Bacterial Bloodstream Infections after Allogeneic Hematopoietic Stem Cell Transplantation: Etiology, Risk Factors and Outcome in a Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Conditioning Regimens and GvHD Prophylaxis
2.3. Definitions
2.4. Supportive Care
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
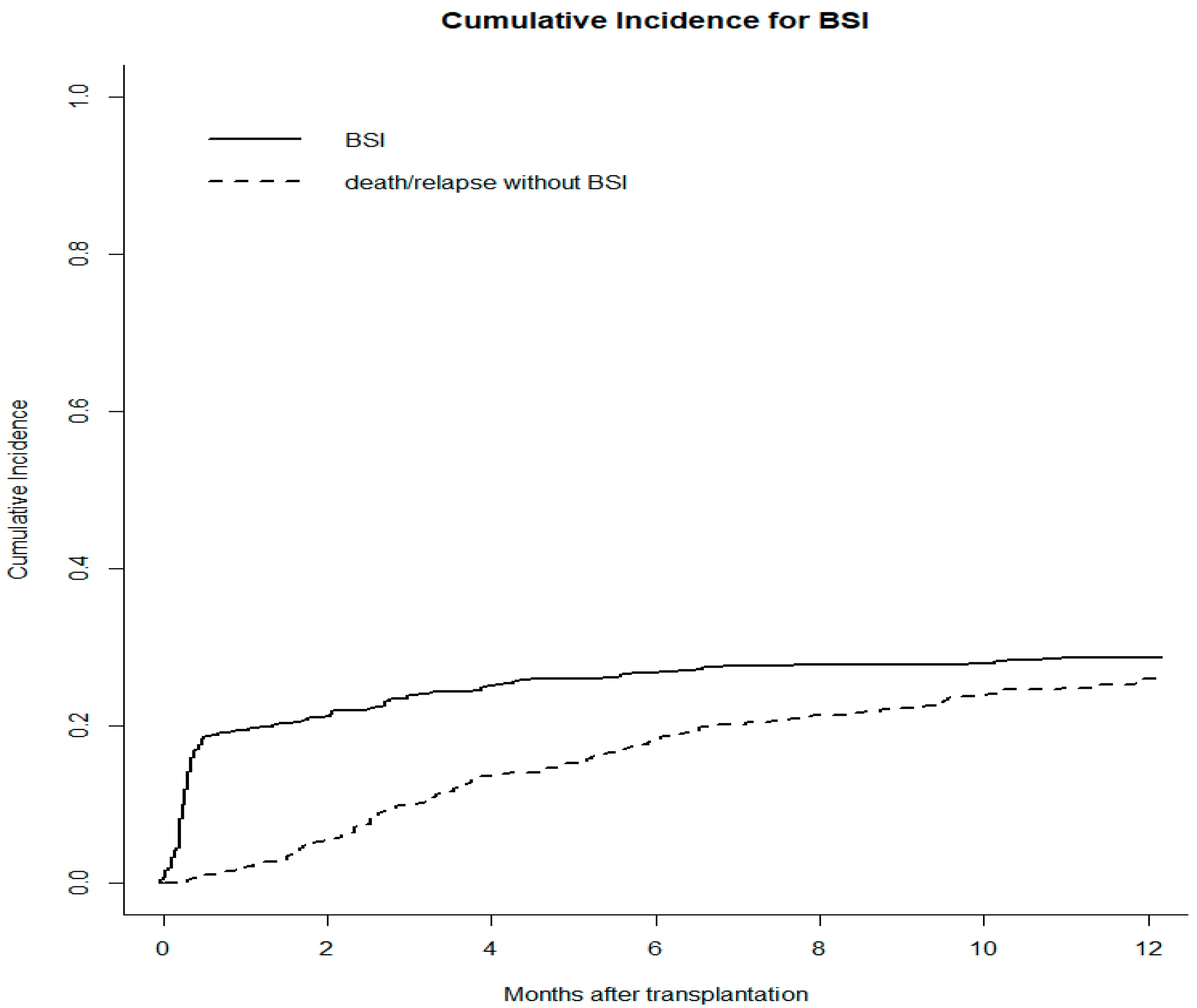
3.2. Incidence and Clinical Characteristics of BSIs
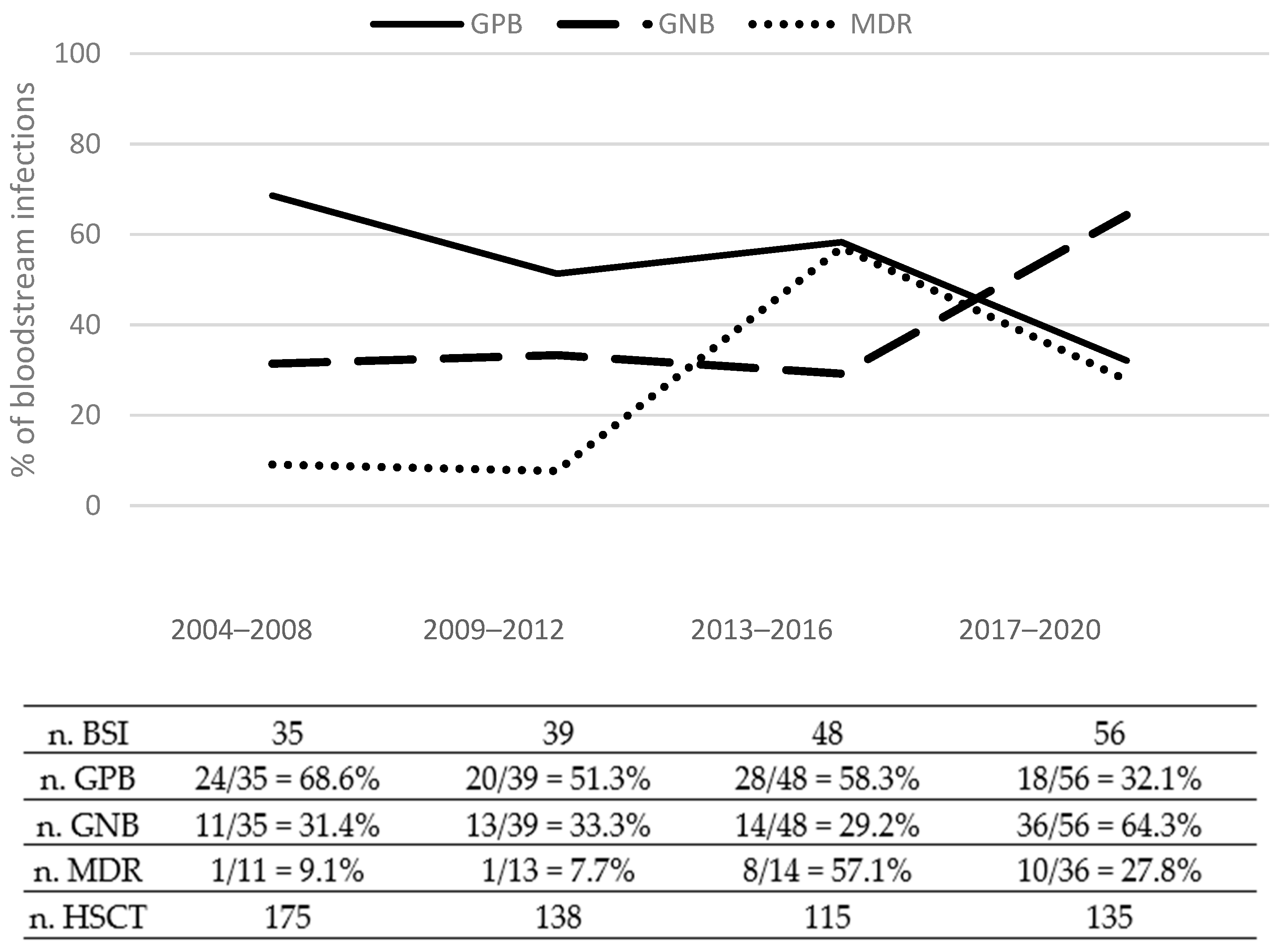
3.3. Etiology of BSIs
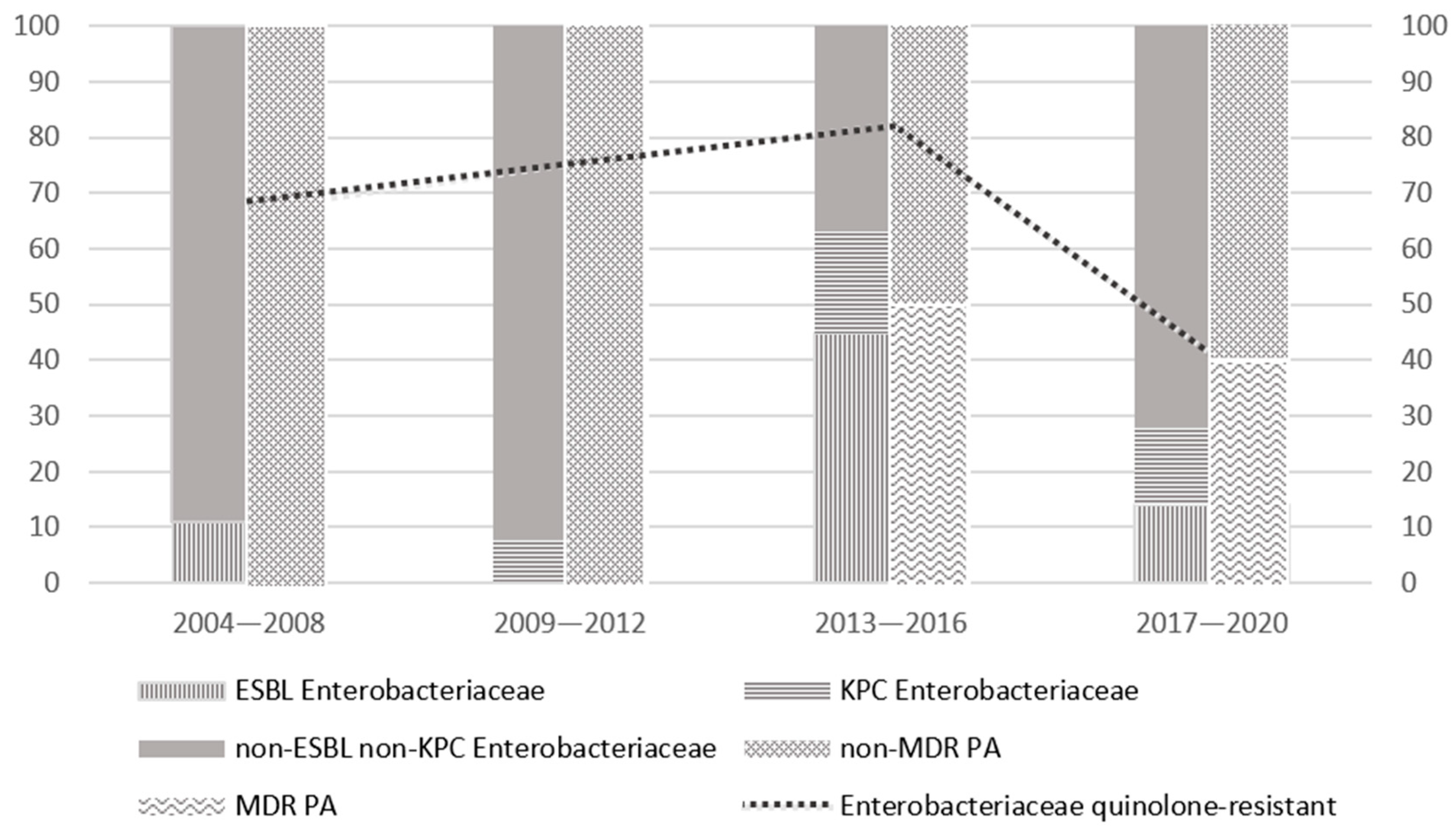
3.4. Drug Resistance
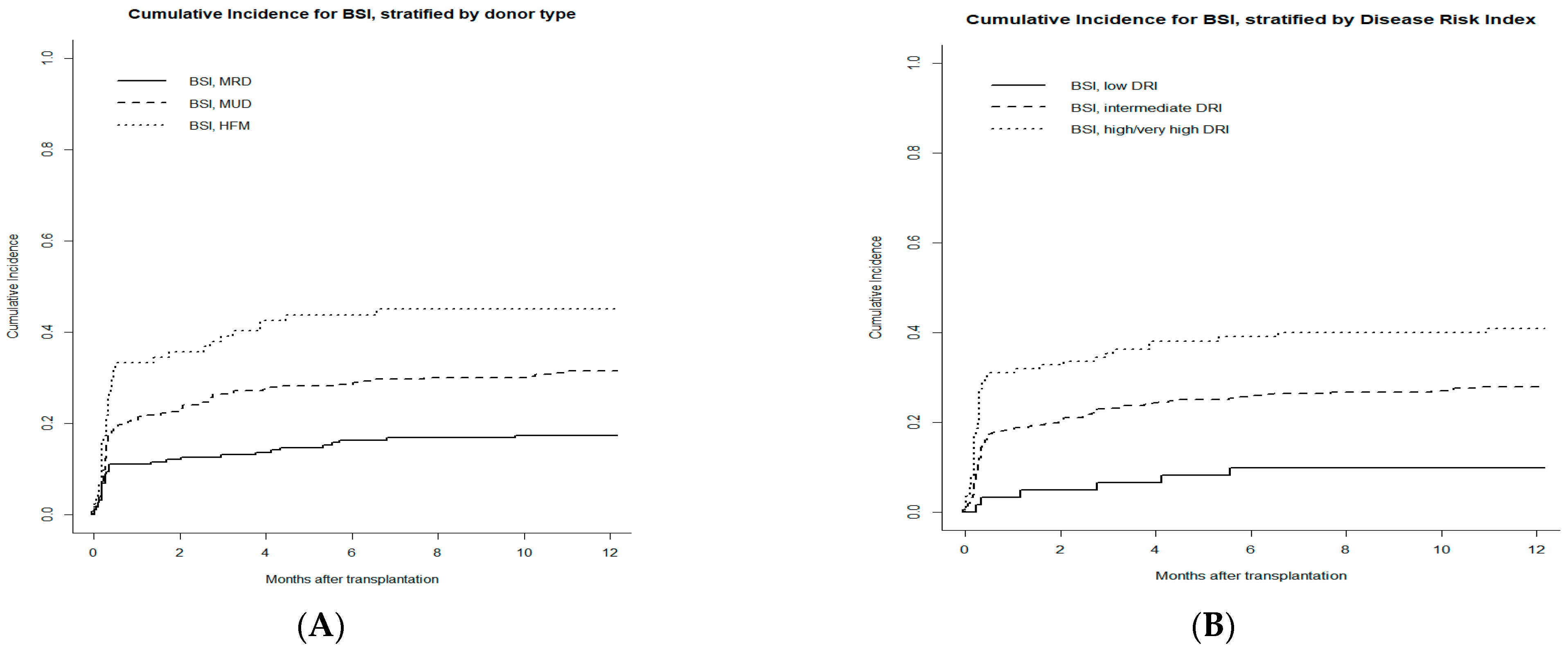
3.5. Risk Factors for Bacterial BSI
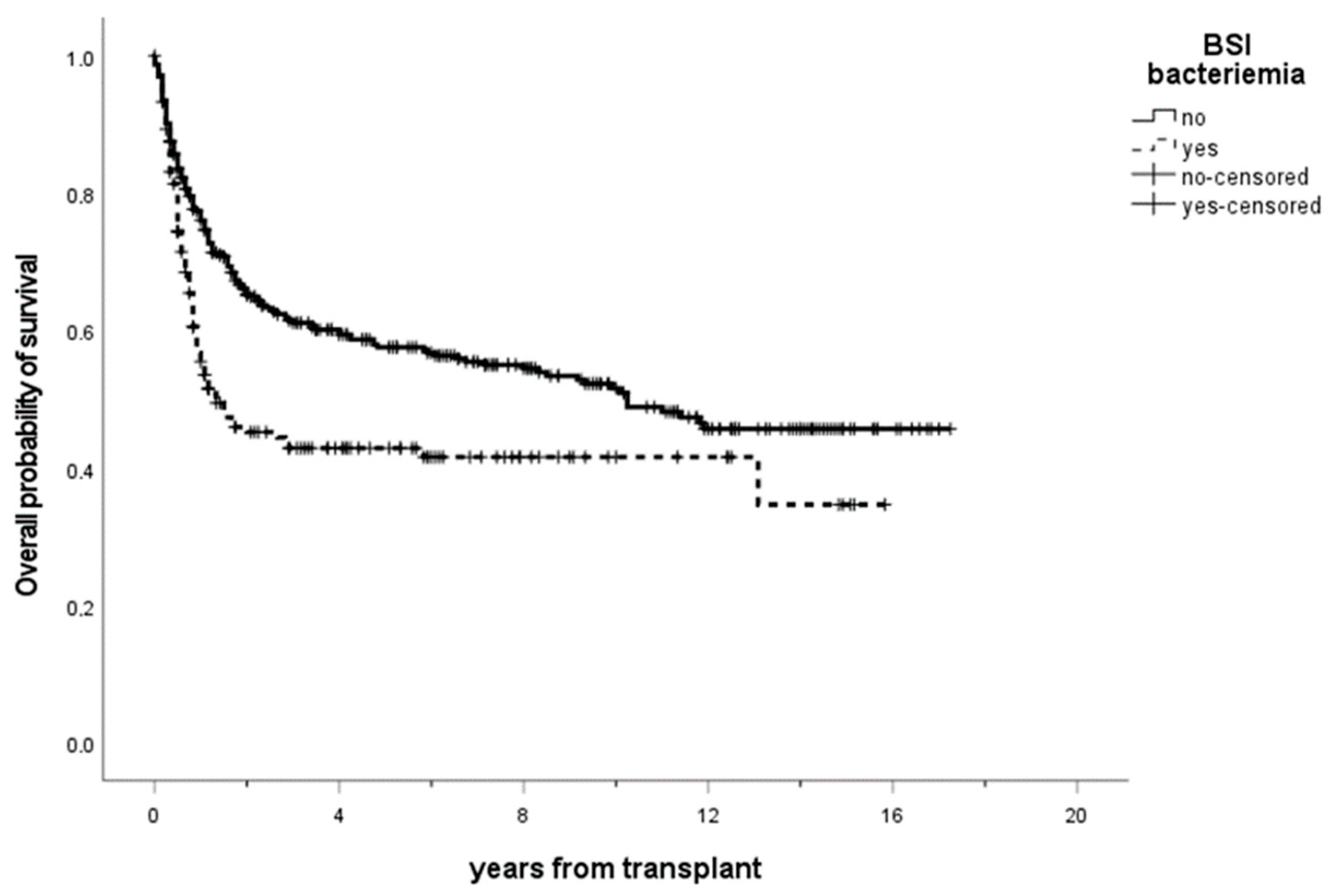
3.6. Outcome and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyurkocza, B.; Rezvani, A.; Storb, R.F. Allogeneic hematopoietic cell transplantation: The state of the art. Expert Rev. Hematol. 2010, 3, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Copelan, E.A.; Chojecki, A.; Lazarus, H.M.; Avalos, B.R. Allogeneic hematopoietic cell transplantation; the current renaissance. Blood Rev. 2018, 34, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Kröger, N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: Can we recommend “when and for whom” in 2021? Haematologica 2021, 106, 1794–1804. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Martinez-Cibrian, N.; Zeiser, R.; Perez-Simon, J.A. Graft-versus-host disease prophylaxis: Pathophysiology-based review on current approaches and future directions. Blood Rev. 2020, 48, 100792. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An Overview of Infectious Complications After Al-logeneic Hematopoietic Stem Cell Transplantation. J. Infect. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Neofytos, D. Antimicrobial Prophylaxis and Preemptive Approaches for the Prevention of Infections in the Stem Cell Transplant Recipient, with Analogies to the Hematologic Malignancy Patient. Infect. Dis. Clin. N. Am. 2019, 33, 361–380. [Google Scholar] [CrossRef]
- Busca, A.; Cinatti, N.; Gill, J.; Passera, R.; Dellacasa, C.M.; Giaccone, L.; Dogliotti, I.; Manetta, S.; Corcione, S.; De Rosa, F.G. Management of Invasive Fungal Infections in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: The Turin Experience. Front. Cell. Infect. Microbiol. 2022, 11, 805514. [Google Scholar] [CrossRef]
- Cao, W.; Guan, L.; Li, X.; Zhang, R.; Li, L.; Zhang, S.; Wang, C.; Xie, X.; Jiang, Z.; Wan, D.; et al. Clinical Analysis of Bloodstream Infections During Agranulocytosis After Allogeneic Hematopoietic Stem Cell Transplantation. Infect. Drug Resist. 2021, 14, 185–192. [Google Scholar] [CrossRef]
- Kern, W.V.; Roth, J.A.; Bertz, H.; Götting, T.; Dettenkofer, M.; Widmer, A.F.; Theilacker, C. The Hospital Infection Surveillance System for Patients with Hematologic/Oncologic Malignancies Study Group (ONKO-KISS); Hospital Infection Surveillance System for Patients with Hematologic/Oncologic Malignancies Study Group (ONKO-KISS) Contribution of specific pathogens to bloodstream infection mortality in neutropenic patients with hematologic malignancies: Results from a multicentric surveillance cohort study. Transpl. Infect. Dis. 2019, 21, e13186. [Google Scholar] [CrossRef] [PubMed]
- Weisser, M.; Theilacker, C.; Tschudin-Sutter, S.; Babikir, R.; Bertz, H.; Götting, T.; Dettenkofer, M.; Kern, W.V.; Widmer, A.F.; Hospital Infection Surveillance System for Patients With Haematologic/Oncologic Malignancies Study Group (ONKO-KISS). Secular trends of bloodstream infections during neutropenia in 15 181 haematopoietic stem cell transplants: 13-year results from a European multicentre surveillance study (ONKO-KISS). Clin. Microbiol. Infect. 2017, 23, 854–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blennow, O.; Ljungman, P.; Sparrelid, E.; Mattsson, J.; Remberger, M. Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl. Infect. Dis. 2014, 16, 106–114. [Google Scholar] [CrossRef]
- Kikuchi, M.; Akahoshi, Y.; Nakano, H.; Ugai, T.; Wada, H.; Yamasaki, R.; Sakamoto, K.; Kawamura, K.; Ishihara, Y.; Sato, M.; et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Yan, C.-H.; Wang, Y.; Mo, X.-D.; Sun, Y.-Q.; Wang, F.-R.; Fu, H.-X.; Chen, Y.; Han, T.-T.; Kong, J.; Cheng, Y.-F.; et al. Incidence, Risk Factors, Microbiology and Outcomes of Pre-engraftment Bloodstream Infection After Haploidentical Hematopoietic Stem Cell Transplantation and Comparison With HLA-identical Sibling Transplantation. Clin. Infect. Dis. 2018, 67, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Italiana del Farmaco-AIFA. Guidelines for Observational Studies; AIFA: Rome, Italy, 2008. [Google Scholar]
- Luznik, L.; Jalla, S.; Engstrom, L.W.; Iannone, R.; Fuchs, E.J. Durable engraftment of major histocompatibility complex–incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 2001, 98, 3456–3464. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kroger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Bookshelf ID: NBK553942; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef] [Green Version]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [Green Version]
- Armand, P.; Kim, H.T.; Logan, B.R.; Wang, Z.; Alyea, E.P.; Kalaycio, M.E.; Maziarz, R.T.; Antin, J.H.; Soiffer, R.J.; Weisdorf, D.J.; et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Klein, J.P.; Barrett, A.J.; Ringden, O.; Antin, J.H.; Cahn, J.-Y.; Carabasi, M.H.; Gale, R.P.; Giralt, S.; Hale, G.A.; et al. Severity of chronic graft-versus-host disease: Association with treatment-related mortality and relapse. Blood 2002, 100, 406–414. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2014, 21, 389–401.e1. [Google Scholar] [CrossRef] [Green Version]
- Oltolini, C.; Greco, R.; Galli, L.; Clerici, D.; Lorentino, F.; Xue, E.; Lupo-Stanghellini, M.T.; Giglio, F.; Uhr, L.; Ripa, M.; et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol. Blood Marrow Transplant. 2020, 26, 1179–1188. [Google Scholar] [CrossRef]
- Bock, A.M.; Cao, Q.; Ferrieri, P.; Young, J.-A.H.; Weisdorf, D.J. Bacteremia in Blood or Marrow Transplantation Patients: Clinical Risk Factors for Infection and Emerging Antibiotic Resistance. Biol. Blood Marrow Transplant. 2013, 19, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.-A.H.; Logan, B.R.; Wu, J.; Wingard, J.R.; Weisdorf, D.J.; Mudrick, C.; Knust, K.; Horowitz, M.M.; Confer, D.L.; Dubberke, E.R.; et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol. Blood Marrow Transplant. 2016, 22, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreira, A.S.; Salas, M.Q.; Remberger, M.; Basso, I.N.; Law, A.D.; Lam, W.; Pasic, I.; Kim, D.; Michelis, F.V.; Viswabandya, A.; et al. Bloodstream Infections and Outcomes Following Allogeneic Hematopoietic Cell Transplantation: A Single-Center Study. Transplant. Cell. Ther. 2022, 28, 50.e1–50.e8. [Google Scholar] [CrossRef]
- Ustun, C.; Young, J.-A.; Papanicolaou, G.A.; Kim, S.; Ahn, K.W.; Chen, M.; Abdel-Azim, H.; Aljurf, M.; Beitinjaneh, A.; Brown, V.; et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019, 54, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.; Tumbarello, M.; Caira, M.; Candoni, A.; Cattaneo, C.; Pastore, D.; Fanci, R.; Nosari, A.; Vianelli, N.; Busca, A.; et al. Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica 2011, 96, e1–e3. [Google Scholar] [CrossRef] [Green Version]
- Arena, F.; Menchinelli, G.; Di Pilato, V.; Torelli, R.; Antonelli, A.; De Angelis, L.H.; Coppi, M.; Sanguinetti, M.; Rossolini, G.M. Resistance and virulence features of hypermucoviscous Klebsiella pneumoniae from bloodstream infections: Results of a nationwide Italian surveillance study. Front. Microbiol. 2022, 13, 983294. [Google Scholar] [CrossRef]
- Kern, W.V.; Weber, S.; Dettenkofer, M.; Kaier, K.; Bertz, H.; Behnke, M.; Weisser, M.; Götting, T.; Widmer, A.F.; Theilacker, C.; et al. Impact of fluoroquinolone prophylaxis during neutropenia on bloodstream infection: Data from a surveillance program in 8755 patients receiving high-dose chemotherapy for haematologic malignancies between 2009 and 2014. J. Infect. 2018, 77, 68–74. [Google Scholar] [CrossRef]
- Jahan, D.; Peile, E.; Sheikh, A.; Islam, S.; Parasnath, S.; Sharma, P.; Iskandar, K.; Dhingra, S.; Charan, J.; Hardcastle, T.C.; et al. Is it time to reconsider prophylactic antimicrobial use for hematopoietic stem cell transplantation? a narrative review of antimicrobials in stem cell transplantation. Expert Rev. Anti-infective Ther. 2021, 19, 1259–1280. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, H.; Lee, R.; Cho, S.-Y.; Lee, D.-G. Bloodstream Infections in Patients with Hematologic Diseases: Causative Organisms and Factors Associated with Resistance. Infect. Chemother. 2022, 54, 340. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Wangjam, T.; Bhatt, A.S.; Weisdorf, D.J.; Holtan, S.G.; on behalf of BMT CTN Investigators. Antibiotic practice patterns in hematopoietic cell transplantation: A survey of blood and marrow transplant clinical trials network centers. Am. J. Hematol. 2018, 93, E348–E350. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Chang, F.-Y.; Chao, T.-Y.; Kao, W.-Y.; Ho, C.-L.; Chen, Y.-C.; Dai, M.-S.; Chang, P.-Y.; Wu, Y.-Y.; Lin, J.-C. Characteristics comparisons of bacteremia in allogeneic and autologous hematopoietic stem cell-transplant recipients with levofloxacin prophylaxis and influence on resistant bacteria emergence. J. Microbiol. Immunol. Infect. 2018, 51, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Fredricks, D.N. The gut microbiota and graft-versus-host disease. J. Clin. Investig. 2019, 129, 1808–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulska, M.; Del Bono, V.; Bruzzi, P.; Raiola, A.M.; Gualandi, F.; Van Lint, M.T.; Bacigalupo, A.; Viscoli, C. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection 2012, 40, 271–278. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Cardozo, C.; Marco, F.; Suárez-Lledó, M.; Moreno-García, E.; Morata, L.; Fernández-Avilés, F.; Gutiérrez-Garcia, G.; Chumbita, M.; Rosiñol, L.; et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: Current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant. 2020, 55, 603–612. [Google Scholar] [CrossRef]
- Patriarca, F.; Cigana, C.; Massimo, D.; Lazzarotto, D.; Geromin, A.; Isola, M.; Battista, M.L.; Medeot, M.; Cerno, M.; Sperotto, A.; et al. Risk Factors and Outcomes of Infections by Multidrug-Resistant Gram-Negative Bacteria in Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 23, 333–339, Erratum in: Biol. Blood Marrow Transplant. 2017, 23, 1040. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, S.; Venturelli, C.; DiGaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and Risk Factors Associated With Mortality in Consecutive Patients With Bacterial Bloodstream Infection: Impact of MDR and XDR Bacteria. Open Forum Infect. Dis. 2020, 7, ofaa461. [Google Scholar] [CrossRef]
- Mellouli, A.; Chebbi, Y.; El Fatmi, R.; Raddaoui, A.; Lakhal, A.; Torjmane, L.; Ben Abdeljelil, N.; Belloumi, D.; Ladeb, S.; Ben Othmen, T.; et al. Multidrug resistant bacteremia in hematopoietic stem cell transplant recipients. Tunis Med. 2021, 99, 269–276. [Google Scholar] [PubMed]
| Characteristics | All Patients | BSI Neg | BSI Pos |
|---|---|---|---|
| Number of patients | 563 | 385 | 178 |
| Age at transplant, median (IQR), years | 49 (40–58) | 49 (39–58) | 49 (40–58) |
| Gender | |||
| Male | 307 (54.5%) | 202 (52.5%) | 105 (59%) |
| Female | 256 (45.5%) | 183 (47.5%) | 73 (41.0%) |
| Underlying disease | |||
| AML/MDS | 319 (56.7%) | 199 (51.7%) | 120 (67.4%) |
| ALL | 84 (14.9%) | 57 (14.8%) | 27 (15.2%) |
| HL/NHL | 101 (17.9%) | 80 (20.8%) | 21 (11.8%) |
| MPN/LMMC | 49 (8.7%) | 39 (10.1%) | 10 (5.6%) |
| MM | 10 (1.8%) | 10 (2.6%) | 0 (0%) |
| Disease status at transplant | |||
| CR | 282 (50.4%) | 194 (50.7%) | 88 (49.7%) |
| CR2 | 96 (17.1%) | 73 (19.1%) | 23 (13%) |
| PIF/relapse | 182 (32.5%) | 116 (30.3%) | 66 (37.3%) |
| HCT-CI | |||
| Low/intermediate (0–2) | 236 (72.6%) | 151 (73.7%) | 85 (70.8%) |
| High (>3) | 89 (27.4%) | 54 (26.3%) | 35 (29.2%) |
| DRI | |||
| Low | 61 (11.3%) | 54 (14.6%) | 7 (4.1%) |
| Intermediate | 367 (67.8%) | 255 (68.7%) | 112 (65.9%) |
| High | 92 (17.0%) | 52 (14.0%) | 40 (23.5%) |
| Very high | 21 (3.9%) | 10 (2.7%) | 11 (6.5%) |
| Donor type | |||
| MRD | 191 (34%) | 151 (39.3%) | 40 (22.5%) |
| MUD | 284 (50.5%) | 188 (49%) | 96 (53.9%) |
| Haploidentical a | 87 (15.5%) | 45 (11.7%) | 42 (24%) |
| Stem cell source | |||
| PBSC | 458 (81.5%) | 323 (84.1%) | 135 (75.8%) |
| BM | 96 (17.1%) | 56 (14.6%) | 40 (22.5%) |
| CB | 8 (1.4%) | 5 (1.3%) | 3 (1.7%) |
| Number of CD34+ cells infused, median (IQR), ×106/kg | 7.0 (5.4–9.1) | 7.2 (5.7–9.1) | 6.6 (5.2–8.6) |
| Number of CD3+ cells infused, median (IQR), ×108/kg | 2.5 (1.5–3.3) | 2.6 (1.7–3.6) | 2.2 (1.2–3.1) |
| Conditioning regimen | |||
| MAC | 390 (69.4%) | 258 (67.2%) | 132 (74.2%) |
| RIC | 172 (30.6%) | 126 (32.8%) | 46 (25.8%) |
| GvHD prophylaxis | |||
| ATG | 280 (50.4%) | 186 (48.9%) | 94 (53.4%) |
| other | 276 (49.6%) | 194 (51.1%) | 82 (46.6%) |
| Time to engraftment b, median (IQR), days | 16 (14–18) | 16 (14–18) | 16 (14–18) |
| FQ prophylaxis | |||
| No | 153 (28.7%) | 88 (23.7%) | 65 (40.1%) |
| Yes | 381 (71.3%) | 284 (76.3%) | 97 (59.9%) |
| Acute GvHD | |||
| 0–I | 175 (65.8%) | 108 (65.9%) | 67 (65.7%) |
| II–IV | 91 (34.2%) | 56 (34.1%) | 35 (34.3%) |
| Chronic GvHD | |||
| absent/mild | 166 (65.6%) | 102 (66.7%) | 64 (64%) |
| moderate/severe | 87 (34.4%) | 51 (33.3%) | 36 (36%) |
| Overall Survival | |||
| Alive | 295 (52.4%) | 213 (55.3%) | 82 (46.1%) |
| Dead | 268 (47.6%) | 172 (44.7%) | 96 (53.9%) |
| Whole Cohort (n = 178) | Pre-Engraftment (day 0–30) (n = 109) | Post-Engraftment (day ≥ 31) (n = 69) | |
|---|---|---|---|
| Gram-positive Bacteria | 90 | 52 | 38 |
| Coagulase-negative staphylococci a | 66 | 34 | 32 |
| Staphylococcus aureus (n. MRSA) | 3 (2 MRSA) | 1 (1 MRSA) | 2 (1 MRSA) |
| Streptococci mitis | 8 | 7 | 1 |
| Other streptococci | 4 | 2 | 2 |
| Enterococcus (n. VRE) | 8 (1 VRE) | 7 (1 VRE) | 1 0 |
| Corynebacterium spp. | 1 | 1 | - |
| Gram-negative Bacteria | 74 | 47 | 27 |
| Escherichia coli (n. ESBL) | 39 (7 ESBL) | 30 (6 ESBL) | 9 (1 ESBL) |
| Klebsiella (n. ESBL) (n. KPC) | 12 (2 ESBL) (7 KPC) | 7 (0 ESBL) (5 KPC) | 5 (2 ESBL) (2 KPC) |
| Enterobacter (n. ESBL) | 4 (1 ESBL) | 3 (1 ESBL) | 1 |
| Stenotrophomonas maltophilia | 3 | - | 3 |
| Citrobacter | 1 | 1 | - |
| Proteus mirabilis | 2 | 1 | 1 |
| Pseudomonas aeruginosa (n. MDR) | 10 (3 MDR) | 3 (1 MDR) | 7 (2 MDR) |
| Fusobacterium | 2 | 2 | - |
| Bacteroides | 1 | - | 1 |
| Polymicrobial BSI | 14 | 10 | 4 |
| MDR-gram-negative bacteria | 20 | 13 | 7 |
| Univariate Analyses | Multivariate Analysis | |||
| SDHR (95% CI) | p | SDHR (95% CI) | p | |
| Age (>60 vs. 40–60 vs. <40 years) | 1.02 (0.78–1.22) | 0.850 | - | - |
| Gender (male vs. female) | 1.27 (0.93–1.74) | 0.130 | - | - |
| Underlying disease (AML vs. other) | 1.69 (1.23–2.34) | 0.001 | 1.31 (0.91–1.90) | 0.150 |
| Disease status at transplant (advanced vs. early disease) | 1.08 (0.79–1.47) | 0.620 | - | - |
| HCT-CI (≥3 vs. 0–2) | 1.11 (0.74–1.68) | 0.610 | - | - |
| DRI (very high + high vs. intermediate vs. low) | 1.91 (1.46–2.49) | <0.001 | 1.70 (1.26–2.31) | <0.001 |
| Donor type (Haploidentical vs. MUD vs. MRD) | 1.76 (1.41–2.20) | <0.001 | 1.51 (1.17–1.93) | 0.001 |
| Stem cell source (PBSC vs. BM) | 0.70 (0.48–1.01) | 0.055 | 0.80 (0.49–1.29) | 0.360 |
| Number of CD34+ cells infused (over vs. under median), 106/kg | 0.77 (0.57–1.06) | 0.110 | - | - |
| Number of CD3+ cells infused (over vs. under median), 108/kg | 0.68 (0.49–0.94) | 0.020 | 0.15 (0.55–1.21) | 0.300 |
| Conditioning regimen (RIC vs. MAC) | 0.71 (0.50–1.02) | 0.061 | 0.87 (0.57–1.32) | 0.640 |
| GvHD prophylaxis (ATG vs. other) | 1.24 (0.91–1.69) | 0.170 | - | - |
| Antibacterial prophylaxis (yes vs. no) | 0.51 (0.37–0.70) | <0.001 | 0.58 (0.40–0.84) | 0.004 |
| Time to engraftment (over vs. under median), days | 0.97 (0.70–1.33) | 0.840 | - | - |
| Acute GvHD (II–IV vs. 0–I) | 1.01 (0.67–1.53) | 0.950 | - | - |
| Chronic GvHD (moderate-severe vs. no and mild) | 1.13 (0.75–1.70) | 0.550 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, J.; Busca, A.; Cinatti, N.; Passera, R.; Dellacasa, C.M.; Giaccone, L.; Dogliotti, I.; Manetta, S.; Corcione, S.; De Rosa, F.G. Bacterial Bloodstream Infections after Allogeneic Hematopoietic Stem Cell Transplantation: Etiology, Risk Factors and Outcome in a Single-Center Study. Microorganisms 2023, 11, 742. https://doi.org/10.3390/microorganisms11030742
Gill J, Busca A, Cinatti N, Passera R, Dellacasa CM, Giaccone L, Dogliotti I, Manetta S, Corcione S, De Rosa FG. Bacterial Bloodstream Infections after Allogeneic Hematopoietic Stem Cell Transplantation: Etiology, Risk Factors and Outcome in a Single-Center Study. Microorganisms. 2023; 11(3):742. https://doi.org/10.3390/microorganisms11030742
Chicago/Turabian StyleGill, Jessica, Alessandro Busca, Natascia Cinatti, Roberto Passera, Chiara Maria Dellacasa, Luisa Giaccone, Irene Dogliotti, Sara Manetta, Silvia Corcione, and Francesco Giuseppe De Rosa. 2023. "Bacterial Bloodstream Infections after Allogeneic Hematopoietic Stem Cell Transplantation: Etiology, Risk Factors and Outcome in a Single-Center Study" Microorganisms 11, no. 3: 742. https://doi.org/10.3390/microorganisms11030742








