Time to Load Up–Resistance Training Can Improve the Health of Women with Polycystic Ovary Syndrome (PCOS): A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
Search Methods for Identification of Studies
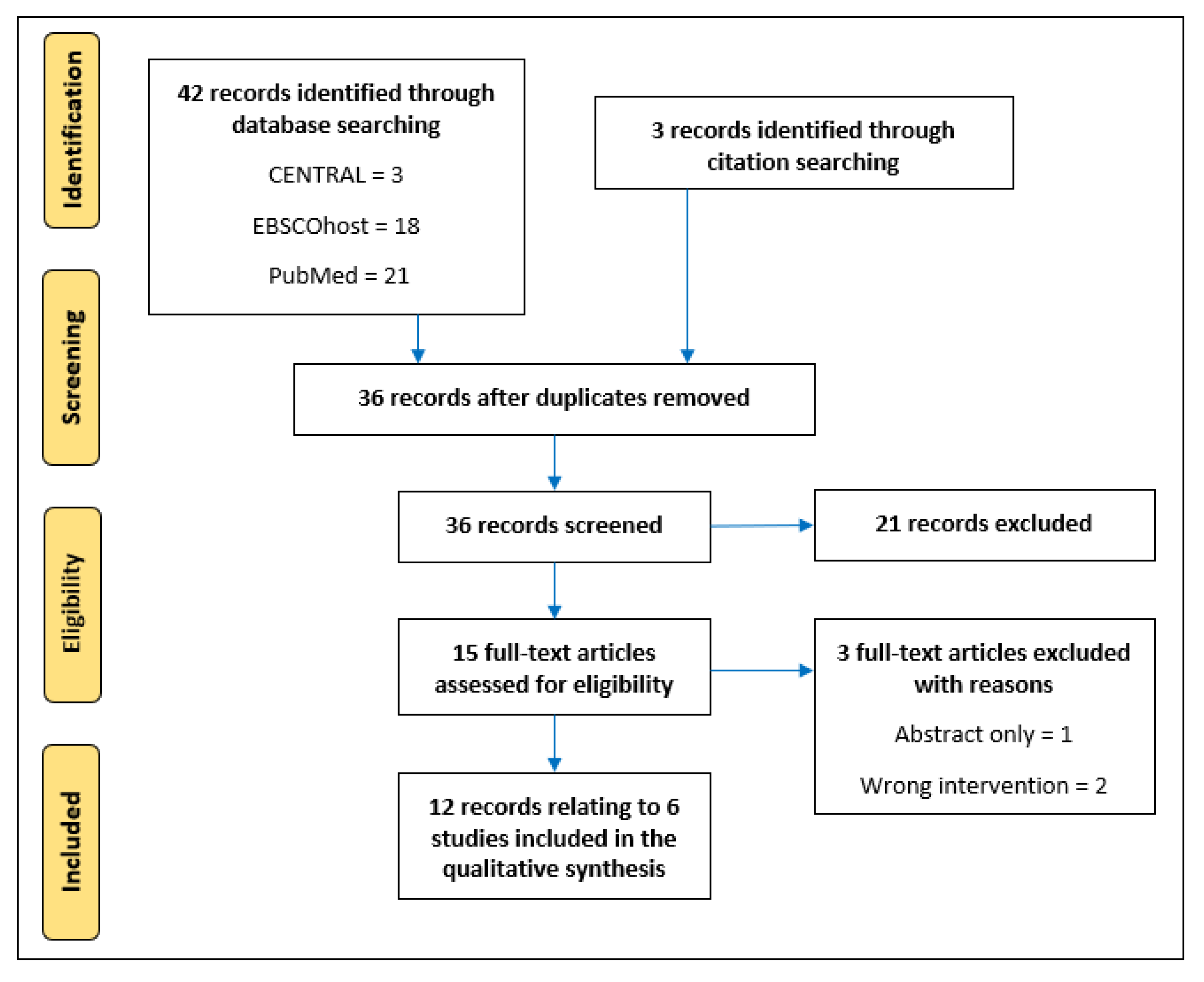
3. Results
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Outcome Measures
3.4. Effects of Resistance Training Interventions
4. Discussion
4.1. Included Primary Studies
4.2. RT Training Composition and Reporting
4.3. Outcomes and Favourable Effects of Interventions
4.4. Strengths and Limitations of the Present Scoping Review
4.5. Summary of Gaps in Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boyle, J.A.; Cunningham, J.; OˈDea, K.; Dunbar, T.; Norman, R.J. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med. J. Aust. 2012, 196, 62–66. [Google Scholar] [CrossRef]
- Kyrou, I.; Weickert, M.O.; Randeva, H.S. Diagnosis and management of polycystic ovary syndrome (PCOS). In Endocrinology and Diabetes; Springer: London, UK, 2015; pp. 99–113. [Google Scholar]
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity, in Endotext [Internet]; MDText. com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Randeva, H.S.; Tan, B.K.; Weickert, M.O.; Lois, K.; Nestler, J.E.; Sattar, N.; Lehnert, H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr. Rev. 2012, 33, 812–841. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Kyrou, I.; Uthman, O.A.; Brown, A.; Johnson, S.; Wall, P.D.; Metcalfe, A.; Parr, D.G.; Tahrani, A.A.; Randeva, H.S. The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: A systematic review and meta-analysis. Sleep Breath. 2020, 24, 339–350. [Google Scholar] [CrossRef]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef]
- Barry, J.A.; Kuczmierczyk, A.R.; Hardiman, P.J. Anxiety and depression in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2011, 26, 2442–2451. [Google Scholar] [CrossRef]
- Karjula, S.; Morin-Papunen, L.; Auvinen, J.; Ruokonen, A.; Puukka, K.; Franks, S.; Järvelin, M.-R.; Tapanainen, J.S.; Jokelainen, J.; Miettunen, J. Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. J. Clin. Endocrinol. Metab. 2017, 102, 1861–1869. [Google Scholar] [CrossRef]
- Tay, C.T.; Teede, H.J.; Hill, B.; Loxton, D.; Joham, A.E. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: A community-based cohort study. Fertil. Steril. 2019, 112, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Kite, C.; Atkinson, L.; McGregor, G.; Clark, C.C.; Brown, J.E.; Kyrou, I.; Randeva, H.S. Sleep disruption and depression, stress and anxiety levels in women with polycystic ovary syndrome (PCOS) during the lockdown measures for COVID-19 in the UK. Front. Glob. Women’s Health 2021, 2, 649104. [Google Scholar] [CrossRef] [PubMed]
- Jedel, E.; Waern, M.; Gustafson, D.; Landen, M.; Eriksson, E.; Holm, G.; Nilsson, L.; Lind, A.-K.; Janson, P.; Stener-Victorin, E. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum. Reprod. 2010, 25, 450–456. [Google Scholar] [CrossRef]
- Podfigurna-Stopa, A.; Luisi, S.; Regini, C.; Katulski, K.; Centini, G.; Meczekalski, B.; Petraglia, F. Mood disorders and quality of life in polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 431–434. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Pirotta, S.; Joham, A.J.; Moran, L.J.; Skouteris, H.; Lim, S.S. Implementation of evidence-based PCOS lifestyle management guidelines: Perceived barriers and facilitators by consumers using the Theoretical Domains Framework and COM-B Model. Patient Educ. Couns. 2021, 104, 2080–2088. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Hyde, R.; Wing, A.L.; Hsieh, C.-C. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef]
- Ekelund, U.; Ward, H.A.; Norat, T.; Luan, J.a.; May, A.M.; Weiderpass, E.; Sharp, S.J.; Overvad, K.; Østergaard, J.N.; Tjønneland, A. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 2015, 101, 613–621. [Google Scholar] [CrossRef]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; De Gonzalez, A.B.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 51. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Almenning, I.; Rieber-Mohn, A.; Lundgren, K.M.; Shetelig Løvvik, T.; Garnæs, K.K.; Moholdt, T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015, 10, e0138793. [Google Scholar] [CrossRef]
- Saremi, A.; Yaghoubi, M.S. Effect of resistance exercises with calcium consumption on level of anti-mullerian hormone and some metabolic indices in women with polycystic ovarian syndrome. Iran. J. Obstet. Gynecol. Infertil. 2016, 18, 7–15. [Google Scholar]
- Vizza, L.; Smith, C.A.; Swaraj, S.; Agho, K.; Cheema, B.S. The feasibility of progressive resistance training in women with polycystic ovary syndrome: A pilot randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Moore, H. UK Chief Medical Officers’ physical activity guidelines 2019: What’s new and how can we get people more active? Nutr. Bull. 2019, 44, 320–328. [Google Scholar] [CrossRef]
- Ross, R.; Chaput, J.-P.; Giangregorio, L.M.; Janssen, I.; Saunders, T.J.; Kho, M.E.; Poitras, V.J.; Tomasone, J.R.; El-Kotob, R.; McLaughlin, E.C. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: An integration of physical activity, sedentary behaviour, and sleep. Appl. Physiol. Nutr. Metab. 2020, 45, S57–S102. [Google Scholar] [CrossRef] [PubMed]
- El-Kotob, R.; Ponzano, M.; Chaput, J.-P.; Janssen, I.; Kho, M.E.; Poitras, V.J.; Ross, R.; Ross-White, A.; Saunders, T.J.; Giangregorio, L.M. Resistance training and health in adults: An overview of systematic reviews. Appl. Physiol. Nutr. Metab. 2020, 45, S165–S179. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Benson, A.; Bird, S.; Fraser, S. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2009, 83, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Lubans, D.R.; Karunamuni, N.; Kennedy, S.; Plotnikoff, R. Factors associated with participation in resistance training: A systematic review. Br. J. Sports Med. 2017, 51, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.; Afonso, J.; Ramirez-Campillo, R.; Murawska-Ciałowciz, E.; Marques, A.; Clemente, F.M. The effects of exclusively resistance training-based supervised programs in people with depression: A systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2020, 17, 6715. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, A.; Mavros, Y.; Heisz, J.J.; Singh, M.A.F. The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Med. Rev. 2018, 39, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Taaffe, D.R.; Galvão, D.A.; Newton, R.U.; Nonemacher, E.R.; Wendt, V.M.; Bassanesi, R.N.; Turella, D.J.; Rech, A. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13428. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Donà, S.; Bacchi, E.; Moghetti, P. Is cardiorespiratory fitness impaired in PCOS women? A review of the literature. J. Endocrinol. Investig. 2017, 40, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Lloyd, R.S.; Faigenbaum, A.D. Age- and Sex-Related Differences and Their Implications for Resistance Exercise. In Essentials of Strength Training and Conditioning; Haff, G.G., Triplett, N.T., Eds.; Human Kinetics: Champaign, IL, USA, 2016; pp. 135–154. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Ramos, F.K.P.; Lara, L.A.D.S.; Kogure, G.S.; Silva, R.C.; Ferriani, R.A.; Silva de Sá, M.F.; Reis, R.M.D. Quality of life in women with polycystic ovary syndrome after a program of resistance exercise training. Rev. Bras. Ginecol. E Obs. 2016, 38, 340–347. [Google Scholar] [CrossRef]
- Kogure, G.S.; Miranda-Furtado, C.L.; Pedroso, D.C.C.; Ribeiro, V.B.; Eiras, M.C.; Silva, R.C.; Caetano, L.C.; Ferriani, R.A.; Calado, R.T.; Dos Reis, R.M. Effects of Progressive Resistance Training on Obesity Indices in Polycystic Ovary Syndrome and the Relationship With Telomere Length. J. Phys. Act. Health 2019, 16, 601–607. [Google Scholar] [CrossRef]
- Kogure, G.S.; Miranda-Furtado, C.L.; Silva, R.C.; Melo, A.S.; Ferriani, R.A.; De Sá, M.F.; Dos Reis, R.M. Resistance Exercise Impacts Lean Muscle Mass in Women with Polycystic Ovary Syndrome. Med. Sci. Sports Exerc. 2016, 48, 589–598. [Google Scholar] [CrossRef]
- Kogure, G.S.; Silva, R.C.; Miranda-Furtado, C.L.; Ribeiro, V.B.; Pedroso, D.C.C.; Melo, A.S.; Ferriani, R.A.; Reis, R.M.D. Hyperandrogenism Enhances Muscle Strength after Progressive Resistance Training, Independent of Body Composition, in Women with Polycystic Ovary Syndrome. J. Strength Cond. Res. 2018, 32, 2642–2651. [Google Scholar] [CrossRef]
- Miranda-Furtado, C.L.; Ramos, F.K.; Kogure, G.S.; Santana-Lemos, B.A.; Ferriani, R.A.; Calado, R.T.; Dos Reis, R.M. A Nonrandomized Trial of Progressive Resistance Training Intervention in Women With Polycystic Ovary Syndrome and Its Implications in Telomere Content. Reprod. Sci. 2016, 23, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.B.; Kogure, G.S.; Reis, R.M.; Gastaldi, A.C.; De AraÚJo, J.E.; Mazon, J.H.; Borghi, A.; Souza, H.C.D. Polycystic Ovary Syndrome Presents Higher Sympathetic Cardiac Autonomic Modulation that is not altered by Strength Training. Int. J. Exerc. Sci. 2016, 9, 554–566. [Google Scholar]
- Lara, L.A.; Ramos, F.K.; Kogure, G.S.; Costa, R.S.; Silva de Sá, M.F.; Ferriani, R.A.; dos Reis, R.M. Impact of Physical Resistance Training on the Sexual Function of Women with Polycystic Ovary Syndrome. J. Sex. Med. 2015, 12, 1584–1590. [Google Scholar] [CrossRef]
- Rao, M.; Khan, A.A.; Adnan, Q.U.A. Effects of high-intensity interval training and strength training on levels of testosterone and physical activity among women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Kazemi, N.; Shadmehri, S.; Jalili, S.; Ahmadi, M. The effect of resistance training in water and land with vitamin D supplementation on anti-mullerian hormone in women with polycystic ovary syndrome. Women’s Health Bull. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Freiman, J.A.; Chalmers, T.C.; Smith, H.A.; Kuebler, R.R. The importance of beta, the type II error, and sample size in the design and interpretation of the randomized controlled trial: Survey of two sets of “negative” trials. In Medical Uses of Statistics; CRC Press: Boca Raton, FL, USA, 2019; pp. 357–389. [Google Scholar]
- Wallace, S.S.; Barak, G.; Truong, G.; Parker, M.W. Hierarchy of Evidence Within the Medical Literature. Hosp. Pediatrics 2022, 12, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Katsukawa, F. FITT principle of exercise in the management of lifestyle-related diseases. Clin. Calcium 2016, 26, 447–451. [Google Scholar]
- Smidt, N.; de Vet, H.C.; Bouter, L.M.; Dekker, J. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust. J. Physiother. 2005, 51, 71–85. [Google Scholar] [CrossRef]
- McLean, S.; Holden, M.A.; Potia, T.; Gee, M.; Mallett, R.; Bhanbhro, S.; Parsons, H.; Haywood, K. Quality and acceptability of measures of exercise adherence in musculoskeletal settings: A systematic review. Rheumatology 2017, 56, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Forbes, C.C.; Trinh, L.; Sellar, C.M.; Friedenreich, C.M.; Reiman, T. Patient satisfaction with participation in a randomized exercise trial: Effects of randomization and a usual care posttrial exercise program. Clin. Trials 2013, 10, 959–966. [Google Scholar] [CrossRef]
- Hertogh, E.M.; Schuit, A.J.; Peeters, P.H.; Monninkhof, E.M. Noncompliance in lifestyle intervention studies: The instrumental variable method provides insight into the bias. J. Clin. Epidemiol. 2010, 63, 900–906. [Google Scholar] [CrossRef]
- Steins Bisschop, C.N.; Courneya, K.S.; Velthuis, M.J.; Monninkhof, E.M.; Jones, L.W.; Friedenreich, C.; Van Der Wall, E.; Peeters, P.H.; May, A.M. Control group design, contamination and drop-out in exercise oncology trials: A systematic review. PLoS ONE 2015, 10, e0120996. [Google Scholar] [CrossRef]
- McGlothlin, A.E.; Lewis, R.J. Minimal clinically important difference: Defining what really matters to patients. JAMA 2014, 312, 1342–1343. [Google Scholar] [CrossRef]
- Hinderliter, A.L.; Sherwood, A.; Craighead, L.W.; Lin, P.-H.; Watkins, L.; Babyak, M.A.; Blumenthal, J.A. The long-term effects of lifestyle change on blood pressure: One-year follow-up of the ENCORE study. Am. J. Hypertens. 2014, 27, 734–741. [Google Scholar] [CrossRef]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration and John Wiley and Sons Ltd.: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P. Assessing risk of bias in a non-randomized study. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration and John Wiley and Sons Ltd.: Chichester, UK, 2019; pp. 621–641. [Google Scholar]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on exercise reporting template (CERT): Explanation and elaboration statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
| Inclusion Criteria |
|---|
| 1. Study design: studies incorporating a resistance training intervention; randomised controlled trials, cross-sectional studies, case–control studies are eligible 2. Participants: reproductive-aged women with a reported diagnosis of PCOS 3. Intervention: any study which incorporates an arm that utilises only resistance/strength training. Can be of any duration, supervised or unsupervised, with follow-up data collection of any duration 4. Outcomes: all reported outcomes |
| Exclusion Criteria |
| 1. Study design: literature reviews, systematics reviews and meta-analyses, editorials, and commentaries 2. Participants: males, adolescent females, post-menopausal women Intervention: aerobic exercise, combined interventions (i.e., resistance training + aerobic training/diet/ pharmacological/etc. where effects of exercise cannot be isolated) |
| Search Algorithm for PubMed |
|---|
| (“Polycystic ovary syndrome” [MeSH Terms] OR “Polycystic ovar * [Title/Abstract]” OR “PCOS” [Title/Abstract] OR “PCOD” [Title/Abstract] OR “Stein levent *” [Title/Abstract] AND “Resistance Training” [MeSH Terms] OR “Muscle training” [Title/Abstract] OR “Strength training” [Title/Abstract] OR “Strengthening” [Title/Abstract]) |
| Study (year) | Study Characteristics | Participant Characteristics | Intervention | Comparator (s) |
|---|---|---|---|---|
| Vizza [26] (2016) | Design: RCT Location: Australia Sample size: 13 (resistance training: 7, control: 6) Diagnosis: Rotterdam PCOS diagnostic criteria | Age: 27 ± 5 years BMI: 37.8 ± 11.4 kg/m2 | Duration: 12 weeks Frequency: 4 times/week (2 × RT, 2 home-based) Intensity: load not defined but progressed with strength gains. Two to three sets of 8–12 reps. Time: ~60 min per session Type: lat pulldown, leg curl, seated row, leg press, calf raise, chest press, split squat, shoulder press, biceps curl, triceps extension, and abdominal curl. Home-based: Callisthenics, 3 sets of 10 reps Participants were supervised for the resistance training but not for the home-based callisthenics | Control Participants did not receive any exercise intervention and were advised to continue with their current lifestyle, and usual healthcare and medical treatments. |
| Almenning [24] (2015) | Design: RCT Location: Norway Sample-size: 25 (resistance training: 8, HIIT: 8, control: 9) Diagnosis: Rotterdam PCOS diagnostic criteria | Age: 27.2 ± 5.5 years BMI: 26.7 ± 6.0 kg/m2 | Duration: 10 weeks Frequency: 3 times/week Intensity: 75% 1-RM, 3 sets of 10 repetitions separated by 1 min rest Time: not specified Type: eight dynamic strength drills Participants were supervised | HIIT Frequency: 2 times/wk Intensity: 4 × 4 min at 90–95% HRmax separated by 3 min at ~70% HRmax Frequency: 1 time/wk Intensity: 10 × 1 min with maximal intensity separated by 1 min of rest/very low activity Type: treadmill, outdoor running/walking and/or cycling. Control Advised to adhere to the recommended ≥150 min per week of moderate intensity physical activity. |
| Lara [42,43,44,45,46,47,48] (2015 [48]; 2016 [42,44,46,47]; 2018 [45]; 2019 [43]) | Design: Case-control Location: Brazil Sample size: PCOS: 45, Control: 52 Diagnosis: Rotterdam PCOS diagnostic criteria | PCOS Age: 28.1 ± 5.4 years BMI: 28.5 ± 6.02 kg/m2Control Age: 29.6 ± 5.3 years BMI: 26.2 ± 6.8 kg/m2 | Duration: 16 weeks Frequency: 3 times/week Intensity: Progression from 60% 1-RM (week 1) to 85% 1-RM. Progression performed over 4 weeks of a microcycle; intensity increased, and volume reduced. Minimum of 3 sets of 8 repetitions Time: 60 min Type: bench press, leg extension, front latissimus pull-down, leg curl, lateral raise, leg press (45°), triceps pulley, calf leg press, arm curl, and abdominal exercise, executed in alternating segments. Participants were supervised. | All participants received the progressive resistance training intervention. Women with PCOS were compared to women without. |
| Rao [49] (2022) | Design: RCT Location: Pakistan Sample-size: 50 (resistance training: 25, HIIT: 25) Diagnosis: Rotterdam PCOS diagnostic criteria | Resistance trainingAge: 30.5 ± 4.8 years BMI: 25.3 ± 1.96 kg/m2HIITAge: 28.1 ± 4.9 years BMI: 26.5 ± 3.09 kg/m2 | Duration: 12 weeks Frequency: 3 times/week Intensity: 60–70% 1-RM, 3 sets of 10–12 repetitions separated by 2 min of rest Time: ~32 min of work Type: squats, deadlifts, lunge, standing bent rowing, shoulder press, bench press, push-ups, and abdominal crunches Participants were supervised | HIIT Frequency: 3 times/wk Intensity: 4 × 4 min at 90–95% HRmax separated by 3 min of moderate intensity activity at ~70% HRmax Time: 45 min including warm-up and cooldown Type: treadmill |
| Hosseini [50] (2019) | Design: RCT Location: Iran Sample-size: 60 (control: 10, water training: 10, land training: 10, Vitamin D: 10, Water/Vitamin D: 10, Land/Vitamin D: 10) Diagnosis: Rotterdam PCOS diagnostic criteria | Water training Age: 31.12 ± 2.42 years BMI: 27.11 ± 0.74 kg/m2 Land training Age: 30.01 ± 1.70 years BMI: 27.01 ± 1.15 kg/m2 Control Age: 29.23 ± 2.11 years BMI: 26.70 ± 0.99 kg/m2 Vitamin D Age: 28.56 ± 1.55 years BMI: 26.71 ± 0.91 kg/m2 Water/Vitamin D Age: 29.43 ± 2.73 years BMI: 27.72 ± 1.47 kg/m2 Land/Vitamin D Age: 30.21 ± 1.65 years BMI: 27.86 ± 1.54 kg/m2 | Duration: 8 weeks Frequency: 3 times/week Land training Intensity: 40% 1-RM progressing to 70% 1-RM at week 8 Time: 15 min warm-up, 30 min of resistance training, 5 min cooldown Type: weight training Water training Intensity: load not specified, 3 sets of 12 repetitions Time: 5–15 min warm-up, 60 min of resistance training, 15 min cooldown Type: trunk strength training with dumbbells Participants were supervised for both land- and water-based training | Control No specific detail provided Vitamin D Consumed Vitamin D3 supplement for 8 weeks, which was dosed by a physician according to the nature and severity of Vitamin D deficiency of subjects. Water/land training and Vitamin D These intervention arms followed the resistance training intervention whilst be supplemented with Vitamin D as above. |
| Saremi [25] (2016) | Design: RCT Location: Iran Sample size: 30 (resistance training and placebo: 10, resistance training and calcium: 10, control: 10) Diagnosis: Rotterdam PCOS diagnostic criteria | Age: 27.1 ± 5.1 years BMI: 25.5 ± 2.7 kg/m2 | Duration: 8 weeks Frequency: 3 times/week Intensity: 40–60% 1RM of 1–2 sets of 15–20 repetitions Type: combination of free weights and machine weights including leg press, bench press, arm curl, and pulldown. Participants also took a placebo alongside the intervention (blinded). Participants were supervised | Control No specific detail provided. Resistance training and calcium Participants received 1000 mg per day of calcium alongside the resistance intervention. |
| Study (Design) | Study Aim(s) | Outcome Measures | Key Findings |
|---|---|---|---|
| Vizza [26] | To evaluate the feasibility of executing a randomised controlled trial of progressive resistance training in women with PCOS. | Body weight, BMI, waist circumference, fat mass, lean mass, fat-free mass, body fat (%), HbA1c, fasting insulin, fasting glucose, HOMA-2, hsCRP, testosterone, sex-hormone binding globulin, free androgen index, upper and lower body strength, PCOSQ (five domains: emotions, body hair, weight, infertility problems, and menstrual problems), SF-36 (eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health), DASS-21 (three domains: depression, anxiety, and stress), and exercise self-efficacy scale. | For those performing progressive resistance training, there were statistical improvements in waist circumference, HbA1c, fasting glucose, lower body strength, and domains from the PCOSQ (emotions, infertility problems), SF-36 (physical functioning) and the DASS-21 (depression). By contrast, there were no statistical changes for any outcome in the control group. The authors concluded that a randomised clinical trial of progressive resistance training in women with PCOS would be feasible to conduct, and that there may be a beneficial effect on a range of key outcomes in this cohort. However, a suitably powered randomised controlled trial is required to confirm these findings. |
| Almenning [24] | To assess the effects of 10 weeks of structured exercise training on metabolic, cardiovascular, and hormonal outcomes in women with PCOS; the primary outcome measure was HOMA-IR. The comparison of high-intensity interval training and strength training (HIIT) was exploratory. | Body weight, BMI, waist circumference, fat mass, visceral fat, fat-free mass, VO2 max, resting heart rate, heart rate recovery, flow-mediated dilation, fasting glucose, fasting insulin, HOMA-IR, testosterone, free androgen index, anti-Mullerian hormone, sex-hormone binding globulin, dehydroepiandrosterone sulphate, cholesterol, HDL-C, LDL-C, triglycerides, homocysteine, hsCRP, adiponectin, and leptin. | For those completing the strength training intervention, there were statistically favourable effects for fat mass (%), fat-free mass (kg), free androgen index, anti-Mullerian hormone, and sex-hormone binding globulin. By contrast, HIIT improved fat mass (kg and %), VO2 max, flow-mediated dilation, fasting insulin, HOMA-IR, dehydroepiandrosterone, HDL-C, and homocysteine; there were no statistical changes in the control group. Observed changes were seen using exercise as a sole treatment (i.e., no dietary or pharmacological intervention) and without any changes in weight. Further research is needed to advance conclusions, and to establish exercise guidelines for these women. |
| Lara [42,43,44,45,46,47,48] | The study aimed to assess sexual function and emotional status of women with PCOS after 16 weeks of progressive resistance training [48]. To evaluate the efficacy of progressive resistance training for improving lean muscle mass, metabolic factors, and steroid hormones in women with PCOS compared to those without PCOS [44]. To investigate resistance training induced changes in telomere content and metabolic disorder in women with PCOS and controls [46]. To assess the effect of a 16-week programme of resistance training on the quality of life of women with PCOS [42]. To investigate the effects of periodized strength training on cardiac autonomic parameters and any correlation with metabolic/endocrine outcomes in women with PCOS [47]. To evaluate the effects of eight and sixteen weeks of progressive resistance training on body composition, indicators of hypertrophy, and muscle strength in women with and without PCOS [45]. Investigate the impact of progressive resistance training on obesity indices in women with PCOS and to assess the relationship between telomere length and obesity indices [43]. | Female Sexual Function Index (six domains: desire, excitement, lubrication, orgasm, satisfaction, pain) [48]. Body weight, BMI, waist circumference, luteinising hormone, follicle stimulating hormone, oestradiol, androstenedione, testosterone, sex-hormone binding globulin, free androgen index, fasting glucose, fasting insulin, and HOMA-IR [44]. Body weight, BMI, waist circumference, body fat (%), fat-free mass, follicle stimulating hormone, luteinising hormone, prolactin, androstenedione, testosterone, oestradiol, sex-hormone binding globulin, free androgen index, glycaemia, fasting insulin, HOMA-IR, homocysteine, telomere length [46]. Body weight, BMI, waist circumference, and SF-36 (eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health) [42]. Heart rate, systolic, diastolic, and mean blood pressure, body weight, BMI, body fat (%), testosterone, androstenedione, testosterone/ androstenedione ratio, sex-hormone binding globulin, free androgen index, fasting glucose, fasting insulin, HOMA-IR, and spectral analysis (supine and tilt test) [47]. Testosterone, androstenedione, sex hormone binding globulin, free androgen index, fasting glucose, insulin, HOMA-IR, arm muscle area, thigh muscle area, sum of skinfolds, body fat (% and kg), lean body mass, chest press, leg extension, and arm curl [45]. Body weight, prolactin, thyroid stimulating hormone, 17-hydroxyprogesterone, luteinising hormone, follicle stimulating hormone, oestradiol, androstenedione, testosterone, sex hormone binding globulin, free androgen index, glycaemia, insulin, HOMA-IR, homocysteine, telomere length, body fat (%), trunk body fat (%), android body fat (%), fat mass/height2, BMI, waist circumference, umbilical waist, waist-to-hip-ratio, waist-to-height-ratio, conicity index [43]. | The sexual function of women with PCOS was statistically improved in all domains apart from orgasm and satisfaction whereas in the control women, statistical improvements were only seen in the pain domain [48]. In women with PCOS, following resistance training, waist circumference, testosterone, sex-hormone binding globulin, and glycaemia were statistically improved, whilst androstenedione concentration was increased. There were no significant differences in anthropometric characteristics, but values were consistently lower in women without PCOS pre-, and post-intervention [44]. Following progressive resistance training, women with PCOS had statistical reductions to waist circumference, body fat (%), testosterone, sex-hormone binding globulin, and free androgen index. Conversely, there were statistical increases in androstenedione, prolactin, and fat-free mass (kg) [46]. Following resistance training, the physical functioning of women with PCOS was statistically improved. No other domain reached statistical significance. By contrast, the control women saw statistical effects for vitality, social functioning, and mental health domains [42]. Women with PCOS who completed the resistance training intervention statistically reduced serum testosterone and exhibited changes to the testosterone/androstenedione ratio; there were no statistical changes in the control group. There were no statistical changes from baseline in the spectral analysis [47]. Women with PCOS had a statistical reduction in fasting glucose, testosterone, sex hormone binding globulin, sum of skinfolds, and body fat (% and kg). By contrast, androstenedione, arm and thigh muscle area, lean body mass, and weight moved during chest press, leg extension and arm curl exercises all increased. Control women also had similar benefits, particularly those relating to body composition and strength. Progressive resistance training was shown to increase strength parameters in women with PCOS and this is likely to the intrinsic hyperandrogenism associated with PCOS [45]. Following the resistance training intervention, women with PCOS had statistical changes to their biochemical profile: androstenedione, testosterone, and glycaemia all differed; these were the same for the control women too. Women with PCOS also had reductions in waist circumference, umbilical waist, waist-to-height-ratio, and conicity index [43]. |
| Rao [49] | To evaluate the efficacy of high-intensity interval training (HIIT) on serum testosterone levels, body fat percentage, and physical activity levels among women with PCOS. HIIT was compared to a strength training intervention. | BMI, testosterone, body fat (%), and minutes per week of physical activity (IPAQ) | For those completing strength training there were favourable changes observed for all four outcomes; pre-post statistical significance is not reported in the results. Similar effects are observed for those completing the HIIT intervention but again, statistical significance is not reported. The authors statistically contrast change from baseline HIIT and strength training and report greater benefit from HIIT for all outcomes apart from BMI (no difference). |
| Hosseini [50] | The study aimed to investigate the effect of resistance training, in water and on land, with and without Vitamin D supplementation, on anti-Mullerian hormone levels in women with PCOS. | Anti-Mullerian hormone and BMI | Those performing resistance training (either on land or water) demonstrated statistical improvements in anti-Mullerian hormone and BMI. When interventions were performed in combination with Vitamin D supplementation, the effects were increased; these changes were not evident in the control group. |
| Saremi [25] | The study aimed to investigate the effect of eight weeks of resistance training, with and without calcium supplementation, on levels of anti-Mullerian hormone and metabolic parameters in women with PCOS. | Body weight, BMI, bench press, leg press, cholesterol, triglycerides, LDL-C, HDL-C, HOMA-IR, fasting glucose, fasting insulin, anti-Mullerian hormone. | Those who completed the strength training (and placebo) intervention had statistical change from baseline improvements for fasting insulin, fasting blood glucose, triglycerides, cholesterol, LDL-C, HOMA-IR, and upper/lower body strength; there was a statistical increase in body weight too. All these changes were also observed in the strength training and calcium combined group. The combined group reported statistical changes to anti-Mullerian, which were not observed in the strength training alone or control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kite, C.; Parkes, E.; Taylor, S.R.; Davies, R.W.; Lagojda, L.; Brown, J.E.; Broom, D.R.; Kyrou, I.; Randeva, H.S. Time to Load Up–Resistance Training Can Improve the Health of Women with Polycystic Ovary Syndrome (PCOS): A Scoping Review. Med. Sci. 2022, 10, 53. https://doi.org/10.3390/medsci10040053
Kite C, Parkes E, Taylor SR, Davies RW, Lagojda L, Brown JE, Broom DR, Kyrou I, Randeva HS. Time to Load Up–Resistance Training Can Improve the Health of Women with Polycystic Ovary Syndrome (PCOS): A Scoping Review. Medical Sciences. 2022; 10(4):53. https://doi.org/10.3390/medsci10040053
Chicago/Turabian StyleKite, Chris, Elizabeth Parkes, Suzan R. Taylor, Robert W. Davies, Lukasz Lagojda, James E. Brown, David R. Broom, Ioannis Kyrou, and Harpal S. Randeva. 2022. "Time to Load Up–Resistance Training Can Improve the Health of Women with Polycystic Ovary Syndrome (PCOS): A Scoping Review" Medical Sciences 10, no. 4: 53. https://doi.org/10.3390/medsci10040053
APA StyleKite, C., Parkes, E., Taylor, S. R., Davies, R. W., Lagojda, L., Brown, J. E., Broom, D. R., Kyrou, I., & Randeva, H. S. (2022). Time to Load Up–Resistance Training Can Improve the Health of Women with Polycystic Ovary Syndrome (PCOS): A Scoping Review. Medical Sciences, 10(4), 53. https://doi.org/10.3390/medsci10040053









