Synergistic Anticandidal Effectiveness of Greenly Synthesized Zinc Oxide Nanoparticles with Antifungal Agents against Nosocomial Candidal Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of S. officinalis Leaf Extract
2.2. Green Biofabrication of the Biogenic ZnO-NPs
2.3. Physicochemical Characterization of the Biogenic ZnO-NPs
2.4. Screening of Antifungal Effectiveness of ZnO-NPs
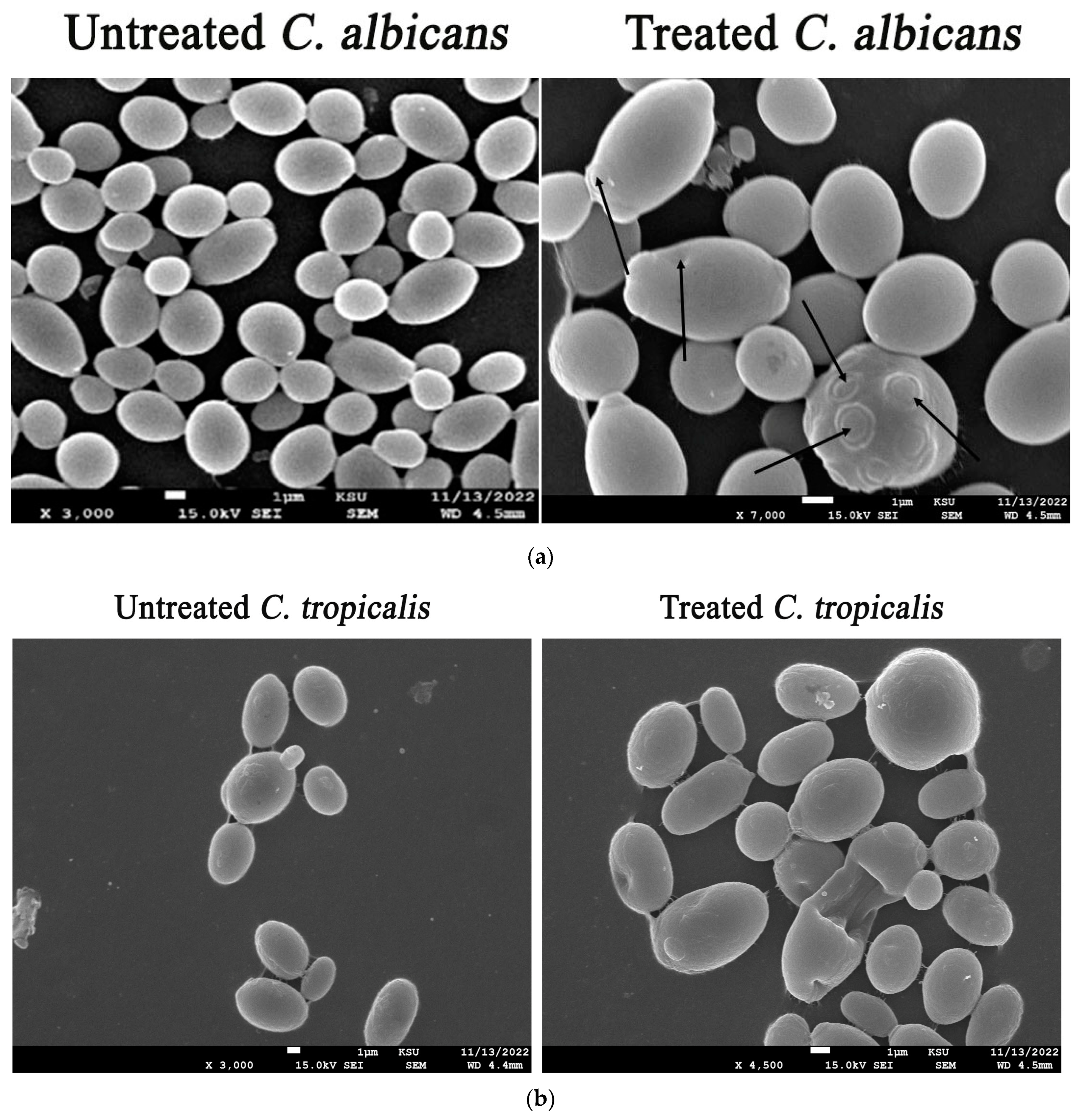
2.5. Detection of Fungal Cell Deformations Utilizing Scanning Electron Microscopy (SEM) Analysis
2.6. Evaluation of Synergistic Efficiency of the Biosynthesized ZnO-NPs with Antifungal Agents
2.7. Cytotoxicity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis of ZnO-NPs
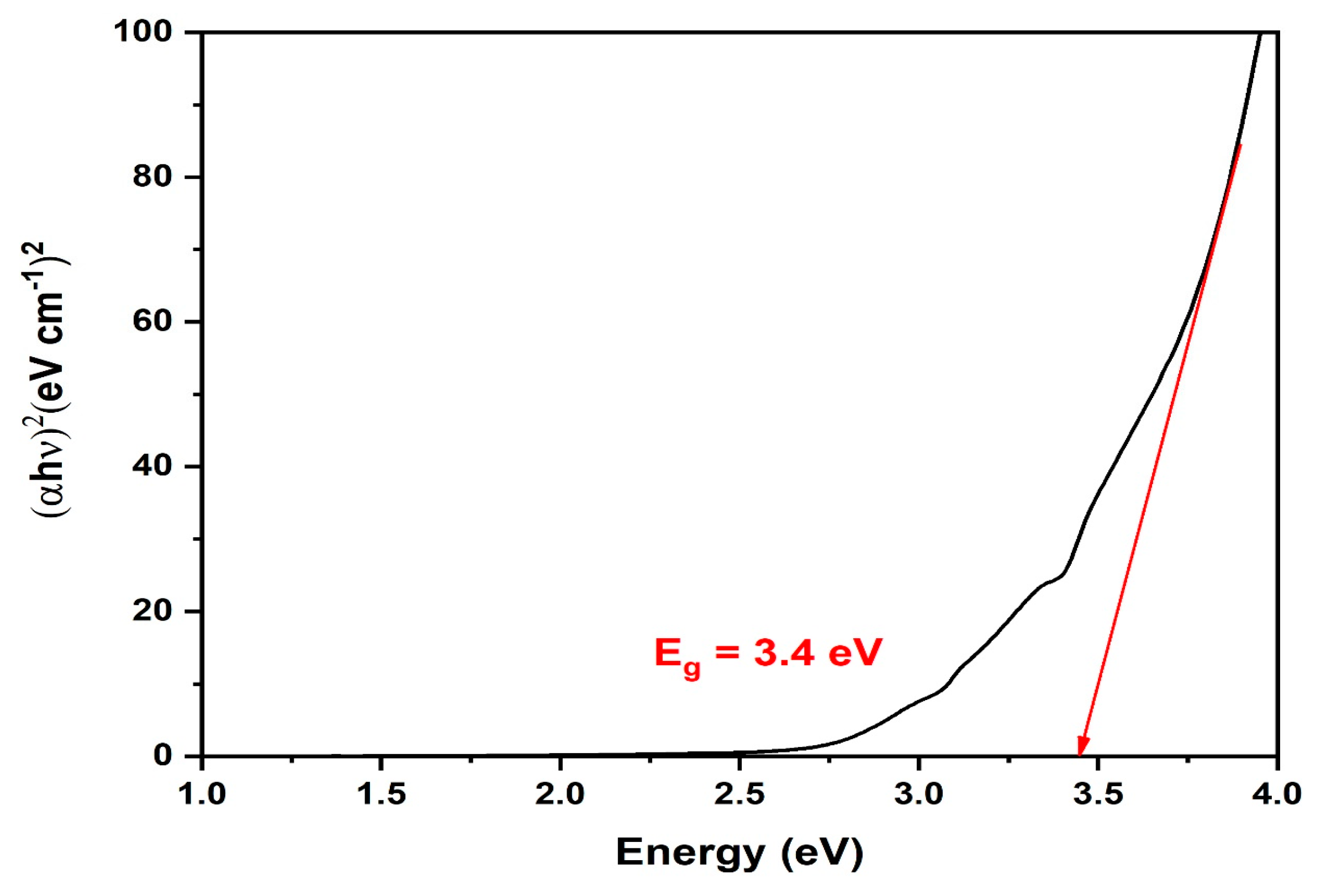
3.2. UV-Vis Spectral Analysis
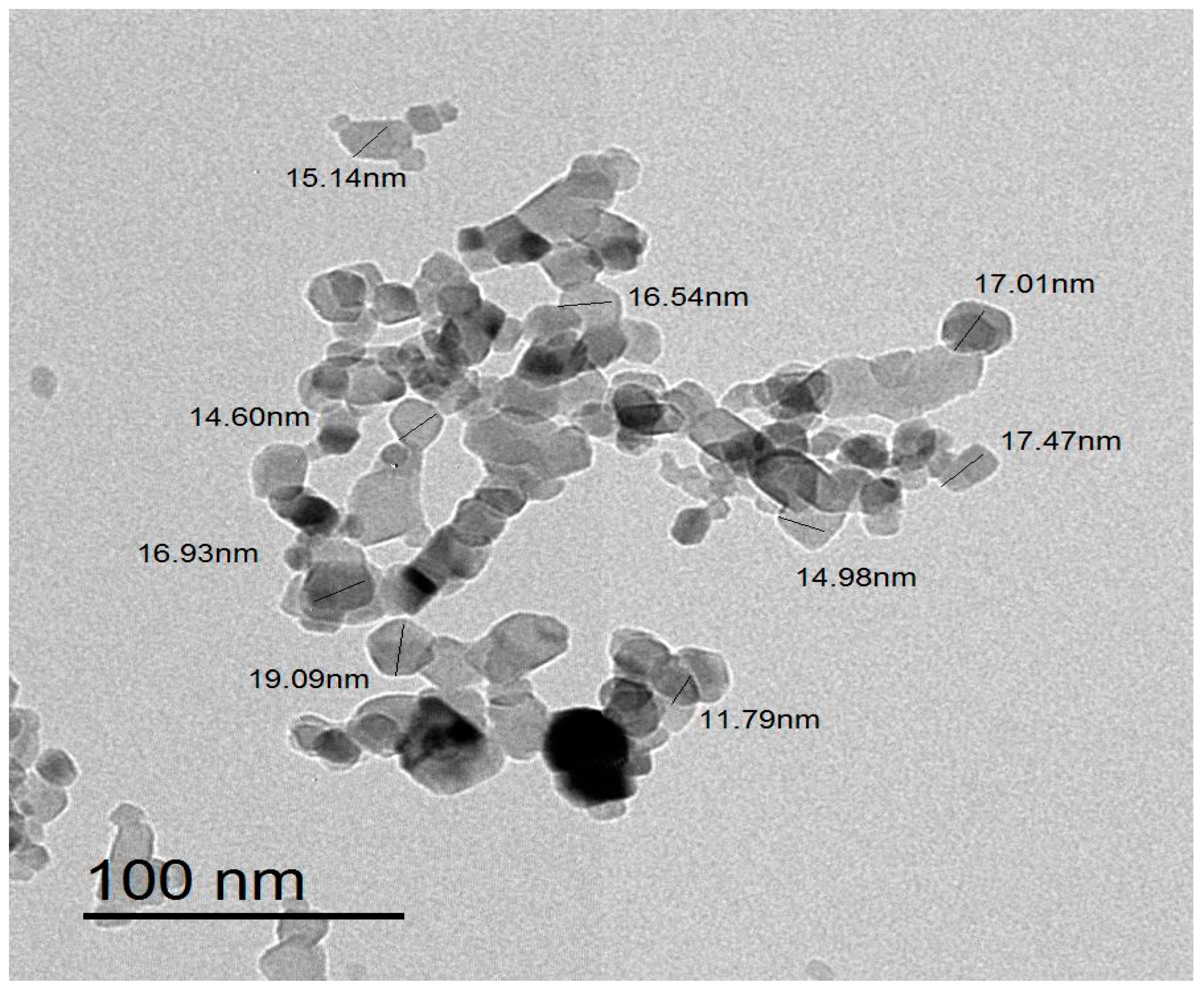
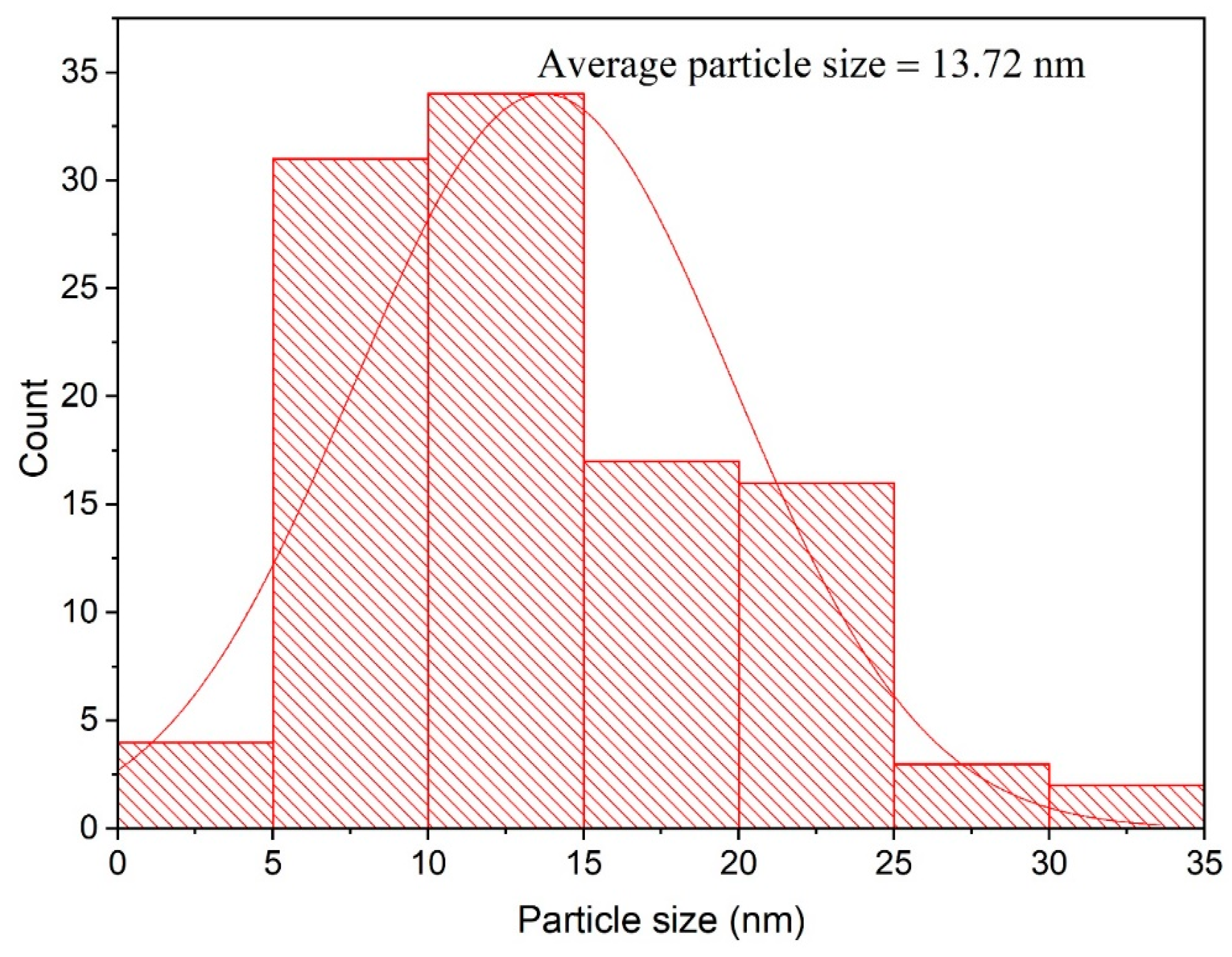
3.3. Transmission Electron Microscopy Analysis
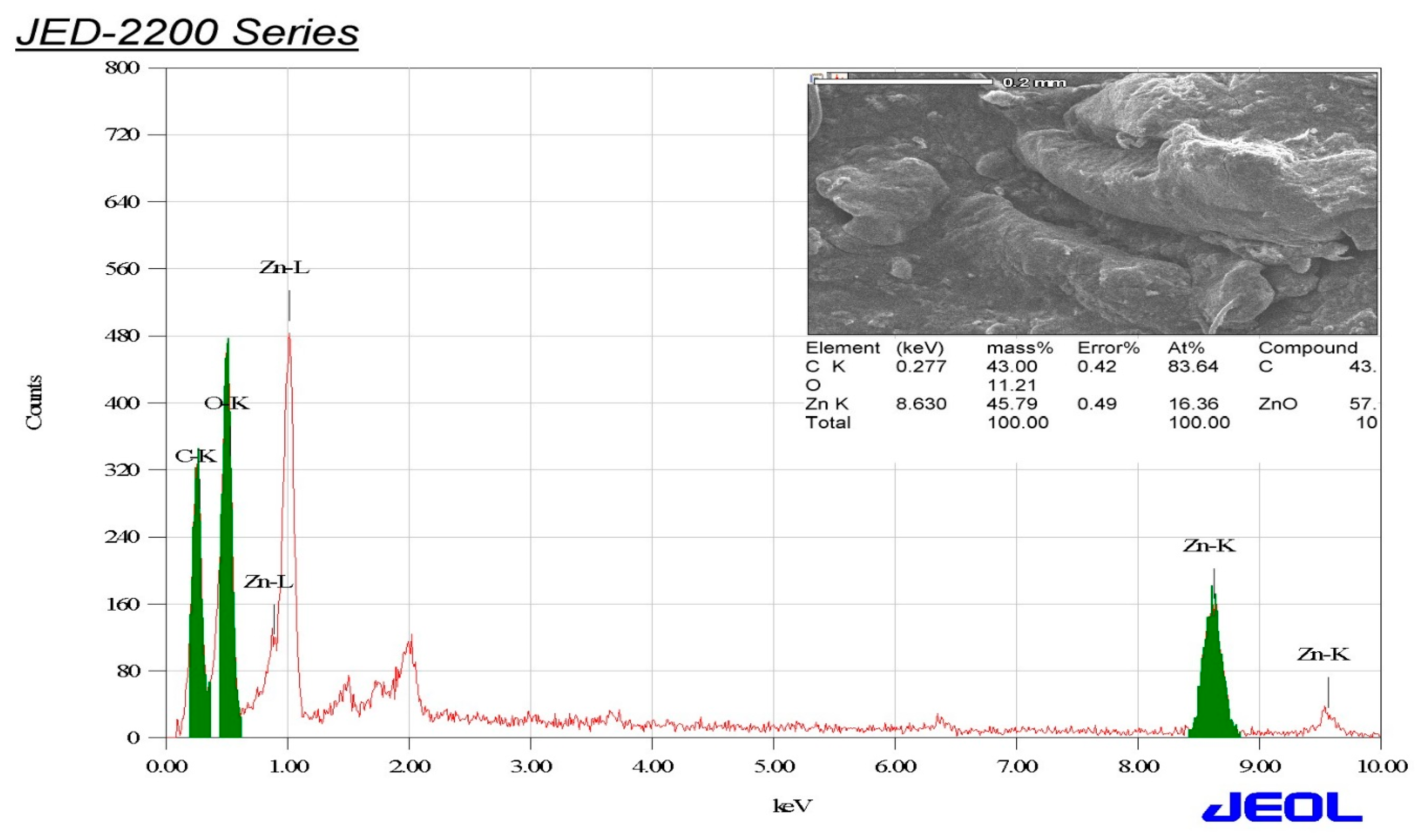
3.4. EDX Elemental Analysis of the Biogenic ZnO-NPs
3.5. Fourier Transform Infrared Spectroscopy Analysis of the Biogenic ZnO-NPs
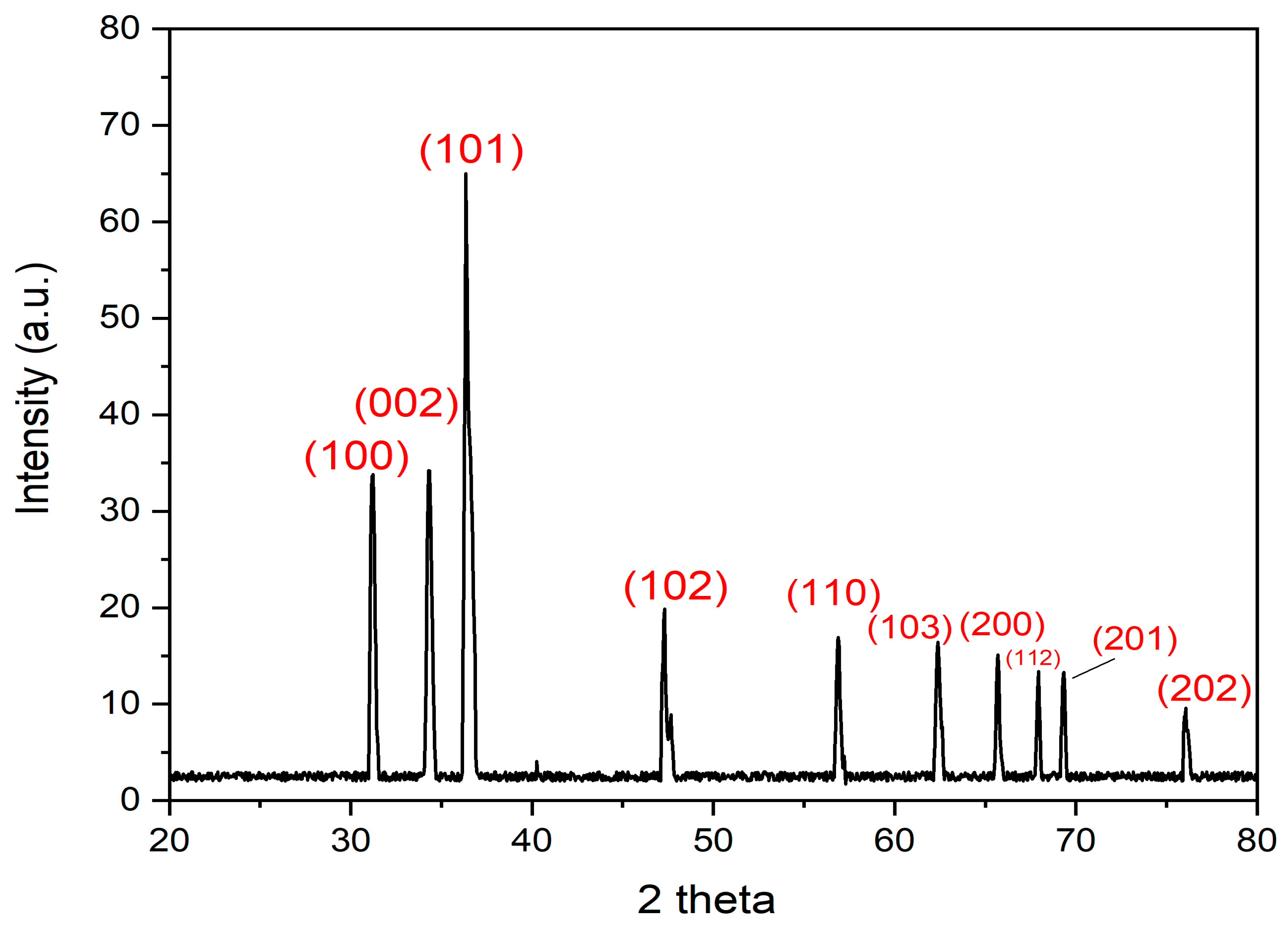
3.6. XRD Analysis of the Biogenic ZnO-NPs
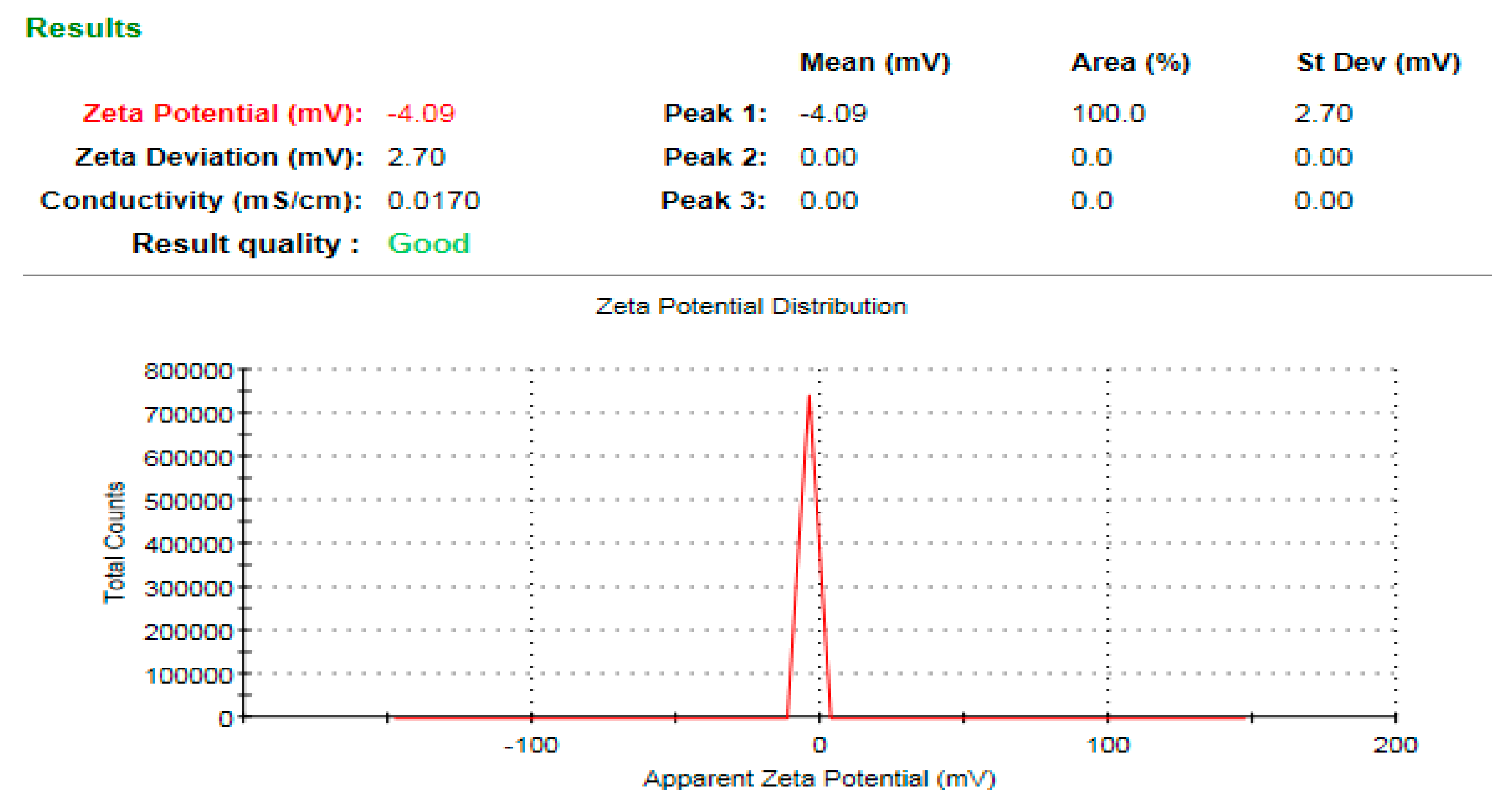
3.7. Zeta Potential Analysis of the Bioinspired ZnO-NPs
3.8. Evaluation of Anticandidal Effectiveness of the Bioinspired ZnO-NPs
3.9. Morphological Deformations of Candidal Cells Treated with the Biogenic ZnO-NPs
3.10. Synergistic Patterns of the Biogenic ZnO-NPs with Commercial Antifungal Agents
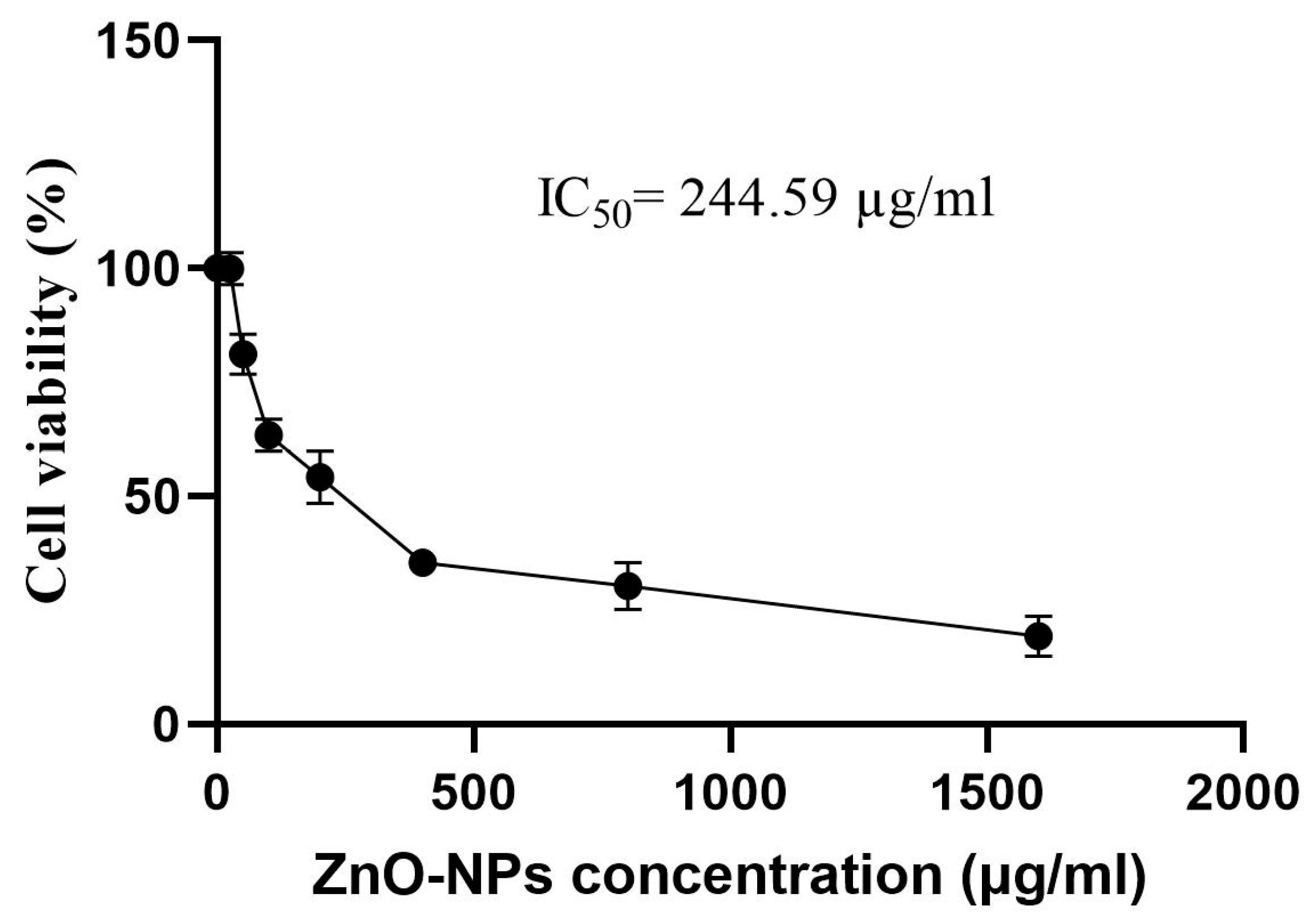
3.11. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, D.; Sun, P.; Li, H.; Zhang, M.; Liu, G.; Strickland, A.B.; Chen, Y.; Fu, Y.; Xu, J.; Yosri, M.; et al. Fungal Dissemination Is Limited by Liver Macrophage Filtration of the Blood. Nat. Commun. 2019, 10, 4566. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the Epidemiological Landscape of Invasive Candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Olusegun-Joseph, T.S.; Killaney, V.M. Survey of Possible Pathogenic Organisms Found in Urine and Vaginal Swab Samples of Selected Female Population in Lagos, Nigeria. Int. J. Biol. Chem. Sci. 2016, 10, 1840–1852. [Google Scholar] [CrossRef][Green Version]
- Chaari, A.; Munir, A.; Sharaf, A.; Khairy, A.; Kauts, V.; Erdem, H. Predictive Factors and Prognostic Value of Candiduria in Critically-Ill Patients with Solid and Hematological Malignancies. J. Med. Mycol. 2023, 33, 101353. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, I.A.; Kechia, F.A.; Iwewe, Y.S.; Ngueguim, A.D.; Nangwat, C.; Dzoyem, J.P. Species Distribution and Antifungal Susceptibility Profile of Candida spp. Isolated from Urine of Hospitalized Patients in Dschang District Hospital, Cameroon. Int. J. Biol. Chem. Sci. 2017, 11, 1212–1221. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Ghaddar, N.; Anastasiadis, E.; Halimeh, R.; Ghaddar, A.; Dhar, R.; Al Fouzan, W.; Yusef, H.; El Chaar, M. Prevalence and Antifungal Susceptibility of Candida Albicans Causing Vaginal Discharge among Pregnant Women in Lebanon. BMC Infect. Dis. 2020, 20, 32. [Google Scholar] [CrossRef]
- Sangaré, I.; Sirima, C.; Bamba, S.; Zida, A.; Cissé, M.; Bazié, W.W.; Sanou, S.; Dao, B.; Menan, H.; Guiguemdé, R.T. Prevalence of Vulvovaginal Candidiasis in Pregnancy at Three Health Centers in Burkina Faso. J. Mycol. Medicale 2018, 28, 186–192. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida Albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Pahwa, N.; Kumar, R.; Nirkhiwale, S.; Bandi, A. Species Distribution and Drug Susceptibility of Candida in Clinical Isolates from a Tertiary Care Centre at Indore. Indian J. Med. Microbiol. 2014, 32, 44–48. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K. Bioinspired Metal/Metal Oxide Nanoparticles: A Road Map to Potential Applications. Mater. Today Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A Review on Nanotechnology: Properties, Applications, and Mechanistic Insights of Cellular Uptake Mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, R.; Kumari, A. A Review on Biogenic Synthesis, Applications and Toxicity Aspects of Zinc Oxide Nanoparticles. EXCLI J. 2020, 19, 1325–1340. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Reshma, V.G.; Syama, S.; Sruthi, S.; Reshma, S.C.; Remya, N.S.; Mohanan, P.V. Engineered Nanoparticles with Antimicrobial Property. Curr. Drug Metab. 2017, 18, 1040–1054. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered Nanomaterials for Antimicrobial Applications: A Review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Suppi, S.; Kasemets, K.; Ivask, A.; Künnis-Beres, K.; Sihtmäe, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A Novel Method for Comparison of Biocidal Properties of Nanomaterials to Bacteria, Yeasts and Algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Mostafa, M.H. Antimicrobial Applications of Nanoparticles. Nanomater. Nanotechnol. Med. 2022, 517–552. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Gnanasekar, S.; Seetharaman, P.; Keppanan, R.; Arockiaswamy, W.; Sivaperumal, S. Formulation of Carica Papaya Latex-Functionalized Silver Nanoparticles for Its Improved Antibacterial and Anticancer Applications. J. Mol. Liq. 2016, 219, 232–238. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I. Novel Green Synthetic Strategy to Prepare ZnO Nanocrystals Using Rambutan (Nephelium Lappaceum L.) Peel Extract and Its Antibacterial Applications. Mater. Sci. Eng. C 2014, 41, 17–27. [Google Scholar] [CrossRef]
- Ghotekar, S.; Basnet, P.; Lin, K.-Y.A.; Rahdar, A.; Larios, A.P.; Gandhi, V.; Oza, R. Green Synthesis of CeVO4 Nanoparticles Using Azadirechta Indica Leaves Extract and Their Promising Applications as an Antioxidant and Anticancer Agent. J. Sol-Gel Sci. Technol. 2023, 106, 726–736. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S. A Review on Green Synthesis of Zinc Oxide Nanoparticles Using Plant Extracts and Its Biomedical Applications. BioNanoScience 2020, 10, 848–863. [Google Scholar] [CrossRef]
- Anitha, R.; Ramesh, K.V.; Ravishankar, T.N.; Sudheer Kumar, K.H.; Ramakrishnappa, T. Cytotoxicity, Antibacterial and Antifungal Activities of ZnO Nanoparticles Prepared by the Artocarpus Gomezianus Fruit Mediated Facile Green Combustion Method. J. Sci. Adv. Mater. Devices 2018, 3, 440–451. [Google Scholar] [CrossRef]
- Ringu, T.; Ghosh, S.; Das, A.; Pramanik, N. Zinc Oxide Nanoparticles: An Excellent Biomaterial for Bioengineering Applications. Emergent Mater. 2022, 5, 1629–1648. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, Characterization and Antimicrobial Activity of Gold Nanoparticles from Leaf Extracts of Annona muricata. J. Nanostruct. Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Negi, A.; Gangwar, R.; Vishwakarma, R.K.; Negi, D.S. Biogenic Zinc Oxide Nanoparticles as an Antibacterial, Antifungal, and Photocatalytic Tool Mediated via Leaves of Girardinia diversifolia. Nanotechnol. Environ. Eng. 2022, 7, 223–233. [Google Scholar] [CrossRef]
- Mehta, M.; Chopra, C.; Sistla, S.; Bhushan, I. Potential of Biosynthesized Silver and Zinc Oxide Nanoparticles from Carissa opaca Extracts for Antimicrobial Activity and Wastewater Treatment. Sustainability 2023, 15, 8911. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia Kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Chand, P.; Kumari, S.; Mondal, N.; Singh, S.P.; Prasad, T. Synergism of Zinc Oxide Quantum Dots with Antifungal Drugs: Potential Approach for Combination Therapy against Drug Resistant Candida albicans. Front. Nanotechnol. 2021, 3, 624564. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, S.; Chen, K.; Tang, J.; Liu, J.; Su, W.; Liu, X.; Zeng, X. Synergistic Antibacterial Effect from Silver Nanoparticles and Anticancer Activity Against Human Lung Cancer Cells. J. Biomed. Nanotechnol. 2022, 18, 2204–2215. [Google Scholar] [CrossRef]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic Antibacterial Activity of Silver Nanoparticles Biosynthesized by Carbapenem-Resistant Gram-Negative Bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef]
- Ben Khalifa, R.; Cacciatore, I.; Dimmito, M.P.; Ciulla, M.; Grande, R.; Puca, V.; Robuffo, I.; De Laurenzi, V.; Chekir-Ghedira, L.; Di Stefano, A.; et al. Multiple Lipid Nanoparticles as Antimicrobial Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102887. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Perez-Larios, A. Antimicrobial Activity of Green Synthesized Se Nanoparticles Using Ginger and Onion Extract: A Laboratory and in Silico Analysis. Part. Sci. Technol. 2022, 41, 319–329. [Google Scholar] [CrossRef]
- Bhoye, M.; Pansambal, S.; Basnet, P.; Lin, K.-Y.A.; Gutierrez-Mercado, K.Y.; Pérez-Larios, A.; Chauhan, A.; Oza, R.; Ghotekar, S. Eco-Friendly Synthesis of Ni/NiO Nanoparticles Using Gymnema Sylvestre Leaves Extract for Antifungal Activity. J. Compos. Sci. 2023, 7, 105. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Aqueous Extract with the Evaluation of Its Antibacterial Activity against Clinical and Food Pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef]
- Fuku, X.; Diallo, A.; Maaza, M. Nanoscaled Electrocatalytic Optically Modulated ZnO Nanoparticles through Green Process of Punica Granatum L. and Their Antibacterial Activities. Int. J. Electrochem. 2016, 2016, 4682967. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO Nanoparticles: A Study of Blueshift of Optical Band Gap and Photocatalytic Degradation of Reactive Yellow 186 Dye under Direct Sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar]
- Dhanemozhi, A.C.; Rajeswari, V.; Sathyajothi, S. Green Synthesis of Zinc Oxide Nanoparticle Using Green Tea Leaf Extract for Supercapacitor Application. Mater. Today Proc. 2017, 4, 660–667. [Google Scholar] [CrossRef]
- Torres-Ramos, M.I.; Martín-Camacho, U.J.; González, J.L.; Yañez-Acosta, M.F.; Becerra-Solano, L.; Gutiérrez-Mercado, Y.K.; Macias-Carballo, M.; Gómez, C.M.; González-Vargas, O.A.; Rivera-Mayorga, J.A.; et al. A Study of Zn-Ca Nanocomposites and Their Antibacterial Properties. Int. J. Mol. Sci. 2022, 23, 7258. [Google Scholar] [CrossRef]
- Limón-Rocha, I.; Guzmán-González, C.A.; Anaya-Esparza, L.M.; Romero-Toledo, R.; Rico, J.L.; González-Vargas, O.A.; Pérez-Larios, A. Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics 2022, 10, 16. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra Tortuosa and Their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Geetha, A.; Sakthivel, R.; Mallika, J.; Kannusamy, R.; Rajendran, R. Green Synthesis of Antibacterial Zinc Oxide Nanoparticles Using Biopolymer Azadirachta Indica Gum. Orient. J. Chem. 2016, 32, 955–963. [Google Scholar] [CrossRef]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green Synthesized Doxorubicin Loaded Zinc Oxide Nanoparticles Regulates the Bax and Bcl-2 Expression in Breast and Colon Carcinoma. Process Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S.; Ataei, M. Water-Based Synthesis of ZnO Nanoparticles via Decomposition of a Ternary Zinc Complex Containing Schiff-Base, Chelating, and Phen Ligands. J. Chin. Chem. Soc. 2018, 65, 1210–1217. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green Synthesis of Zinc Oxide Nanoparticles Using Atalantia Monophylla Leaf Extracts: Characterization and Antimicrobial Analysis. Mater. Sci. Semicond. Process. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Sultana, F.; Barman, J.; Kalita, M.C. Biogenic Synthesis of ZnO Nanoparticles Using Polygonum Chinense Leaf Extract and Their Antibacterial Activity. Int. J. Nanotechnol. Appl. 2017, 11, 155–165. [Google Scholar]
- Akhter, S.M.H.; Mahmood, Z.; Ahmad, S.; Mohammad, F. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Swertia Chirayita Leaf Extract, Characterization and Its Antibacterial Efficacy against Some Common Pathogenic Bacteria. Bionanoscience 2018, 8, 811–817. [Google Scholar] [CrossRef]
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronomy 2021, 11, 729. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired Green Synthesis of Chitosan and Zinc Oxide Nanoparticles with Strong Antibacterial Activity against Rice Pathogen Xanthomonas oryzae Pv. Oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Kaliamurthi, S.; Selvaraj, G.; Cakmak, Z.E.; Korkmaz, A.D.; Cakmak, T. The Relationship between Chlorella Sp. and Zinc Oxide Nanoparticles: Changes in Biochemical, Oxygen Evolution, and Lipid Production Ability. Process Biochem. 2019, 85, 43–50. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kumar, N.; Kumar, R.; Salar, R.K. Antimicrobial Activity of Zinc Oxide Nanoparticles Synthesized from Aloe Vera Peel Extract. SN Appl. Sci. 2018, 1, 136. [Google Scholar] [CrossRef]
- Banumathi, B.; Malaikozhundan, B.; Vaseeharan, B. Invitro Acaricidal Activity of Ethnoveterinary Plants and Green Synthesis of Zinc Oxide Nanoparticles against Rhipicephalus (Boophilus) Microplus. Vet. Parasitol. 2016, 216, 93–100. [Google Scholar] [CrossRef]
- Degefa, A.; Bekele, B.; Jule, L.T.; Fikadu, B.; Ramaswamy, S.; Dwarampudi, L.P.; Nagaprasad, N.; Ramaswamy, K. Green Synthesis, Characterization of Zinc Oxide Nanoparticles, and Examination of Properties for Dye-Sensitive Solar Cells Using Various Vegetable Extracts. J. Nanomater. 2021, 2021, e3941923. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum Perforatum L.-Mediated Green Synthesis of Silver Nanoparticles Exhibiting Antioxidant and Anticancer Activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green Tea Extract Mediated Biogenic Synthesis of Silver Nanoparticles: Characterization, Cytotoxicity Evaluation and Antibacterial Activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Rosbero, T.M.S.; Camacho, D.H. Green Preparation and Characterization of Tentacle-like Silver/Copper Nanoparticles for Catalytic Degradation of Toxic Chlorpyrifos in Water. J. Environ. Chem. Eng. 2017, 5, 2524–2532. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Sánchez, E.; Debut, A.; Cumbal, L. Plukenetia Volubilis L. Seed Flour Mediated Biofabrication and Characterization of Silver Nanoparticles. Chem. Phys. Lett. 2021, 781, 138993. [Google Scholar] [CrossRef]
- Neamah, S.A.; Albukhaty, S.; Falih, I.Q.; Dewir, Y.H.; Mahood, H.B. Biosynthesis of Zinc Oxide Nanoparticles Using Capparis spinosa L. Fruit Extract: Characterization, Biocompatibility, and Antioxidant Activity. Appl. Sci. 2023, 13, 6604. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green Biosynthesis of Silver Nanoparticles Using Novel Endophytic Rothia Endophytica: Characterization and Anticandidal Activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Begum, I.; Shamim, S.; Ameen, F.; Hussain, Z.; Bhat, S.A.; Qadri, T.; Hussain, M. A Combinatorial Approach towards Antibacterial and Antioxidant Activity Using Tartaric Acid Capped Silver Nanoparticles. Processes 2022, 10, 716. [Google Scholar] [CrossRef]
- Preedia Babu, E.; Subastri, A.; Suyavaran, A.; Premkumar, K.; Sujatha, V.; Aristatile, B.; Alshammari, G.M.; Dharuman, V.; Thirunavukkarasu, C. Size Dependent Uptake and Hemolytic Effect of Zinc Oxide Nanoparticles on Erythrocytes and Biomedical Potential of ZnO-Ferulic Acid Conjugates. Sci. Rep. 2017, 7, 4203. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N.; Pabarja, A.; Abadi, M.F.S.; Tahroudi, M.H.M. Biosynthesis of Zinc Oxide Nanoparticles Using Hertia Intermedia and Evaluation of Its Cytotoxic and Antimicrobial Activities. BioNanoScience 2021, 11, 245–255. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green Synthesis of Silver Nanoparticles by the Cyanobacteria synechocystis sp.: Characterization, Antimicrobial and Diabetic Wound-Healing Actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Yahya, R.; Bakri, M.M.; Al Abboud, M.A.; Yahya, R.; Qanash, H.; Bazaid, A.S.; Salem, S.S. Phytofabrication of Zinc Oxide Nanoparticles with Advanced Characterization and Its Antioxidant, Anticancer, and Antimicrobial Activity against Pathogenic Microorganisms. Biomass Convers. Biorefinery 2023, 13, 417–430. [Google Scholar] [CrossRef]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia Officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Younes, S.; Mourad, N.; Rahal, M. Allylamines, Benzylamines, and Fungal Cell Permeability: A Review of Mechanistic Effects and Usefulness against Fungal Pathogens. Membranes 2022, 12, 1171. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Borah, P.; Hazarika, S.; Sharma, D.; Venugopala, K.N.; Chopra, D.; Al-Shar’i, N.A.; Hemalatha, S.; Shakya, A.K.; Chandra Acharya, P.; Deb, P.K. Chapter 9—Systemic and Topical Antifungal Drugs. In Medicinal Chemistry of Chemotherapeutic Agents; Acharya, P.C., Kurosu, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 285–315. ISBN 978-0-323-90575-6. [Google Scholar]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Yassin, M.T.; Elgorban, A.M.; Al-Askar, A.A.; Sholkamy, E.N.; Ameen, F.; Maniah, K. Synergistic Anticandidal Activities of Greenly Synthesized ZnO Nanomaterials with Commercial Antifungal Agents against Candidal Infections. Micromachines 2023, 14, 209. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From Plants to Antimicrobials: Natural Products against Bacterial Membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef]
- Haro-Reyes, T.; Díaz-Peralta, L.; Galván-Hernández, A.; Rodríguez-López, A.; Rodríguez-Fragoso, L.; Ortega-Blake, I. Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications. Membranes 2022, 12, 681. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and Their Antimicrobial Properties against Pathogens Including Bacteria, Fungi, Parasites and Viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Jha, S.; Rani, R.; Singh, S. Biogenic Zinc Oxide Nanoparticles and Their Biomedical Applications: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef]
- Tan, G.; İlk, S.; Emul, E.; Asik, M.D.; Sam, M.; Altindag, S.; Birhanli, E.; Apohan, E.; Yesilada, O.; Verma, S.K.; et al. Green Synthesis and Biogenic Materials, Characterization, and Their Applications. In Microbial Nanobionics: Volume 1, State-of-the-Art; Prasad, R., Ed.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 29–61. ISBN 978-3-030-16383-9. [Google Scholar]
- Lagunes-Castro, M.d.l.S.; Trigos, Á.; López-Monteon, A.; Mendoza, G.; Ramos-Ligonio, A. Cytotoxic activity and induction of inflammatory mediators of the methanol:chloroform extract of Fusarium moniliforme. Rev. Iberoam. Micol. 2015, 32, 235–241. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]


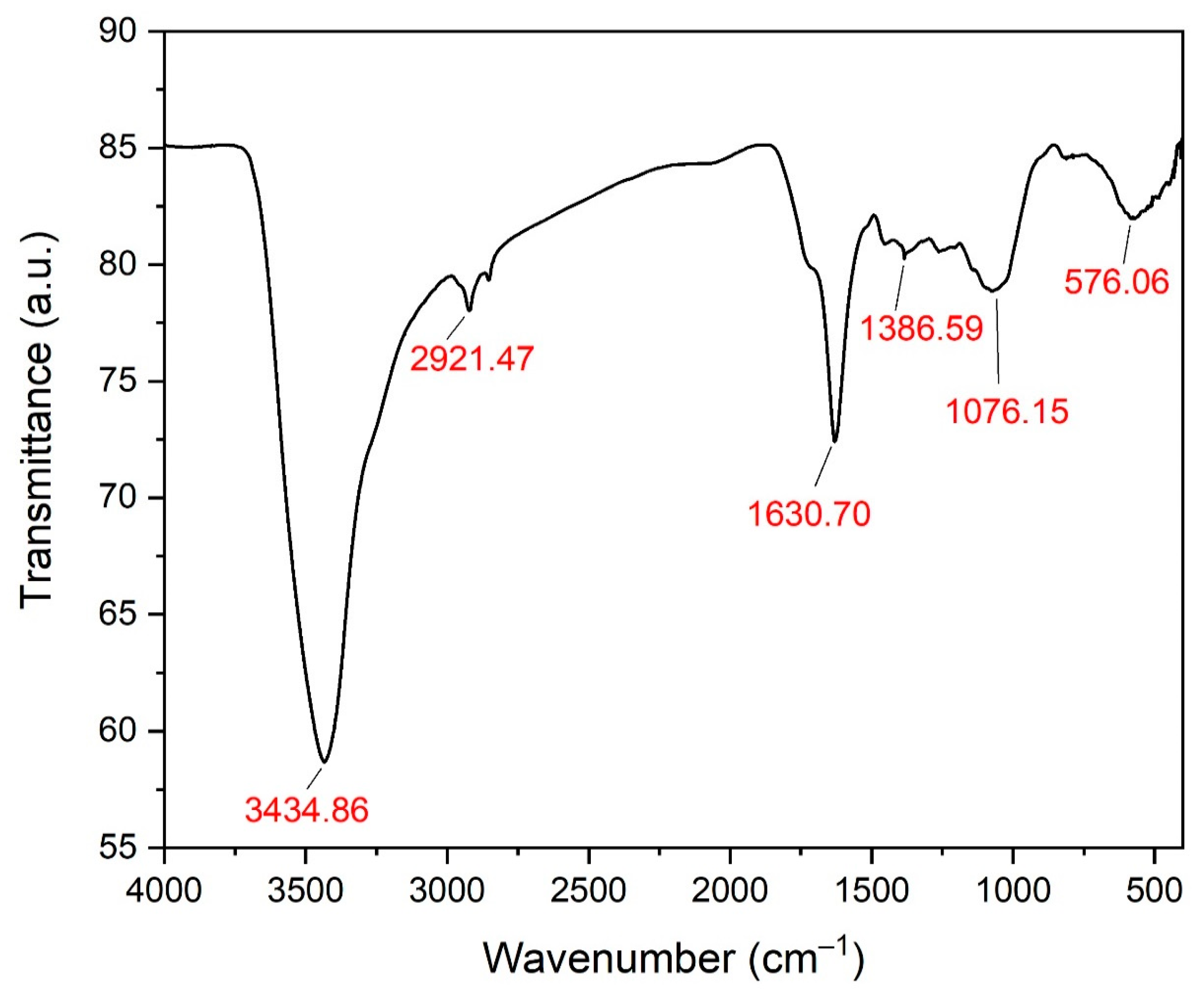

| No. | Absorption Peak (cm−1) | Appearance | Functional Groups | Molecular Motion |
|---|---|---|---|---|
| 1 | 3434.86 | Strong | Phenolics | O-H stretching |
| 2 | 2921.47 | Medium | Alkanes | C-H stretching |
| 3 | 1630.70 | Medium | Alkenes | C=C stretching |
| 4 | 1386.59 | Weak | Aldehydes | C-H bending |
| 5 | 1076.15 | Medium | Primary alcohols | C-O stretching |
| 6 | 576.06 | Weak, broad | Metal oxide bonds | Zn-O stretching |
| Candidal Strains | Inhibition Zone Diameters (mm) | |||
|---|---|---|---|---|
| ZnO-NPs (50 μg/disk) | ZnO-NPs (100 μg/disk) | Terbinafine (30 µg/disk) | Negative Control | |
| C. albicans | 18.27 ± 0.16 a | 22.56 ± 0.51 a | 25.73 ± 0.27 a | 0.00 ± 0.00 a |
| C. glabrata | 13.53 ± 0.64 b | 15.12 ± 0.38 b | 8.54 ± 0.21 b | 0.00 ± 0.00 a |
| C. tropicalis | 19.68 ± 0.32 a | 23.17 ± 0.45 a | 34.82 ± 0.12 c | 0.00 ± 0.00 a |
| Concentrations (µg/disk) | Inhibition Zone Diameter (mm) | ||
|---|---|---|---|
| C. albicans (IFA) | C. glabrata (IFA) | C. tropicalis (IFA) | |
| CLO (10 µg) | 16.11 ± 0.63 | 26.15 ± 0.38 | 32.19 ± 0.53 |
| CLO (10 µg) + ZnONPs (10 µg) | 19.57 ± 0.42 (0.48) * | 23.14 ± 0.45 (−0.22) * | 27.15 ± 0.24 (−0.41) * |
| FLU (25 µg) | 8.97 ± 0.54 | 23.49 ± 0.21 | 30.19 ± 0.12 |
| FLU (25 µg) + ZnONPs (10 µg) | 14.57 ± 0.12 (1.63) * | 23.87 ± 0.23 (0.03) ns | 32.84 ± 0.56 (0.81) * |
| ITZ (10 µg) | 20.68 ± 0.18 | 11.98 ± 0.52 | 25.12 ± 0.37 |
| ITZ (10 µg) + ZnONPs (10 µg) | 21.39 ± 0.56 (0.07) ns | 15.96 ± 0.83 (0.77) * | 23.16 ± 0.43 (−0.18) * |
| NST (25 µg) | 15.33 ± 0.19 | 8.76 ± 0.54 | 9.13 ± 0.23 |
| NST (25 µg) + ZnONPs (10 µg) | 20.12 ± 0.17 (0.72) * | 16.14 ± 0.29 (2.39) * | 13.12 ± 0.34 (1.06) * |
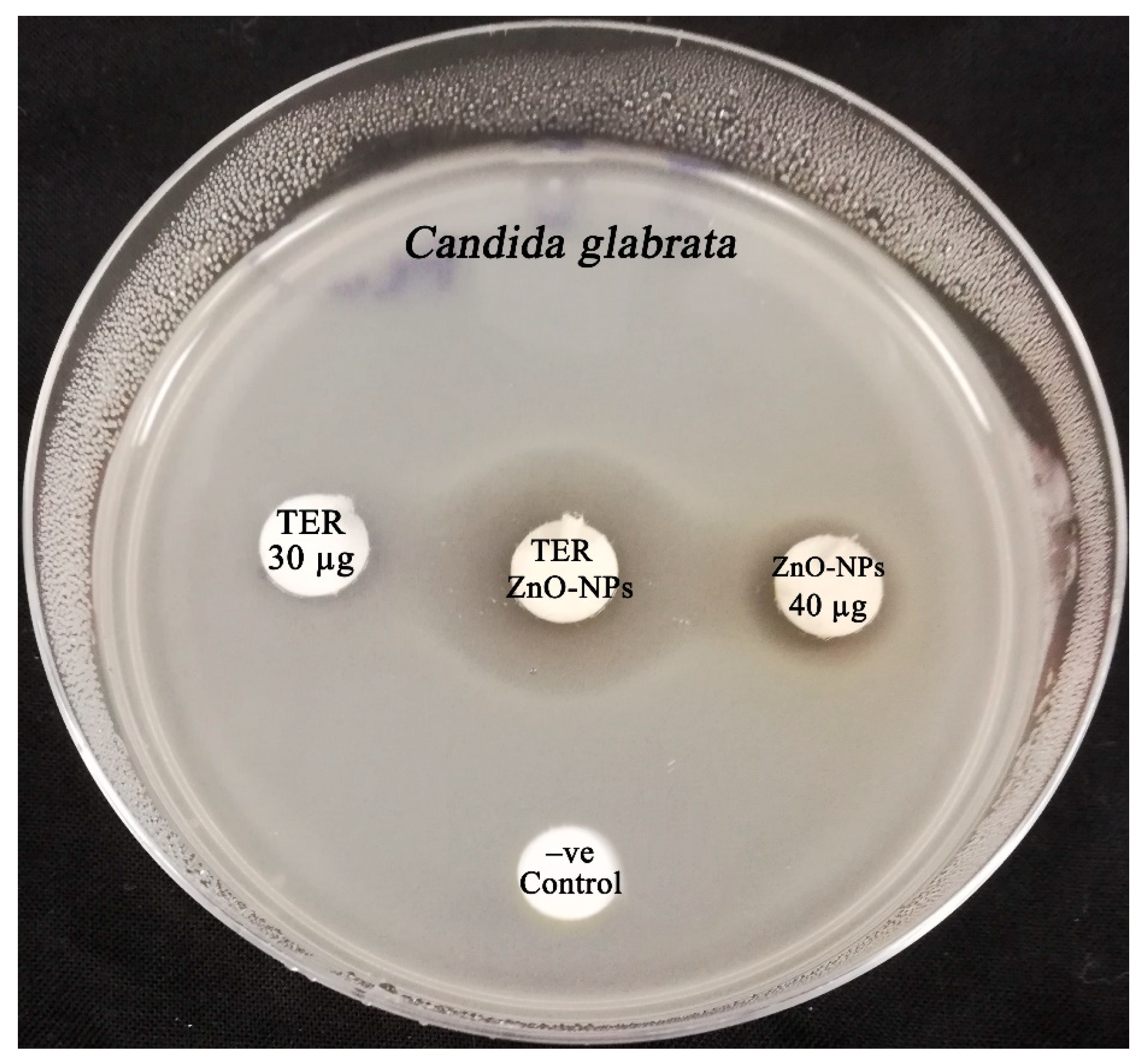
| TER (30 µg) | 27.12 ± 0.56 | 8.34 ± 0.42 | 36.14 ± 0.29 |
| TER (30 µg) + ZnONPs (10 µg) | 29.17 ± 0.21 (0.16) * | 24.16 ± 0.54 (6.82) * | 37.15 ± 0.42 (0.06) ns |
| Candidal Strains | Combined ZnO+ Antifungal Agent | FICI | Action |
|---|---|---|---|
| C. albicans | ZnO-NPs + clotrimazole | 0.75 | Additive |
| ZnO-NPs + fluconazole | 0.38 | Synergistic | |
| ZnO-NPs + itraconazole | 1.50 | No effect | |
| ZnO-NPs + nystatin | 0.75 | Additive | |
| ZnO-NPs + terbinafine | 1.25 | No effect | |
| C. glabrata | ZnO-NPs + clotrimazole | 1.25 | No effect |
| ZnO-NPs + fluconazole | 1.50 | No effect | |
| ZnO-NPs + itraconazole | 0.63 | Additive | |
| ZnO-NPs + nystatin | 0.38 | Synergistic | |
| ZnO-NPs + terbinafine | 0.25 | Synergistic | |
| C. tropicalis | ZnO-NPs + clotrimazole | 1.50 | No effect |
| ZnO-NPs + fluconazole | 1.00 | Additive | |
| ZnO-NPs + itraconazole | 2.00 | No effect | |
| ZnO-NPs + nystatin | 0.50 | Synergistic | |
| ZnO-NPs + terbinafine | 1.50 | No effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, M.T.; Al-Otibi, F.O.; Al-Askar, A.A.; Elmaghrabi, M.M. Synergistic Anticandidal Effectiveness of Greenly Synthesized Zinc Oxide Nanoparticles with Antifungal Agents against Nosocomial Candidal Pathogens. Microorganisms 2023, 11, 1957. https://doi.org/10.3390/microorganisms11081957
Yassin MT, Al-Otibi FO, Al-Askar AA, Elmaghrabi MM. Synergistic Anticandidal Effectiveness of Greenly Synthesized Zinc Oxide Nanoparticles with Antifungal Agents against Nosocomial Candidal Pathogens. Microorganisms. 2023; 11(8):1957. https://doi.org/10.3390/microorganisms11081957
Chicago/Turabian StyleYassin, Mohamed Taha, Fatimah O. Al-Otibi, Abdulaziz A. Al-Askar, and Marwa M. Elmaghrabi. 2023. "Synergistic Anticandidal Effectiveness of Greenly Synthesized Zinc Oxide Nanoparticles with Antifungal Agents against Nosocomial Candidal Pathogens" Microorganisms 11, no. 8: 1957. https://doi.org/10.3390/microorganisms11081957
APA StyleYassin, M. T., Al-Otibi, F. O., Al-Askar, A. A., & Elmaghrabi, M. M. (2023). Synergistic Anticandidal Effectiveness of Greenly Synthesized Zinc Oxide Nanoparticles with Antifungal Agents against Nosocomial Candidal Pathogens. Microorganisms, 11(8), 1957. https://doi.org/10.3390/microorganisms11081957






