The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic and Antioxidant Characterization of Grape Seeds
2.2. Phenolic Profile of Wines and Evolution
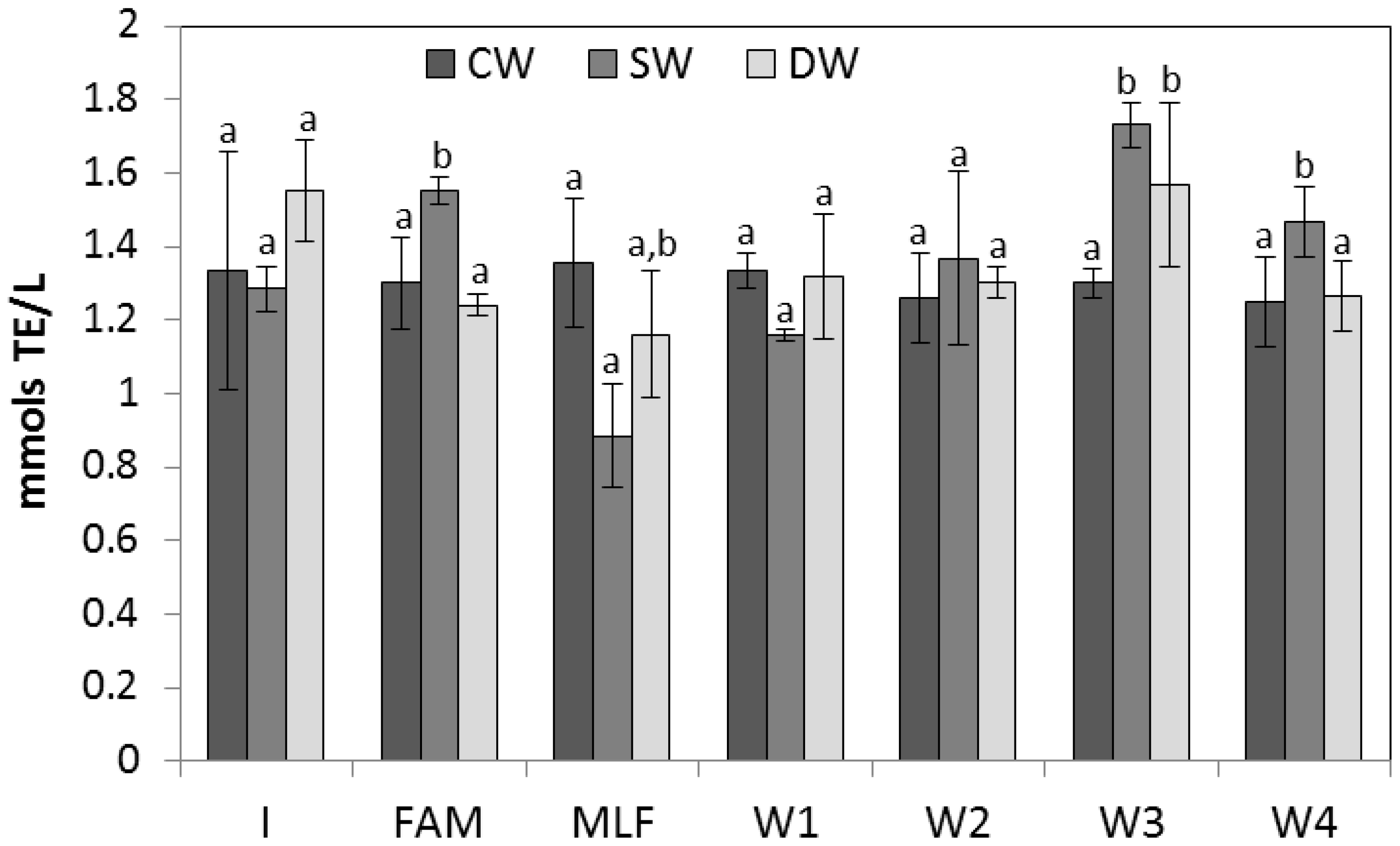
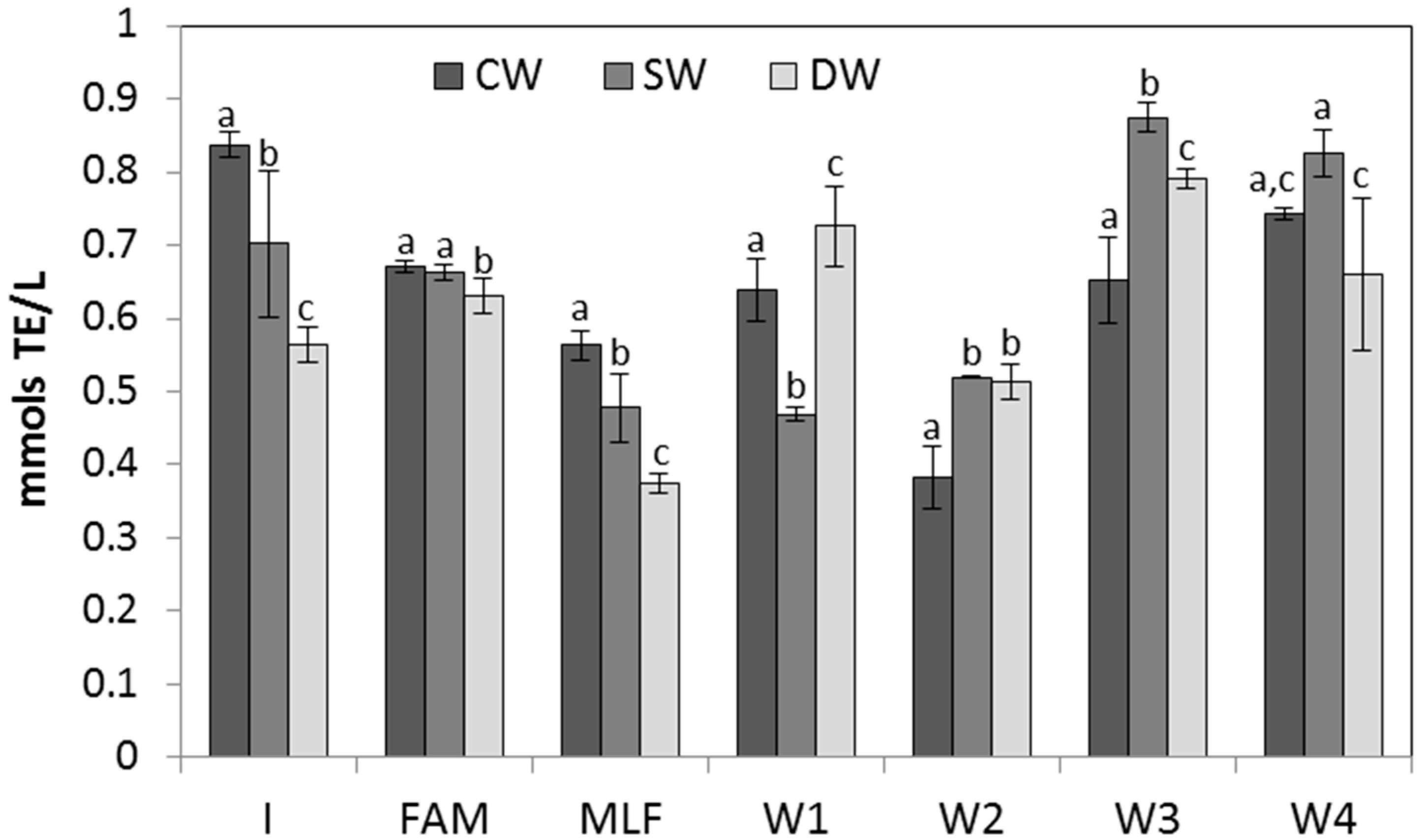
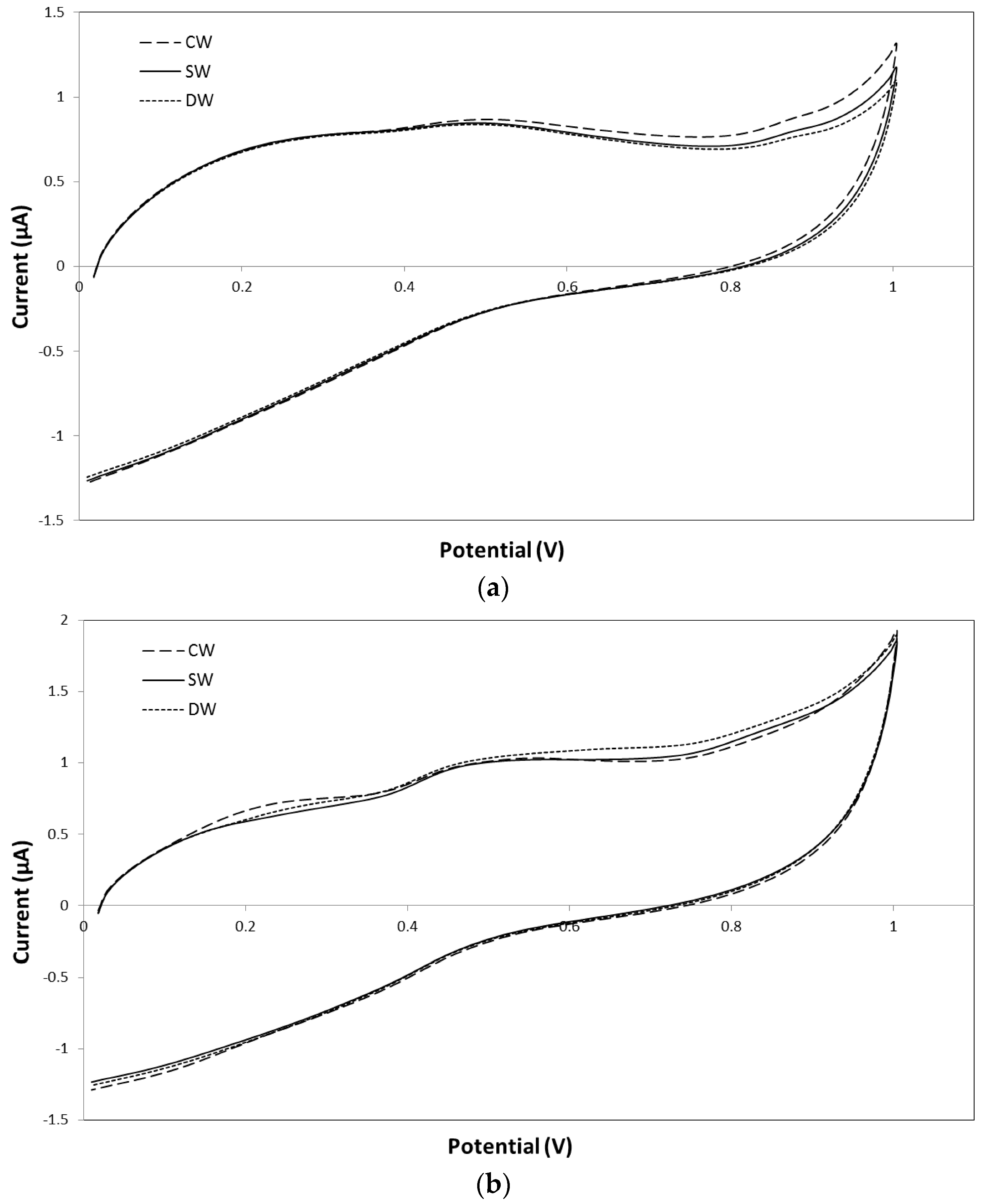
2.3. Antioxidant Activity of Wines and Evolution
3. Experimental Section
3.1. Chemical and Reagents
3.2. Samples and Winemaking
3.3. Extraction of Phenolic Compounds from Seeds
3.4. Determination of Total Phenolic Content
3.5. Determination of Total Flavonoids
3.6. UHPLC-DAD Analysis of Phenolic Compounds
3.7. ABTS/Persulfate Assay
3.8. FRAP Assay
3.9. Cyclic Voltammetry
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.A.; Nitzke, J.A.; Klipel, C.B.; de Jong, E.V. Chocolate and red wine—A comparison between flavonoids content. Food Chem. 2010, 120, 109–112. [Google Scholar] [CrossRef]
- Crozier, A.; del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, F.M.; Bearden, M.M.; Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hoensch, H.P.; Reinhard, O. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nut. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Hu, H.-L.; Forsey, R.J.; Blades, T.J.; Barratt, M.E.J.; Parmar, P.; Powell, J.R. Antioxidants may contribute in the fight against ageing: An in vitro model. Mech. Ageing Dev. 2000, 121, 217–230. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Serafini, M.; Estruch, R.; Lamuela-Raventós, R.M.; Martínez-González, M.A.; Salas-Salvadó, J.; Fiol, M.; Lapetra, J.; Arós, F.; Covas, M.I.; et al. PREDIMED Study Investigators. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: Evidence for am mechanism of antioxidant tuning. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, A.; Nuviala, A.; Fernandez-Martinez, A.; Porcel-Galvez, A.M.; Moral-Garcia, J.E.; Martinez-Lopez, E.J. Adherence to the Mediterranean diet in rural and urban adolescents of southern Spain, life satisfaction, anthropometry, and physical and sedentary activities. Nutr. Hosp. 2013, 28, 1129–1135. [Google Scholar] [PubMed]
- Tauchen, J.; Marsik, P.; Kvasnicova, M.; Maghradze, D.; Kokoska, L.; Vanek, T.; Landa, P. In vitro antioxidant activity and phenolic composition of Georgian, Central and West European wines. J. Food Compos. Anal. 2015, 41, 113–121. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marino, M.; Hernandez-Hierro, J.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Escribano-Bailon, M.T. Multivariate analysis of the polyphenol composition of Tempranillo and Graciano red wines. Talanta 2011, 85, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Saliba, A.J.; Prenzler, P.D. Should Red Wine Be Considered a Functional Food? Compr. Rev. Food Sci. Food Saf. 2010, 9, 530–551. [Google Scholar] [CrossRef]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. ESPEN 2015, 10, e224–e233. [Google Scholar] [CrossRef]
- Biagi, M.; Bertelli, A.A. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Prenzler, P.D.; Saliba, A.J.; Ryan, D. Assessment of some Australian red wines for price, phenolic content, antioxidant activity, and vintage in relation to functional food prospects. J. Food Sci. 2011, 76, C1355–C1364. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Saliba, A.J.; Prenzler, P.D.; Ryan, D. Total phenolic content, antioxidant activity, and cross-cultural consumer rejection threshold in white and red wines functionally enhanced with catechin-rich extracts. J. Agric. Food Chem. 2012, 60, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Saliba, A.J.; MacDonald, J.B.; Prenzler, P.D.; Ryan, D. A cross-cultural study of wine consumers with respect to health benefits of wine. Food Qual. Preference 2013, 28, 531–538. [Google Scholar] [CrossRef]
- Saliba, A.J.; Moran, C.C. The influence of perceived healthiness on wine consumption patterns. Food Qual. Preference 2010, 21, 692–696. [Google Scholar] [CrossRef]
- Gordillo, B.; Rodriguez-Pulido, F.J.; Mateus, N.; Escudero-Gilete, M.L.; Gonzalez-Miret, M.L.; Heredia, F.J.; de Freitas, V. Application of LC-MS and tristimulus colorimetry to assess the ageing aptitude of Syrah wine in the Condado de Huelva D.O. (Spain), a typical warm climate region. Anal. Chim. Acta 2012, 732, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J. Application of the differential colorimetry and polyphenolic profile to the evaluation of the chromatic quality of Tempranillo red wines elaborated in warm climate. Influence of the presence of oak wood chips during fermentation. Food Chem. 2013, 141, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, B.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Quijada-Morín, N.; Rivas-Gonzalo, J.C.; García-Estévez, I.; Heredia, F.J.; Escribano-Bailón, M.T. Application of differential colorimetry to evaluate anthocyanin-flavonol-flavanol ternary copigmentation interactions in model solutions. J. Agric. Food Chem. 2015, 63, 7645–7653. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pulido, F.J.; Barbin, D.F.; Sun, D.-W.; Gordillo, B.; González-Miret, M.L.; Heredia, F.J. Grape seed characterization by NIR hyperspectral imaging. Postharvest Biol. Technol. 2013, 76, 74–82. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Hernández-Hierro, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J. Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: A preliminary approach. Food Chem. 2014, 152, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodriguez-Pulido, F.J.; Jara-Palacios, M.J.; Ramirez-Perez, P.; Gonzalez-Miret, M.L.; Heredia, F.J. Impact of adding white pomace to red grapes on the phenolic composition and color stability of Syrah wines from a warm climate. J. Agric. Food Chem. 2014, 62, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Rodriguez-Morgado, B.; Jara-Palacios, M.J.; Rivas-Gonzalo, J.C.; Parrado, J.; Heredia, F.J. Pre-fermentative addition of an enzymatic grape seed hydrolysate in warm climate winemaking. Effect on the differential colorimetry, copigmentation and polyphenolic profiles. Food Chem. 2016, 209, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Del Carmen Llaudy, M.; Canals, J.M.; Zamora, F. Influence of the elimination and addition of seeds on the colour, phenolic composition and astringency of red wine. Eur. Food Res. Technol. 2008, 226, 1183–1190. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Carmona, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Pre-bottling use of dehydrated waste grape skins to improve colour, phenolic and aroma composition of red wines. Food Chem. 2013, 136, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In vitro and in vivo evaluation of the antioxidant and prooxidant activity of phenolic compounds obtained from grape (Vitis vinifera) pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Escudero-Gilete, M.L.; Heredia, F.J. Detailed phenolic composition of white grape by-products by RRLC/MS and measurement of the antioxidant activity. Talanta 2014, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Žemlička, M.; Rivas-Gonzalo, J.C. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. Int. J. Mol. Sci. 2007, 8, 797–809. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y.; Romeo, T.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between grape-derived proanthocyanidins and cell wall material. 2. Implications for vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between grapederived proanthocyanidins and cell wall material. 1. Effect on proanthocyanidin composition and molecular mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Brás, N.F.; García-Estévez, I.; Mateus, N.; Rivas-Gonzalo, J.C.; de Freitas, V.; Escribano-Bailón, M.T. Effect of flavonols on wine astringency and their interaction with human saliva. Food Chem. 2016, 209, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P.A. Electrochemistry applied to the analysis of wine: A mini-review. Electrochem. Commun. 2016, 67, 39–42. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.J.; Rego, R.; Ferreira, M.; Oliveira, M.C. Comparative study of the antioxidant capacity and polyphenol content of Douro wines by chemical and electrochemical methods. Food Chem. 2013, 141, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Hosu, A.; Cristea, V.M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
| Phenolic Compounds | Concentration (mg/100 g) |
|---|---|
| Benzoic acids | |
| Gallic acid | 35.84 ± 0.07 |
| Protocatechuic acid | 9.25 ± 0.07 |
| Hydroxycinnamic acids | |
| Caffeic acid | 7.60 ± 0.01 |
| p-Coumaric acid | 10.00 ± 0.03 |
| t-Coutaric acid | 10.59 ± 0.02 |
| Flavanols | |
| Catechin | 87.59 ± 0.72 |
| Epicatechin | 85.04 ± 2.31 |
| Epicatechin gallate | 48.90 ± 0.35 |
| Procyanidin B1 | 74.62 ± 0.21 |
| Procyanidin B2 | 61.54 ± 1.05 |
| Procyanidin B3 | 31.50 ± 0.63 |
| Procyanidin B4 | 26.03 ± 0.08 |
| Procyanidin trimer 1 | 27.98 ± 0.06 |
| Procyanidin trimer 2 | 38.09 ± 0.02 |
| Procyanidin tetramer 1 | 27.43 ± 0.29 |
| Procyanidin tetramer 1 | 41.78 ± 0.06 |
| Procyanidin B2-3-O-gallate | 78.35 ± 1.26 |
| Galloyled procyanidin 1 | 30.46 ± 0.21 |
| Galloyled procyanidin 2 | 45.07 ± 0.42 |
| ΣPhenols | 780.64 ± 0.74 |
| TPC (mg/100 g) | 5534.67 ± 17.80 |
| ABTS (mmol TE/100 g) | 48.24 ± 1.40 |
| FRAP (mmol TE/100 g) | 22.69 ± 3.12 |
| Stage | CW | SW | DW | |
|---|---|---|---|---|
| TPC | I | 2379.72 a ± 109.95 | 2279.07 a ± 116.51 | 2281.97 a ± 46.81 |
| FAM | 2046.72 a ± 52.18 | 2221.79 a ± 150.75 | 2083.11 a ± 80.42 | |
| MLF | 2073.42 a ± 104.95 | 1928.82 a ± 40.71 | 1996.15 a ± 200.05 | |
| W1 | 2230.32 a ± 107.68 | 2280.46 a ± 230.09 | 2299.98 a ± 329.48 | |
| W2 | 2313.81 a ± 62.51 | 2387.15 a ± 31.66 | 2274.15 a ± 175.65 | |
| W3 | 2457.68 a ± 245.46 | 2833.98 b ± 212.47 | 2472.35 a ± 257.45 | |
| W4 | 2291.38 a ± 152.81 | 2344.54 a ± 88.37 | 2320.39 a ± 64.19 | |
| TFC | I | 1074.20 a ± 30.30 | 870.16 b ± 3.54 | 867.39 b ± 3.59 |
| FAM | 941.23 a ± 87.57 | 968.44 a ± 36.78 | 706.25 b ± 49.94 | |
| MLF | 1004.12 a ± 88.16 | 743.88 b ± 49.35 | 848.56 b ± 46.14 | |
| W1 | 955.87 a ± 20.13 | 924.51 a ± 49.43 | 939.33 a ± 23.89 | |
| W2 | 1032.73 a ± 47.19 | 934.43 b ± 33.82 | 1027.73 a ± 63.03 | |
| W3 | 1036.24 a ± 18.13 | 1152.88 b ± 95.23 | 923.51 c ± 1.68 | |
| W4 | 1140.15 a ± 34.95 | 1192.96 a ± 46.01 | 1046.38 b ± 64.40 |
| Stage | CW | SW | DW | |
|---|---|---|---|---|
| I | QT | 0.740 | 0.708 | 0.693 |
| QI | 0.191 | 0.191 | 0.189 | |
| QII | 0.282 | 0.274 | 0.271 | |
| QIII | 0.267 | 0.243 | 0.233 | |
| W4 | QT | 0.900 | 0.887 | 0.925 |
| QI | 0.184 | 0.170 | 0.175 | |
| QII | 0.330 | 0.328 | 0.344 | |
| QIII | 0.386 | 0.389 | 0.405 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines. Molecules 2016, 21, 1526. https://doi.org/10.3390/molecules21111526
Jara-Palacios MJ, Hernanz D, Escudero-Gilete ML, Heredia FJ. The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines. Molecules. 2016; 21(11):1526. https://doi.org/10.3390/molecules21111526
Chicago/Turabian StyleJara-Palacios, María José, Dolores Hernanz, María Luisa Escudero-Gilete, and Francisco J. Heredia. 2016. "The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines" Molecules 21, no. 11: 1526. https://doi.org/10.3390/molecules21111526