Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro α-Amilase and α-Glucosidase Inhibitory Activities
Abstract
:1. Introduction
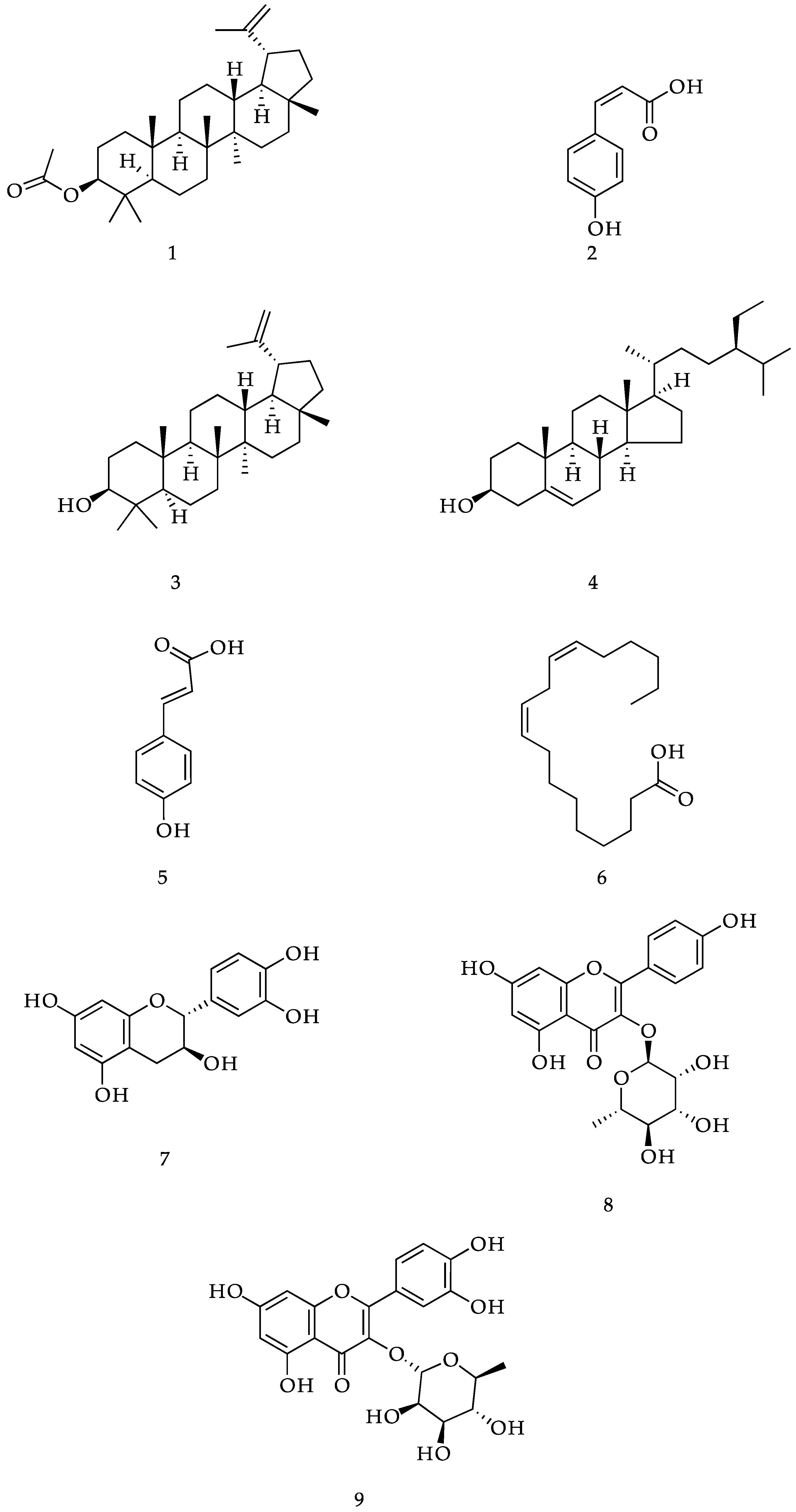
2. Results and Discussion
α-Amylase and α-Glucosidase Inhibition Activity
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation of Compounds
3.4. Measurement of α-Amylase Inhibitory Activity
3.5. α-Glucosidase Inhibition Assay
3.6. Physical and Spectral Data of Isolated Compounds
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Global Report on Diabetes. Available online: http://www.who.int/diabetes/global-report/en/ (accessed on 15 July 2016).
- WHO. Diabetes Country Profiles 2016. Available online: www.who.int/diabetes/country-profiles/en/ (accessed on 15 July 2016).
- Bhutkar, M.A.; Bhise, S.B. In vitro assay of α-amylase inhibitory activity of some indigenous plants. Int. J. Chem. Sci. 2012, 10, 457–462. [Google Scholar]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The genus rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Trojan-Rodrigues, M.; Alves, T.L.S.; Soares, G.L.G.; Ritter, M.R. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, Southern Brazil. J. Ethnopharmacol. 2012, 139, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Povi, L.E.; Batomayena, B.; Hodé, T.A.; Kwashie, E.G.; Kodjo, A.; Messanvi, G. Phytochemical screening, antioxidant and hypoglycemic activity of Coccoloba uvifera leaves and Waltheria indica roots extracts. Int. J. Pharm. Pharm. Sci. 2015, 7, 279–283. [Google Scholar]
- Chen, Z.Q.; Wang, J.J. Hypoglycemic and antioxidant effects of Rheum franzenbachii extract in streptozotocin-induced diabetic rats. Pharm. Biol. 2010, 48, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Naqishbandi, A.M.; Josefsen, K.; Pedersen, M.E.; Jger, A.K. Hypoglycemic activity of Iraqi Rheum ribes root extract. Pharm. Biol. 2009, 47, 380–383. [Google Scholar] [CrossRef]
- Choi, S.Z.; Lee, S.O.; Jang, K.U.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch. Pharm. Res. 2005, 28, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Jørgesen, P.M.; León-Yánez, S. Catalogue of The Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 1–1181. [Google Scholar]
- Burnie, G.; Forrester, S.; Greig, D. Botánica Guía Ilustrada de Plantas : Más de 10000 Especies de la A la Z y Cómo Cultivarlas; Konemann: Hong Kong, China, 2006. [Google Scholar]
- Fagundes, L.L.; Vieira, G.D.-V.; de Pinho, J.D.J.R.G.; Yamamoto, C.H.; Alves, M.S.; Stringheta, P.C.; de Sousa, O.V. Pharmacological proprieties of the ethanol extract of Muehlenbeckia platyclada (F. Muell.) meisn. Leaves. Int. J. Mol. Sci. 2010, 11, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Madrid, A.; Peña-Cortés, H.; López, R.; Jara, C.; Espinoza, L. Antioxidant activity of anthraquinones isolated from leaves of Muehlenbeckia hastulata (J.E. Sm.) johnst. (polygonaceae). J. Chil. Chem. Soc. 2013, 58, 1767–1770. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Muriel, M.P.; Macia, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador; Herbario QCA & Herbario AAU: Quito, Ecuador; Aarhus, Denmark, 2008. [Google Scholar]
- Pablo, M.; Lilia, G.; Judith, H.; Carmela, G. Anthraquinone pigments in Muehlenbeckia tamnifolia and Muehlenbeckia vulcanica. Politecnica 1973, 3, 111–122. [Google Scholar]
- Yen, C.-T.; Hsieh, P.-W.; Hwang, T.-L.; Lan, Y.-H.; Chang, F.-R.; Wu, Y.-C. Flavonol glycosides from Muehlenbeckia platyclada and their anti-inflammatory activity. Chem. Pharm. Bull. 2009, 57, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.K.; Yaacob, W.A.; Din, L.B. A chemical study on phyllanthus reticulatus. J. Phys. Sci. 2008, 19, 45–50. [Google Scholar]
- Kort, R.; Vonk, H.; Xu, X.; Hoff, W.D.; Crielaard, W.; Hellingwerf, K.J. Evidence for trans-cis isomerization of the p-coumaric acid chromophore as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 1996, 382, 73–78. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rahman, S.; Akhtar, P.; Islam, F.; Rahman, M.; Yaakob, Z. Isolation of steroids from N-hexane extract of the leaves of Saurauia roxburghii. Int. Food Res. J. 2013, 20, 2939–2943. [Google Scholar]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Tu, P.-F. Preparative isolation and purification of trans-3,5,4′-trihydroxystilbene-4′-O-β-d-glucopyranoside and (+)catechin from Rheum tanguticum maxim. ex balf. Using high-speed counter-current chromatography by stepwise elution and stepwise increasing the flow-rate of the mobile phase. J. Chromatogr. A 2005, 1092, 241–245. [Google Scholar] [PubMed]
- Lee, S.Y.; So, Y.J.; Shin, M.S.; Cho, J.Y.; Lee, J. Antibacterial effects of afzelin isolated from cornus macrophylla on Pseudomonas aeruginosa, a leading cause of illness in immunocompromised individuals. Molecules 2014, 19, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J.; Park, J.H.; Park, H.J.; Cho, J.G.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Lee, D.Y.; Kim, H.S.; Byun, S.Y.; et al. Flavonoids from machilus Japonica stems and their inhibitory effects on ldl oxidation. Int. J. Mol. Sci. 2014, 15, 16418–16429. [Google Scholar] [CrossRef] [PubMed]
- Ajish, K.R.; Antu, K.A.; Riya, M.P.; Preetharani, M.R.; Raghu, K.G.; Dhanya, B.P.; Radhakrishnan, K.V. Studies on α-glucosidase, aldose reductase and glycation inhibitory properties of sesquiterpenes and flavonoids of Zingiber zerumbet smith. Nat. Prod. Res. 2015, 29, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, J.; He, P.; Chen, Y.; Li, L.; Zhang, L.; Li, Y.; Fu, Y.; Dai, R.; Meng, W.; et al. The α-glucosidase inhibiting isoflavones isolated from Belamcanda chinensis leaf extract. Rec. Nat. Prod. 2012, 6, 110–120. [Google Scholar]
- Hong, H.-C.; Li, S.-L.; Zhang, X.-Q.; Ye, W.-C.; Zhang, Q.-W. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of morus atropurpurea. Chin. Med. 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch-iodine method for measuring α-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. Α-amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J. Nat. Prod. 2011, 74, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chrom. 2013, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-I.; Lee, S.R.; Kim, K.H. Antioxidant and α-glucosidase inhibitory activities of constituents from euonymus alatus twigs. Ind. Crop. Prod. 2015, 76, 1055–1060. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
| No. | Extract/Compound | IC50 (μg/mL, μM *) | |
|---|---|---|---|
| α-amylase | α-glucosidase | ||
| A | Hexane extract | 625 | 48.22 |
| B | EtOAc extract | - | 416.67 |
| C | MeOH extract | - | 19.22 |
| 1 | Lupeol Acetate | - | - |
| 2 | cis-p-coumaric | - | - |
| 3 | Lupeol | - | - |
| 4 | β-sitosterol | - | - |
| 5 | trans-p-coumaric | - | - |
| 6 | Linoleic * | - | 0.42 |
| 7 | (+)-Catechin * | - | 5.50 |
| 8 | Afzelin * | - | 3.56 |
| 9 | Quercitrin * | - | 7.77 |
| D | Acarbose | 10 | 377 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Naranjo, M.; Suárez, A.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro α-Amilase and α-Glucosidase Inhibitory Activities. Molecules 2016, 21, 1461. https://doi.org/10.3390/molecules21111461
Torres-Naranjo M, Suárez A, Gilardoni G, Cartuche L, Flores P, Morocho V. Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro α-Amilase and α-Glucosidase Inhibitory Activities. Molecules. 2016; 21(11):1461. https://doi.org/10.3390/molecules21111461
Chicago/Turabian StyleTorres-Naranjo, María, Alirica Suárez, Gianluca Gilardoni, Luis Cartuche, Paola Flores, and Vladimir Morocho. 2016. "Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro α-Amilase and α-Glucosidase Inhibitory Activities" Molecules 21, no. 11: 1461. https://doi.org/10.3390/molecules21111461
APA StyleTorres-Naranjo, M., Suárez, A., Gilardoni, G., Cartuche, L., Flores, P., & Morocho, V. (2016). Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro α-Amilase and α-Glucosidase Inhibitory Activities. Molecules, 21(11), 1461. https://doi.org/10.3390/molecules21111461









