Soil Microbial Adaptation and Biogeochemical Feedback in Degraded Alpine Meadows of the Qinghai–Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Information
2.2. Soil Physicochemical Properties Analysis
2.3. Soil DNA Extraction and Metagenome Sequencing
2.4. Network Analysis
2.5. Statistical Analysis
3. Results and Discussion
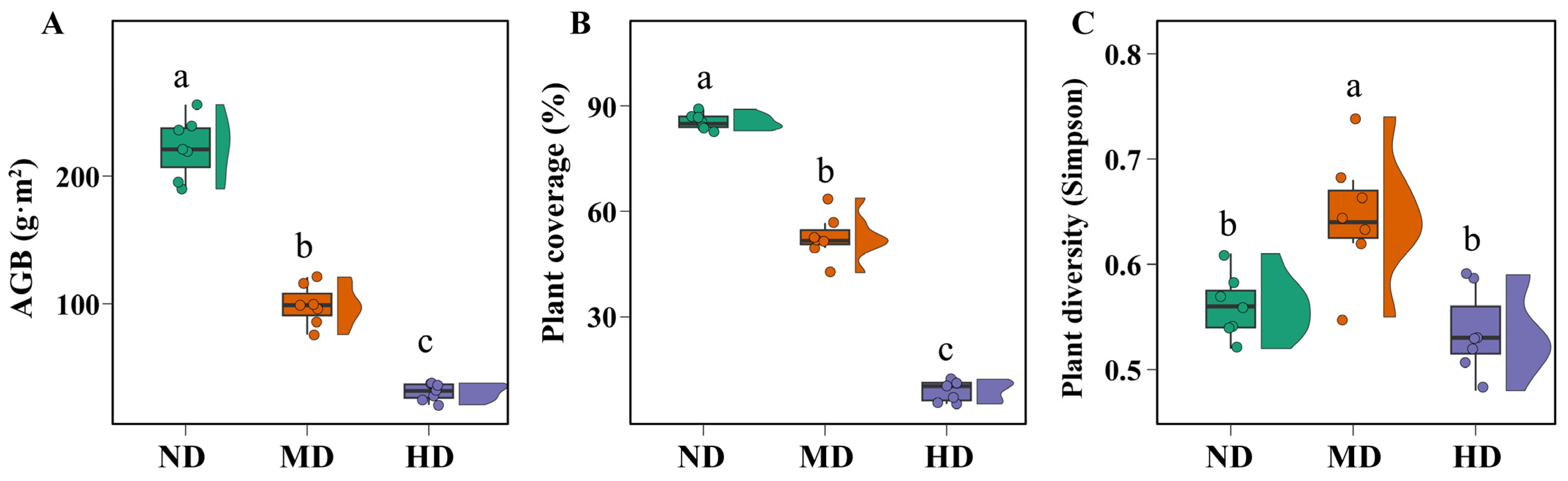
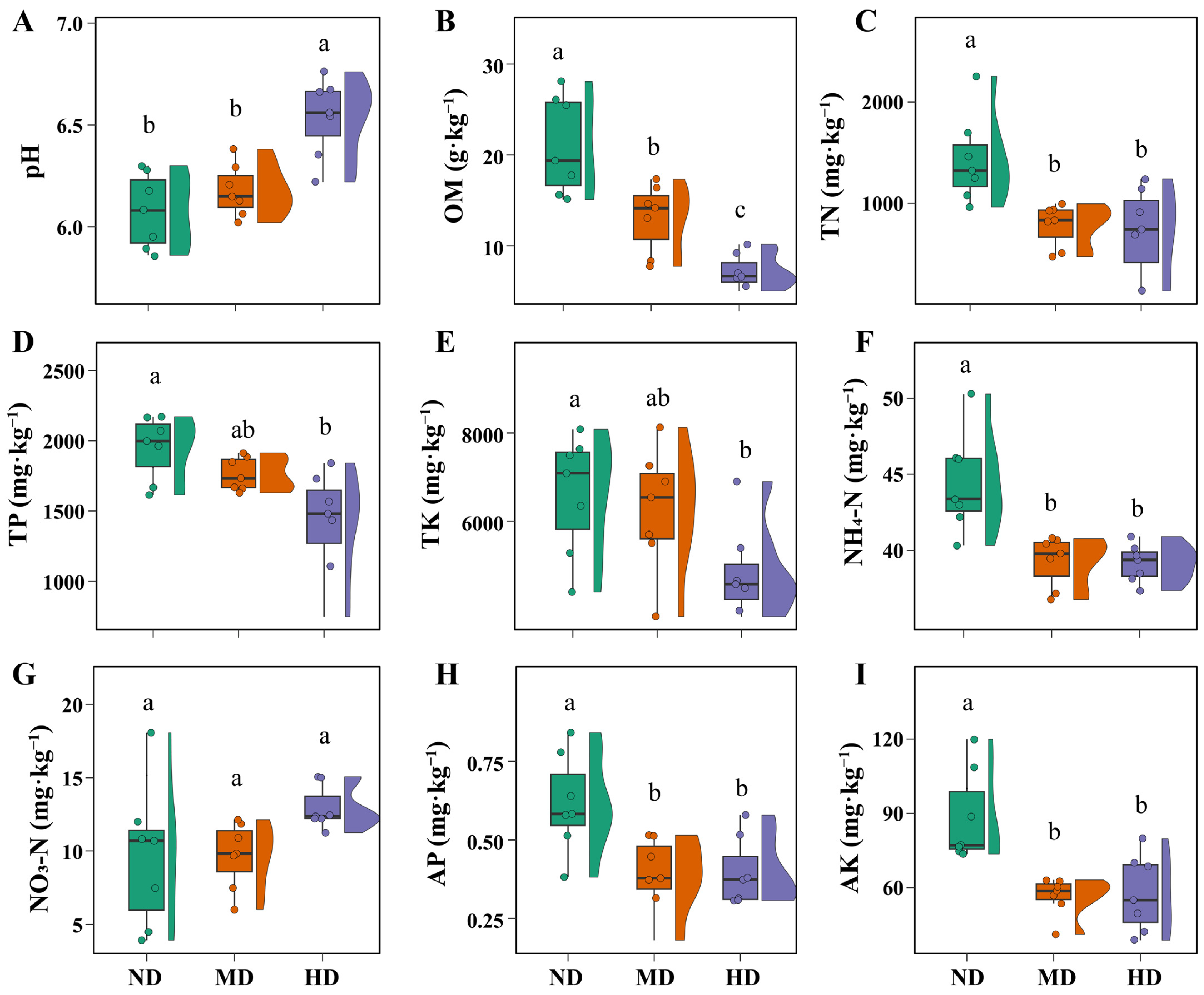
3.1. Changes in Vegetation and Soil Properties Across Meadow Degradation Levels
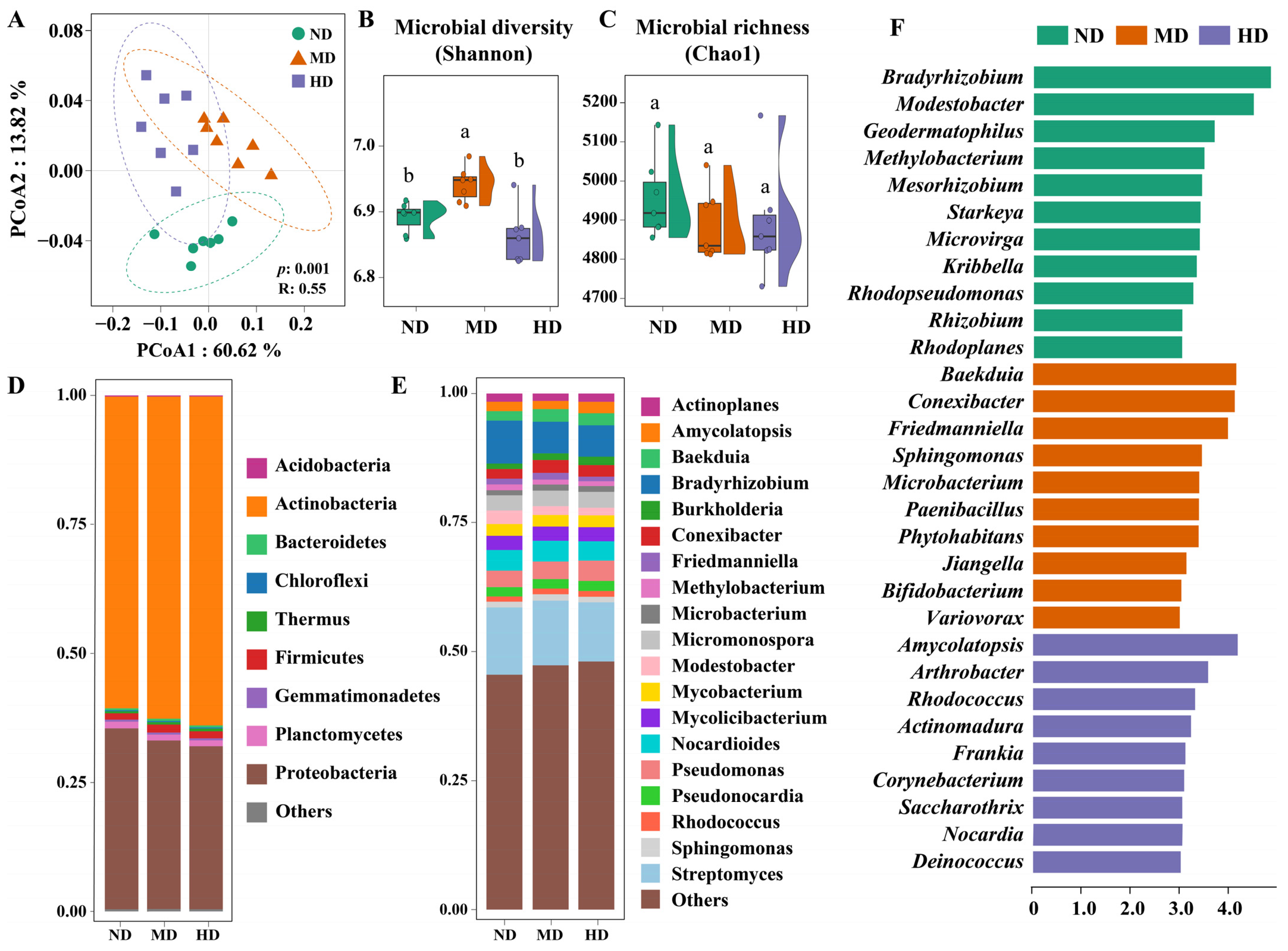
3.2. Soil Microbial Community Succession Across Meadow Degradation Levels
3.3. Microbial Interaction Patterns Across Meadow Degradation Levels
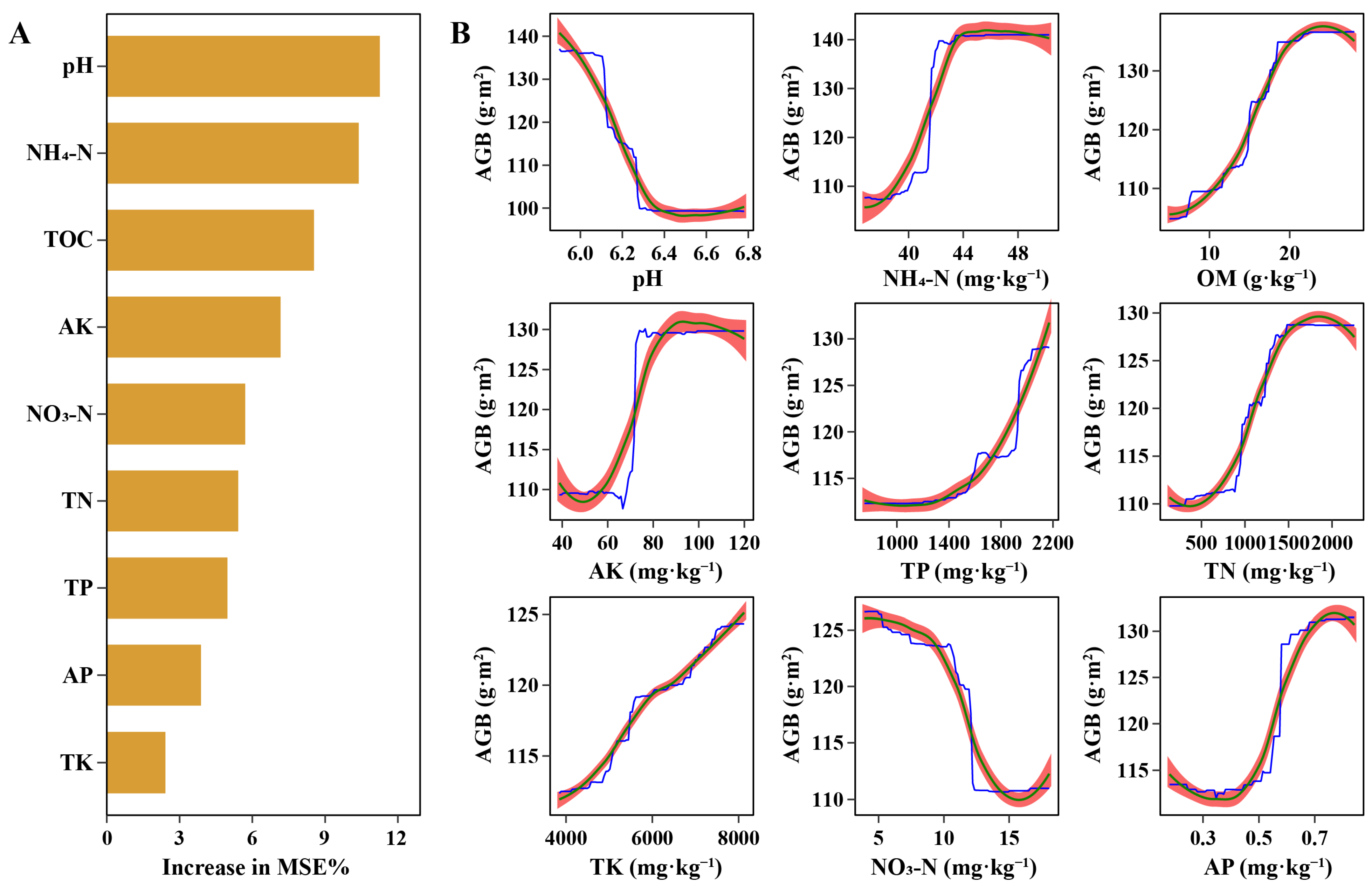
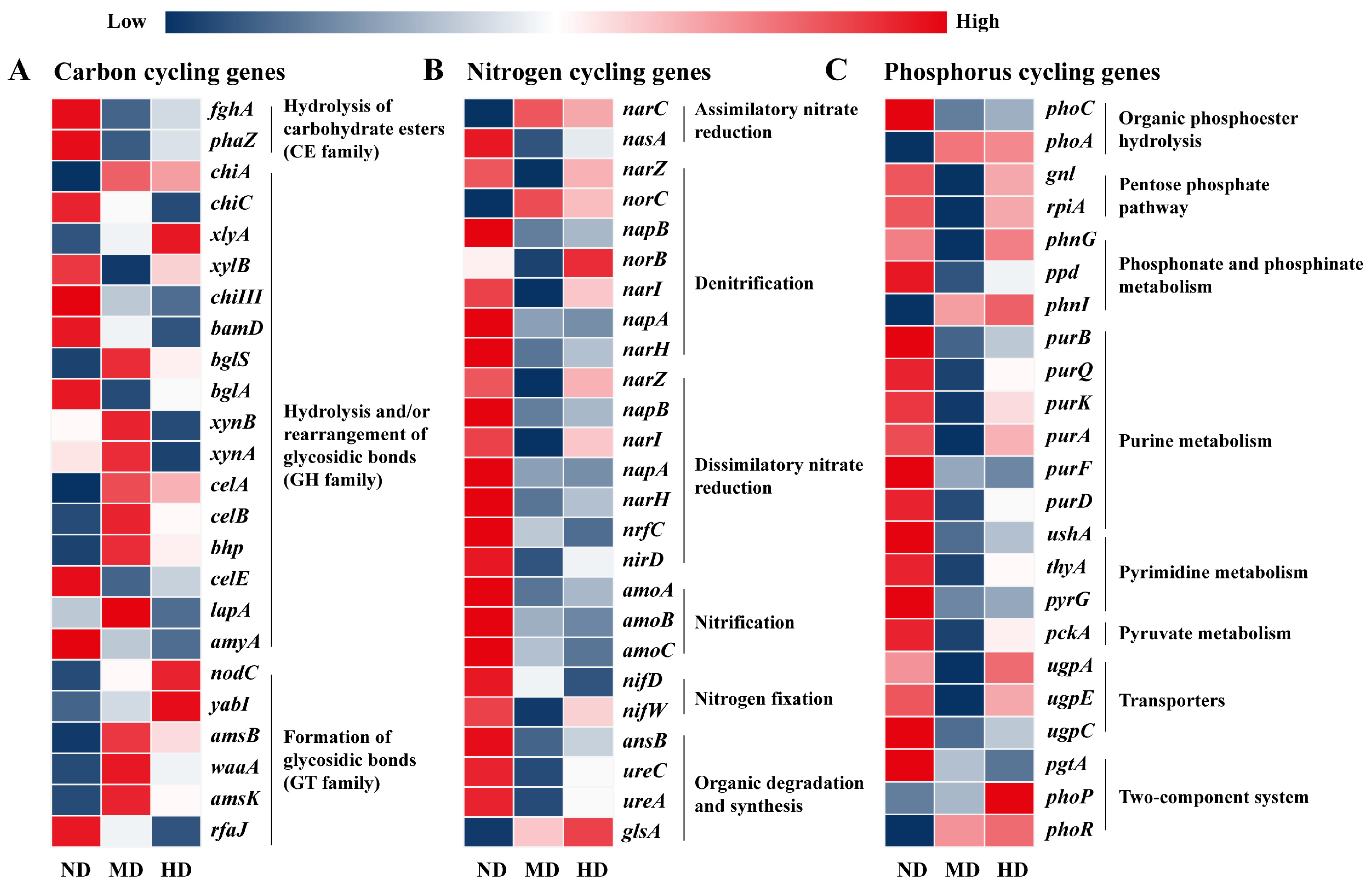
3.4. Soil Biogeochemical Cycles Affected by Degradation Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Liu, J.; Wu, J.; Hu, H.; Chen, Q.; Fang, H.; Tao, K. Effects of alpine grassland degradation on soil microbial community structure and metabolic activity in the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2024, 200, 105458. [Google Scholar] [CrossRef]
- Li, C.; de Jong, R.; Schmid, B.; Wulf, H.; Schaepman, M.E. Changes in grassland cover and in its spatial heterogeneity indicate degradation on the Qinghai-Tibetan Plateau. Ecol. Indic. 2020, 119, 106641. [Google Scholar] [CrossRef]
- Qiao, Y.; Duan, Z. Understanding alpine meadow ecosystems. In Landscape and Ecosystem Diversity, Dynamics and Management in the Yellow River Source Zone; Springer: Cham, Switzerland, 2016; pp. 117–135. [Google Scholar]
- Schuur, E.A.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, W.; Zhan, T.; Hua, T.; Pereira, P. Alpine meadow and alpine steppe plant-soil network in Qinghai-Tibet Plateau, China. Catena 2024, 244, 108235. [Google Scholar] [CrossRef]
- Dai, L.; Guo, X.; Ke, X.; Du, Y.; Zhang, F.; Cao, G. The variation in soil water retention of alpine shrub meadow under different degrees of degradation on northeastern Qinghai-Tibetan plateau. Plant Soil 2021, 458, 231–244. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.; Duan, X.; Zhang, S.; Chen, M.; Hou, H.; Wang, Z.; Yu, A.; Chen, D.; Zhang, X.; et al. The degradation of subalpine meadows significantly changed the soil microbiome. Agric. Ecosyst. Environ. 2023, 349, 108470. [Google Scholar] [CrossRef]
- Li, H.; Geng, H.; Zhang, Z.; Yi, L.; Wang, J.; Zhang, F. Root biomass and altitude jointly regulate the response of topsoil organic carbon density to severe degradation of high-altitude alpine meadows. Grass Forage Sci. 2024, 79, 69–77. [Google Scholar] [CrossRef]
- Qian, D.; Du, Y.; Li, Q.; Guo, X.; Fan, B.; Cao, G. Impacts of alpine shrub-meadow degradation on its ecosystem services and spatial patterns in Qinghai-Tibetan Plateau. Ecol. Indic. 2022, 135, 108541. [Google Scholar] [CrossRef]
- Zhou, T.; Zong, N.; Sun, J.; Hou, G.; Shi, P. Plant nitrogen concentration is more sensitive in response to degradation than phosphorus concentration in alpine meadow. Ecol. Eng. 2021, 169, 106323. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Jiang, Z.; Sun, P.; Xiao, H.; Wu, Y.; Chen, J. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2019, 651, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, A.; Schleuss, P.-M.; Liu, S.; Schneider, D.; Dippold, M.A.; de la Haye, T.; Miehe, G.; Heitkamp, F.; Seeber, E.; Mason-Jones, K. Microbial functional changes mark irreversible course of Tibetan grassland degradation. Nat. Commun. 2022, 13, 2681. [Google Scholar] [CrossRef]
- Che, R.; Wang, Y.; Li, K.; Xu, Z.; Hu, J.; Wang, F.; Rui, Y.; Li, L.; Pang, Z.; Cui, X. Degraded patch formation significantly changed microbial community composition in alpine meadow soils. Soil Tillage Res. 2019, 195, 104426. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Han, D.; Gao, Y.; Zhang, J.; Ma, Y.; Zhang, H.; Yang, X. Nutrients available in the soil regulate the changes of soil microbial community alongside degradation of alpine meadows in the northeast of the Qinghai-Tibet Plateau. Sci Total Environ. 2021, 792, 148363. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, A.; Ding, M.; Zhang, H.; Xu, H.; Zhang, Y. Meadow degradation reduces microbial β diversity and network complexity while enhancing network stability. Appl. Soil Ecol. 2024, 204, 105733. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, J.; An, Z.; Zhang, Y.; Yu, Y. Effects of alpine meadow degradation on the soil physical and chemical properties in Maqu, China. Res. Cold Arid Reg. 2024, 16, 269–277. [Google Scholar] [CrossRef]
- Sah, R. Nitrate-nitrogen determination—A critical review. Commun. Soil Sci. Plant Anal. 1994, 25, 2841–2869. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Barnes, R.B.; Richardson, D.; Berry, J.W.; Hood, R.L. Flame photometry a rapid analytical procedure. Ind. Eng. Chem. Anal. Ed. 1945, 17, 605–611. [Google Scholar] [CrossRef]
- Bai, D.; Chen, T.; Xun, J.; Ma, C.; Luo, H.; Yang, H.; Cao, C.; Cao, X.; Cui, J.; Deng, Y.P. EasyMetagenome: A user-friendly and flexible pipeline for shotgun metagenomic analysis in microbiome research. iMeta 2025, 4, e70001. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ge, Q.; Yan, Y.; Zhang, X.; Huang, L.; Yin, Y. dbCAN3: Automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 2023, 51, W115–W121. [Google Scholar] [CrossRef]
- Tu, Q.; Lin, L.; Cheng, L.; Deng, Y.; He, Z. NCycDB: A curated integrative database for fast and accurate metagenomic profiling of nitrogen cycling genes. Bioinformatics 2018, 35, 1040–1048. [Google Scholar] [CrossRef]
- Zeng, J.; Tu, Q.; Yu, X.; Qian, L.; Wang, C.; Shu, L.; Liu, F.; Liu, S.; Huang, Z.; He, J.; et al. PCycDB: A comprehensive and accurate database for fast analysis of phosphorus cycling genes. Microbiome 2022, 10, 101. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Du, S.; Feng, J.; Bi, L.; Hu, H.W.; Hao, X.; Huang, Q.; Liu, Y.R. Tracking soil resistance and virulence genes in rice-crayfish co-culture systems across China. Environ. Int. 2023, 172, 107789. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Gu, Y.; Wu, Y.; Liu, Y.; Tang, Z.; Xu, Y.; Mao, X.; Zhang, J.; Tian, W. Deciphering the mechanism of rhizosphere microecosystem in modulating rice cadmium accumulation via integrating metabolomics and metagenomics. Sci. Total Environ. 2025, 959, 178181. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Haggblom, M.; Krumins, V.; Dong, Y.; Sun, X.; Li, F.; Wang, Q.; Li, B.; Yan, B. Bacterial Survival Strategies in an Alkaline Tailing Site and the Physiological Mechanisms of Dominant Phylotypes as Revealed by Metagenomic Analyses. Environ. Sci. Technol. 2018, 52, 13370–13380. [Google Scholar] [CrossRef]
- Moi, D.A.; García-Ríos, R.; Hong, Z.; Daquila, B.V.; Mormul, R.P. Intermediate disturbance hypothesis in ecology: A literature review. Ann. Zool. Fenn. 2020, 57, 67–78. [Google Scholar] [CrossRef]
- Comerford, N. Soil factors affecting nutrient bioavailability. In Nutrient Acquisition by Plants: An Ecological Perspective; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Wang, C.; Kuzyakov, Y. Soil organic matter priming: The pH effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, S.; Dengzeng, Z.; Zhang, D.; Zhang, T.; Ma, X. Root exudates contribute to belowground ecosystem hotspots: A review. Front. Microbiol. 2022, 13, 937940. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Yao, T.; Li, H.; Wang, X.; Liu, X.; Li, C.; Li, S.; Bai, J. Meadow Degradation Affects Microbial Community Structure and Vegetation Characteristics by Increasing Soil pH. Land Degrad. Dev. 2025, 36, 790–801. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root exudates mediate the processes of soil organic carbon input and efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, H.; Liu, J.; Yao, Y.; Wang, X.; Rong, G.; Zhao, X.; Shao, M.; Wei, X. Soil erosion significantly reduces organic carbon and nitrogen mineralization in a simulated experiment. Agric. Ecosyst. Environ. 2021, 307, 107232. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, J.; Tang, C.; Zhang, J.; Brookes, P.C. Differences in carbon and nitrogen mineralization in soils of differing initial pH induced by electrokinesis and receiving crop residue amendments. Soil Biol. Biochem. 2013, 67, 70–84. [Google Scholar] [CrossRef]
- Sepp, S.-K.; Vasar, M.; Davison, J.; Oja, J.; Anslan, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; Fabiano, E.C. Global diversity and distribution of nitrogen-fixing bacteria in the soil. Front. Plant Sci. 2023, 14, 1100235. [Google Scholar] [CrossRef]
- Amadou, I.; Faucon, M.-P.; Houben, D. Role of soil minerals on organic phosphorus availability and phosphorus uptake by plants. Geoderma 2022, 428, 116125. [Google Scholar] [CrossRef]
- Mouhamad, R.; Alsaede, A.; Iqbal, M. Behavior of potassium in soil: A mini review. Chem. Int. 2016, 2, 58–69. [Google Scholar]
- Peng, J.; Liu, H.; Hu, Y.; Sun, Y.; Liu, Q.; Li, J.; Dong, Y. Shift in soil bacterial communities from K- to r-strategists facilitates adaptation to grassland degradation. Land Degrad. Dev. 2022, 33, 2076–2091. [Google Scholar] [CrossRef]
- Stock, S.C.; Köster, M.; Dippold, M.A.; Nájera, F.; Matus, F.; Merino, C.; Boy, J.; Spielvogel, S.; Gorbushina, A.; Kuzyakov, Y. Environmental drivers and stoichiometric constraints on enzyme activities in soils from rhizosphere to continental scale. Geoderma 2019, 337, 973–982. [Google Scholar] [CrossRef]
- Zheng, J.; Luan, L.; Luo, Y.; Fan, J.; Xu, Q.; Sun, B.; Jiang, Y. Biochar and lime amendments promote soil nitrification and nitrogen use efficiency by differentially mediating ammonia-oxidizer community in an acidic soil. Appl. Soil Ecol. 2022, 180, 104619. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Y.; Zhu-Barker, X.; He, X.; Horwath, W.R.; Luo, H.; Zhao, Y.; Jiang, X. Responses of nitrification and ammonia oxidizers to a range of background and adjusted pH in purple soils. Geoderma 2019, 334, 9–14. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef]
- Peng, Y.; Peng, Z.; Zeng, X.; Houx, J.H. Effects of nitrogen-phosphorus imbalance on plant biomass production: A global perspective. Plant Soil 2019, 436, 245–252. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Kobayashi, T.; Xu, K.-Q. The role of rice husk biochar addition in anaerobic digestion for sweet sorghum under high loading condition. Biotechnol. Rep. 2020, 27, e00515. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Li, Z.; Bai, X.; Jiao, S.; Li, Y.; Li, P.; Yang, Y.; Zhang, H.; Wei, G. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome 2021, 9, 217. [Google Scholar] [CrossRef]
- Parasar, B.J.; Sharma, I.; Agarwala, N. Root exudation drives abiotic stress tolerance in plants by recruiting beneficial microbes. Appl. Soil Ecol. 2024, 198, 105351. [Google Scholar] [CrossRef]
- Cao, C.; Tao, S.; Cui, Z.; Zhang, Y. Response of Soil Properties and Microbial Communities to Increasing Salinization in the Meadow Grassland of Northeast China. Microb. Ecol. 2021, 82, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Wagg, C.; Castellano, M.J.; Zhang, N.; Ding, W. Soil organic carbon loss decreases biodiversity but stimulates multitrophic interactions that promote belowground metabolism. Glob. Change Biol. 2024, 30, e17101. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhong, Z.; He, Q.; Yun, X.; Yang, L.; Wang, X.; Ji, D.; Wang, G.; Zhao, J.; Zhang, X. Methane emission fluxes and associated microbial community characteristics in a temperate seagrass meadow. Sci. Total Environ. 2025, 958, 177991. [Google Scholar] [CrossRef]
- Wu, D.; Ren, C.; Ren, D.; Tian, Y.; Li, Y.; Wu, C.; Li, Q. New insights into carbon mineralization in tropical paddy soil under land use conversion: Coupled roles of soil microbial community, metabolism, and dissolved organic matter chemodiversity. Geoderma 2023, 432, 116393. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Solyanikova, I.; Golovleva, L. Physiological and biochemical properties of actinobacteria as the basis of their high biodegradative activity. Appl. Biochem. Microbiol. 2015, 51, 143–149. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, G.; Hu, C.; Xu, C.; Zhang, Z.; Ning, K. Comparative genomics analysis reveals genetic characteristics and nitrogen fixation profile of Bradyrhizobium. iScience 2024, 27, 108948. [Google Scholar] [CrossRef]
- Kumar, P.S.; Rangasamy, G.; Gayathri, K.V.; Parthasarathy, V. Rhizobium mayense sp. nov., an efficient plant growth-promoting nitrogen-fixing bacteria isolated from rhizosphere soil. Environ. Res. 2023, 220, 115200. [Google Scholar]
- Zhou, M.; Liu, Z.; Wang, J.; Zhao, Y.; Hu, B. Sphingomonas relies on chemotaxis to degrade polycyclic aromatic hydrocarbons and maintain dominance in coking sites. Microorganisms 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.S.; Yörüklü, H.C.; Bozkurt, K.; Denktaş, C.; Bozdoğan, A.; Özdemir, O.; Özkaya, B. Biodegradation of microplastic by probiotic bifidobacterium. Int. J. Glob. Warm. 2022, 26, 429–443. [Google Scholar] [CrossRef]
- Ortega-González, D.K.; Martínez-González, G.; Flores, C.M.; Zaragoza, D.; Cancino-Diaz, J.C.; Cruz-Maya, J.A.; Jan-Roblero, J. Amycolatopsis sp. Poz14 isolated from oil-contaminated soil degrades polycyclic aromatic hydrocarbons. Int. Biodeterior. Biodegrad. 2015, 99, 165–173. [Google Scholar] [CrossRef]
- Hacker, E.; Antunes, C.A.; Mattos-Guaraldi, A.L.; Burkovski, A.; Tauch, A. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016, 11, 1191–1208. [Google Scholar] [CrossRef]
- Mehta, H.H.; Shamoo, Y. Pathogenic Nocardia: A diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020, 16, e1008280. [Google Scholar] [CrossRef]
- Mo, Y.; Peng, F.; Jeppesen, E.; Gamfeldt, L.; Xiao, P.; Al, M.A.; Yang, J. Microbial network complexity drives non-linear shift in biodiversity-nutrient cycling in a saline urban reservoir. Sci. Total Environ. 2022, 850, 158011. [Google Scholar] [CrossRef]
- Montoya, D.; Yallop, M.L.; Memmott, J. Functional group diversity increases with modularity in complex food webs. Nat. Commun. 2015, 6, 7379. [Google Scholar] [CrossRef]
- Hashemi, R.; Darabi, H.; Hashemi, M.; Wang, J. Graph theory in ecological network analysis: A systematic review for connectivity assessment. J. Clean. Prod. 2024, 472, 143504. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Liu, Y.; Huang, S.; Zhao, C.; Jiang, Z.; Gu, Y.; Xiao, J.; Wu, Y.; Ying, R.; et al. Deciphering soil resistance and virulence gene risks in conventional and organic farming systems. J. Hazard. Mater. 2024, 468, 133788. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, H.; Gao, Z.; Li, D.; Lin, Y.; Wu, C.; Wei, L. Uncovering the toxic effects and adaptive mechanisms of aminated polystyrene nanoplastics on microbes in sludge anaerobic digestion system: Insight from extracellular to intracellular. J. Hazard. Mater. 2024, 480, 136163. [Google Scholar] [CrossRef]
- Liu, P.; Li, A.; Wang, Y.; Cai, Q.; Yu, H.; Li, Y.; Peng, H.; Li, Q.; Wang, Y.; Wei, X. Distinct Miscanthus lignocellulose improves fungus secreting cellulases and xylanases for consistently enhanced biomass saccharification of diverse bioenergy crops. Renew. Energy 2021, 174, 799–809. [Google Scholar] [CrossRef]
- Can, A.; Baysal, Ö. Unveiling novel biocontrol strategies: Serratia marcescens chiA gene against Myzus persicae. bioRxiv 2024. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Classen, A.T.; Dukes, J.S.; Kardol, P.; Liu, L.; Luo, Y.; Rustad, L.; Sun, J.; Tang, J.; Templer, P.H. Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol. Lett. 2016, 19, 697–709. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Apel, A.K.; Sola-Landa, A.; Rodríguez-García, A.; Martín, J.F. Phosphate control of phoA, phoC and phoD gene expression in Streptomyces coelicolor reveals significant differences in binding of PhoP to their promoter regions. Microbiology 2007, 153, 3527–3537. [Google Scholar] [CrossRef]
- White Andrea, K.; Metcalf William, W. Two C—P Lyase Operons in Pseudomonas stutzeri and Their Roles in the Oxidation of Phosphonates, Phosphite, and Hypophosphite. J. Bacteriol. 2004, 186, 4730–4739. [Google Scholar] [CrossRef]
- Poisot, T. The theory of ecological specialization. In The Theory of Evolution: Principles, Concepts, and Assumptions; The University of Chicago Press: Chicago, IL, USA, 2020; pp. 232–253. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Gesang, Q.; Sun, Y.; Wang, Y.; Nan, J.; Xu, J. Soil Microbial Adaptation and Biogeochemical Feedback in Degraded Alpine Meadows of the Qinghai–Tibetan Plateau. Microorganisms 2025, 13, 1142. https://doi.org/10.3390/microorganisms13051142
Li B, Gesang Q, Sun Y, Wang Y, Nan J, Xu J. Soil Microbial Adaptation and Biogeochemical Feedback in Degraded Alpine Meadows of the Qinghai–Tibetan Plateau. Microorganisms. 2025; 13(5):1142. https://doi.org/10.3390/microorganisms13051142
Chicago/Turabian StyleLi, Bingzhang, Quzhen Gesang, Yan Sun, Yuting Wang, Jibin Nan, and Jun Xu. 2025. "Soil Microbial Adaptation and Biogeochemical Feedback in Degraded Alpine Meadows of the Qinghai–Tibetan Plateau" Microorganisms 13, no. 5: 1142. https://doi.org/10.3390/microorganisms13051142
APA StyleLi, B., Gesang, Q., Sun, Y., Wang, Y., Nan, J., & Xu, J. (2025). Soil Microbial Adaptation and Biogeochemical Feedback in Degraded Alpine Meadows of the Qinghai–Tibetan Plateau. Microorganisms, 13(5), 1142. https://doi.org/10.3390/microorganisms13051142




