Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study
Abstract
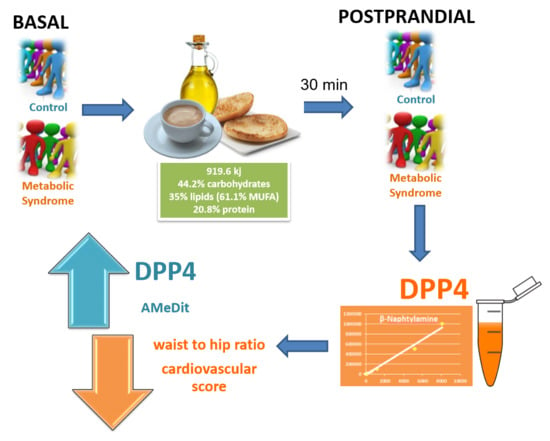
1. Introduction
2. Results
2.1. Anthropometric Indices, Cardiovascular Risk Score and Adherence to Mediterranean Diet
2.2. Biochemical Parameters
2.3. Dipeptidyl Peptidase 4 Activity
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Data Collection
4.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, P.S.; de Gasparo, M. Involvement of the pancreatic renin-angiotensin system in insulin resistance and the metabolic syndrome. J. Cardiometab. Syndr. 2006, 1, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, C.; Segura, J.; Praga, M.; Ruilope, L.M. Guidelines updates in the treatment of obesity or metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2013, 15, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Einarson, T.R.; Machado, M.; Henk Hemels, M.E. Blood glucose and subsequent cardiovascular disease: Update of a meta-analysis. Curr. Med. Res. Opin. 2011, 27, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: The European prospective investigation into cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.J.; Koska, J.; Reaven, P.; Migrino, R.Q. Vascular protective effects of diabetes medications that mimic or increase glucagon-like peptide-1 activity. Recent Pat. Cardiovasc. Drug Discov. 2012, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, E.; Rotella, C.M. Future perspectives on glucagon-like peptide-1, diabetes and cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase 4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Poreba, M.A.; Dong, C.X.; Li, S.K.; Stahl, A.; Miner, J.H.; Brubaker, P.L. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E899–E907. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, J.A.; de la Sacristana, A.G.; Sánchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Peréz-Martinez, P.; López-Miranda, J.; et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin resistant subjects. J. Am. Coll. Nutr. 2007, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Rotella, C.M.; Pala, L.; Mannucci, E. Glucagon-like peptide 1 (GLP-1) and metabolic diseases. J. Endocrinol. Investig. 2005, 28, 746–758. [Google Scholar] [CrossRef]
- De Meester, I.; Lambeir, A.M.; Proost, P.; Scharpé, S. Dipeptidyl peptidase IV substrate: An update on in vitro peptide hydrolysis by human DPPIV. Ad. Exp. Med. Biol. 2003, 524, 3–17. [Google Scholar]
- Zhong, J.; Maiseyeu, A.; Davis, S.N.; Rajagopalan, S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015, 116, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.K.; Lin, S.R.; Kankala, R.K.; Lee, C.H.; Weng, C.F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Lamers, D.; Famulla, S.; Wronkowitz, N.; Hartwig, S.; Lehr, S.; Ouwens, D.M.; Eckardt, K.; Kaufman, J.M.; Ryden, M.; Müller, S.; et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 2011, 60, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, T.; Gao, Y.; Baskota, A.; Chen, T.; Ran, X.; Tian, H. Increased Plasma DPP4 Activity is an independent predictor of the onset of Metabolic Syndrome in chinese over 4 years: Result from the China National Diabetes and Metabolic Disorders Study. PLoS ONE 2014, 9, e92222. [Google Scholar] [CrossRef] [PubMed]
- Arimura, S.T.; Moura, B.M.; Pimentel, G.D.; Silva, M.E.; Sousa, M.V. Waist circumference is better associated with high density lipoprotein (HDL-c) than with body mass index (BMI) in adults with metabolic syndrome. Nutr. Hosp. 2011, 26, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Bullo, M.; Salas-Salvado, J. Mediterranean diet and metabolic syndrome: The evidence. Public Health Nutr. 2009, 12, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D. Sex differences in the metabolic syndrome: Implications for cardiovascular health in women. Clin. Chem. 2014, 60, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E. Sex differences in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, L.; Alagona, C.; Palermo, F.; Piro, S.; Calanna, S.; Parrinello, G.; Purrello, F.; Rabuazzo, A.M. Early phase insulin secretion is increased in subjects with normal fasting glucose and metabolic syndrome: A premature feature of beta-cell dysfunction. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M.; Tojo, T.; Takahira, N.; Matsunaga, A.; Aoyama, N.; Masuda, T.; Izumi, T. Elevated circulating levels of an incretin hormone, glucagon-like peptide-1, are associated with metabolic components in high-risk patients with cardiovascular disease. Cardiovasc Diabetol. 2010, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, E.; Ognibene, A.; Cremasco, F.; Bardini, G.; Mencucci, A.; Pierazzuoli, E.; Ciani, S.; Fanelli, A.; Messeri, G.; Rotella, C.M. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with Type 2 diabetes mellitus. Diabet. Med. 2000, 17, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Deacon, C.F.; Madsbad, S.; Holst, J.J. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001, 50, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Sonne, J.; Madsbad, S.; Vølund, A.; Juul, A.G.; Holst, J.J. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, E.; Pala, L.; Ciani, S.; Bardini, G.; Pezzatini, A.; Sposato, I.; Cremasco, F.; Ognibene, A.; Rotella, C.M. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia 2005, 48, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Hayashi, H.; Sato, T.; Yamada, R.; Hiratsuka, M.; Hirasawa, N. Regulation of dipeptidyl peptidase 4 production in adipocytes by glucose. Diabetes Metab. Syndr. Obes. 2014, 7, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia 2013, 56, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Control Men | MS Men | Control Women | MS Women | |
|---|---|---|---|---|
| Body weight (kg) | 79.3 ± 4.06 | 90.1 ± 1.27 | 65.7 ± 2.87 | 80.6 ± 4.97 * |
| Body Mass Index (kg/m2) | 27.8 ± 1.16 | 33.5 ± 1.66 | 26.7 ± 1.38 | 32.8 ± 1.95 * |
| Waist circunference (cm) | 96.9 ± 3.68 | 112.9 ± 3.81 * | 85.2 ± 2.48 | 106.1 ± 4.12 *** |
| Waist to hip ratio | 1 ± 0.02 | 1.1 ± 0.03 ** | 0.9 ± 0.01 | 1 ± 0.01 *** aaa |
| Bicipital skinfold (mm) | 7.4 ± 0.85 | 13.8 ± 1.55 * | 13.1 ± 0.74 a | 18.3 ± 2.09 * |
| Tricipital skinfold (mm) | 10.9 ± 1.27 | 13.4 ± 1.56 | 23.1 ± 1.58 aaa | 28.3 ± 2.65 aaa |
| Subscapular skinfold (mm) | 18.2 ± 2.30 | 31.2 ± 1.98 ** | 20.2 ± 1.73 | 31.6 ± 2.64 ** |
| Suprailiac skinfold (mm) | 10.2 ± 1.07 | 19.9 ± 2.63 ** | 16.1 ± 1.43 | 23.8 ± 1.63 ** |
| Cardiovascular score | 2.3 ± 0.40 | 7.8 ± 1.67 ** | 1.2 ± 0.21 | 4.7 ± 1.47 * |
| AMeDit | 8.7 ± 0.59 | 7.0 ± 0.67 | 8.6 ± 0.51 | 6.1 ± 0.58 * |
| Control Men | MS Men | Control Women | MS Women | |
|---|---|---|---|---|
| Fasting glucose (mg/dL) | 96.4 ± 3.60 | 174.1 ± 17.99 *** | 90.4 ± 2.14 | 115.1 ± 9.96 aa |
| Fasting insulin (µU/mL) | 7.8 ± 1.15 | 11.6 ± 2.76 | 5.6 ± 0.68 | 10.9 ± 1.34 |
| HbA1 (%)c | 5.4 ± 0.12 | 7.9 ± 0.58 ** | 5.5 ± 0.07 | 6.5 ± 0.53 |
| Postprandial insulin (µU/mL) | 9.5 ± 1.09 | 17.5 ± 2.66 *** | 13.0 ± 1.27 | 17.2 ± 2.35 |
| GLP-1 (pg/mL) | 34.7 ± 4.20 | 53.6 ± 8.28 | 68 ± 9.66 a | 52.9 ± 6.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Durillo, F.T.; Segarra, A.B.; Villarejo, A.B.; Ramírez-Sánchez, M.; Prieto, I. Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules 2018, 23, 1564. https://doi.org/10.3390/molecules23071564
Pérez-Durillo FT, Segarra AB, Villarejo AB, Ramírez-Sánchez M, Prieto I. Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules. 2018; 23(7):1564. https://doi.org/10.3390/molecules23071564
Chicago/Turabian StylePérez-Durillo, Francisco Tomás, Ana Belén Segarra, Ana Belén Villarejo, Manuel Ramírez-Sánchez, and Isabel Prieto. 2018. "Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study" Molecules 23, no. 7: 1564. https://doi.org/10.3390/molecules23071564
APA StylePérez-Durillo, F. T., Segarra, A. B., Villarejo, A. B., Ramírez-Sánchez, M., & Prieto, I. (2018). Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules, 23(7), 1564. https://doi.org/10.3390/molecules23071564