been destroyed, as required by the Human Tissue Act 2004 (UK). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Slater, R.; Lewis, S.; Probert, C. Using Volatile Organic Compounds to Investigate the Effect of Oral Iron Supplementation on the Human Intestinal Metabolome. Molecules 2020, 25, 5113. https://doi.org/10.3390/molecules25215113
Ahmed A, Slater R, Lewis S, Probert C. Using Volatile Organic Compounds to Investigate the Effect of Oral Iron Supplementation on the Human Intestinal Metabolome. Molecules. 2020; 25(21):5113. https://doi.org/10.3390/molecules25215113
Chicago/Turabian StyleAhmed, Ammar, Rachael Slater, Stephen Lewis, and Chris Probert. 2020. "Using Volatile Organic Compounds to Investigate the Effect of Oral Iron Supplementation on the Human Intestinal Metabolome" Molecules 25, no. 21: 5113. https://doi.org/10.3390/molecules25215113





