Abstract
Background: The potential positive interaction between intermittent fasting (IF) and brain-derived neurotrophic factor (BDNF) on cognitive function has been widely discussed. This systematic review tried to assess the efficacy of interventions with different IF regimens on BDNF levels and their association with cognitive functions in humans. Interventions with different forms of IF such as caloric restriction (CR), alternate-day fasting (ADF), time-restricted eating (TRE), and the Ramadan model of intermittent fasting (RIF) were targeted. Methods: A systematic review was conducted for experimental and observational studies on healthy people and patients with diseases published in EMBASE, Scopus, PubMed, and Google Scholar databases from January 2000 to December 2023. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statements (PRISMA) for writing this review. Results: Sixteen research works conducted on healthy people and patients with metabolic disorders met the inclusion criteria for this systematic review. Five studies showed a significant increase in BDNF after the intervention, while five studies reported a significant decrease in BDNF levels, and the other six studies showed no significant changes in BDNF levels due to IF regimens. Moreover, five studies examined the RIF protocol, of which, three studies showed a significant reduction, while two showed a significant increase in BDNF levels, along with an improvement in cognitive function after RIF. Conclusions: The current findings suggest that IF has varying effects on BDNF levels and cognitive functions in healthy, overweight/obese individuals and patients with metabolic conditions. However, few human studies have shown that IF increases BDNF levels, with controversial results. In humans, IF has yet to be fully investigated in terms of its long-term effect on BDNF and cognitive functions. Large-scale, well-controlled studies with high-quality data are warranted to elucidate the impact of the IF regimens on BDNF levels and cognitive functions.
1. Introduction
Brain-derived neurotrophic factor (BDNF) is a protein (neurotrophin) that is mainly produced in the central nervous system. Its function depends on the stage of brain development and it is involved in processes such as synaptic transmission and synaptic plasticity, which contribute to cognitive function [1,2]. Furthermore, BDNF modulates metabolism and controls eating patterns and food intake behaviors as well as contributing to energy homeostasis [3]. In addition, in a zebrafish model, BDNF was found to affect physical performance and glucose metabolism, which may influence food appetite, insulin sensitivity, and parasympathetic cardiovascular tone [4]. Previous studies have shown that lower BDNF concentrations are associated with cognitive impairment, obesity, and metabolic syndrome, while higher BDNF concentrations are associated with improved cognitive performance and metabolic health [5,6,7]. As a result of its effect on glucose oxidation and food intake, BDNF may lower blood glucose levels and increase insulin sensitivity [8]. Healthy diet and lifestyle behaviors, such as physical activity, are well known to preserve cognitive function and metabolic health [9].
Fasting has been advocated as one of the candidate therapies for neurological disorders. This comes by virtue of the fasting effect in improving cognition, slowing down neurodegeneration, reducing brain damage, enhancing functional recovery after stroke, and mitigating the pathological and clinical features of epilepsy and multiple sclerosis in animal models [10]. Among the emerging healthy diets, numerous studies have demonstrated that intermittent fasting (IF) has significant effects on weight changes and metabolic parameters associated with type 2 diabetes, cardiovascular disease, oxidative stress, and cancer [11,12,13,14]. Furthermore, many animal studies have shown that IF reduces cognitive deficits by stimulating a reduction in BDNF production in the hippocampus, cerebral cortex, and striatum by suppressing the expression of proinflammatory cytokines, such as IL-1β, and enhancing neurotrophic support [15,16,17,18].
Intriguingly, recent research unraveled that as humans age, the brain experiences a decline in neurogenesis and synaptic plasticity, contributing to cognitive decline. However, BDNF was found to improve brain function by promoting both neurogenesis and synaptic plasticity, particularly through a process called long-term potentiation (LTP), a process involving persistent strengthening of synapses that leads to a long-lasting increase in signal transmission between neurons [2]. This is achieved by BDNF acting directly binding to a receptor called tropomyosin receptor kinase B, also known as tyrosine receptor kinase B (trkB). This BDNF/TrkB signaling pathway supports neuronal survival, plasticity, differentiation, and growth via the activation of several functional downstream cascades, and ends with triggering both pre-and postsynaptic changes that enhance communication between brain cells [19]. Interestingly, IF is a potent inducer of BDNF signaling, along with an adaptive stress response. This upregulates protein synthesis and further boosts neuroplasticity, leading to improved learning and memory. Therefore, understanding the interplay between brain aging, BDNF, and IF will open exciting avenues for promoting cognitive health and potentially mitigating age-related cognitive decline [20].
A previous review on athletes at rest and during exercise demonstrated that BDNF signaling in the brain can affect some behavioral and metabolic reactions in response to IF, including exercise and activity levels, appetite regulation, cognitive development, and glucose metabolism [21]. However, few human studies have shown the effect of IF on BDNF production. In contrast, a solicited study examining the effects of IF and long-term food restriction on BDNF in human subjects pointed toward a negative impact of IF and long-term food restriction on cognitive performance. To the best of our knowledge, no published systematic review has exclusively examined the effectiveness of interventions with IF on BDNF levels and the associated changes in cognitive functions in human subjects. In this structured systematic review, the main aim was to investigate the effect of interventions with different IF regimens on the concentration of BDNF in human subjects as well as to examine the impact of IF on cognitive functions through the BDNF pathway.
2. Materials and Methods
2.1. Search Strategy
To identify the available studies, a detailed search relating to CR, IF, and BDNF was conducted according to the PRISMA guidelines [22]. A systematic literature search was performed in the electronic databases EMBASE, Scopus, PubMed, and Google Scholar, using the following search terms in all possible combinations: intermittent fasting OR calorie restriction AND brain-derived neurotrophic factor OR BDNF AND cognitive function OR mental health. Additionally, a manual search was conducted through the reference lists of all collected articles to ensure that all relevant studies were identified and to avoid any missing relevant data.
2.2. Data Extraction and Quality Assessment
2.2.1. Study Selection and Data Extraction
All clinical studies evaluating the impact of IF and CR on BDNF levels in humans were reviewed and read carefully to identify their relevancy. To identify the eligible studies, the titles and abstracts were screened in the first phase of the selection procedure by two independent researchers (LM, MF). In the second phase, the full articles were screened for eligibility. The eligible criteria for the inclusion of the articles in this review were clinical trials, observational studies, and correlations between serum/plasma BDNF levels and IF and CR in healthy individuals or individuals with comorbidities. Studies conducted in animal models, case reports, reviews, and duplicate studies were excluded. We extracted items for the characteristics of the articles including the first author, publication year, study design, disease condition, sample size with male/female ratio, IF protocol applied, duration, exercise intervention, assessment of BDNF levels, and cognitive function tested. A flow diagram of the literature search and selection is shown in Figure 1. Potentially relevant studies (n = 601) were identified by searching electronic databases. Duplicates were removed and those studies that only included humans were selected.
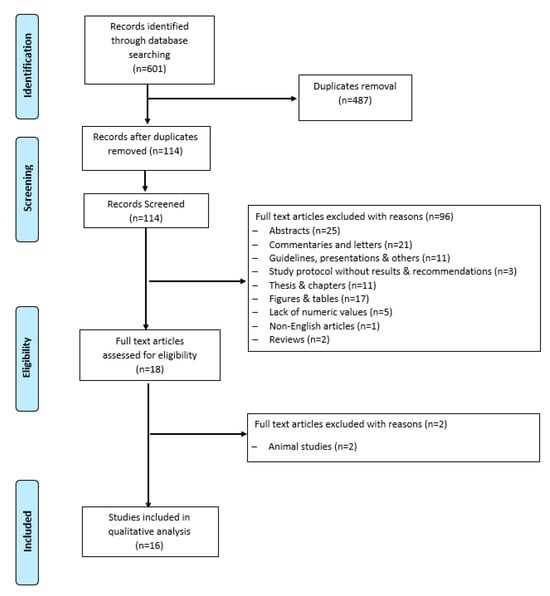
Figure 1.
Flow diagram of literature search and study selection.
2.2.2. Assessment of the Quality of Studies
To assess the quality of the included studies, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was used. We used a tool recommended by the Cochrane Collaboration for assessing the selection bias, performance bias, detection bias, attribution bias, and reporting bias of the included studies [23].
3. Results
The characteristics of the included studies are summarized in Table 1. The sixteen studies were conducted between 2007 and 2023. Two of the studies [24,25] were conducted on patients with schizophrenia, while the rest were conducted on people without a psychological disorder, two of which were conducted on people diagnosed with metabolic syndrome [26,27]. Nine of the sixteen studies were experimental, while the other seven studies were observational. Five of the sixteen studies were executed during the month of Ramadan [25,26,28,29,30]. Three of the five RIF studies revealed reductions in BDNF levels [25,26,30], while the rest (two studies) revealed an increase in BDNF levels during/after the observance of RIF [28,29]. Among the sixteen selected studies, eleven studies did not involve calorie restriction [15,25,26,27,28,29,30,31,32,33,34], while the other five studies did [24,35,36,37,38]. Six studies were conducted in the USA [15,26,27,31,33,36], four in Germany [29,30,32,37], and one in each of the following countries: Egypt [25], UK [35], Brazil [24], New Zealand [34], Iran [28], and Denmark [38].

Table 1.
Characteristics of the included studies.
In this review, BDNF was the primary outcome of interest in association with IF in human studies. In total, 36 relevant publications were found using the literature search. However, 16 studies were experimental studies examining the effect of different types of IF including CR, TRE, RIF, and ADF on BNDF levels in healthy individuals; patients with metabolic syndrome or a neurodegenerative disorder; and overweight or obese individuals. Overall, six of the included studies showed no significant changes in the serum BNDF concentration after the IF intervention. Five studies showed a significant increase and the other five showed a significant decrease in BDNF concentration. Nevertheless, five out of the sixteen studies used the RIF protocol, and by observing RIF, we found that three studies showed significant decreases and two studies showed significant increases in BDNF levels. In addition, only four studies showed that IF therapy may positively influence cognitive function while the rest did not assess cognitive performance.
Interestingly, the results of Catenacci et al. [36] suggested that ADF induced significant changes in BDNF secretion at the 24-week follow up and this alteration may be due to weight loss as BDNF can play a role in the regulation of energy balance that ultimately reduces adiposity. However, this result should be carefully construed, as changes in BDNF may correlate with changes in weight or body composition. Similarly, Bastani et al. [28] observed a significant increase in plasma BDNF on the 14th day (second group) and 29th day (third group) of RIF compared to pre-Ramadan levels. The BDNF level in the second group was increased significantly by 25% and by 47% in the third group compared to the control group (p < 0.05). Another study suggested that TRE may have a direct impact on the central circadian clock and has the tendency to affect hormonal levels depending on meal timing. They also showed that TRE reduced cortisol levels by 1.4 ± 0.6 μg/dL (p = 0.03) and tended to increase BDNF levels by 2.46 ± 1.34 ng/mL (p = 0.09) in the evening [15]. BDNF is a well-recognized protein for the regulation and adaptation of energy balance at the cellular level [39]. However, many lifestyle interventions like IF exert changes in energy balance that may influence the level of BDNF.
A study demonstrated that RIF significantly increases the BDNF level at the end of Ramadan; however, the BDNF level decreased and returned to the baseline value one week post Ramadan [29]. This could be explained by the fact that BDNF can be altered in the energy balance adaptation process. Similarly, the CR diet also induced an increase in BDNF levels in schizophrenic patients and it was suggested that the improvement in dietary nutrients and food quantity may modify important markers of brain plasticity. However, the BDNF level may also increase in patients taking high antipsychotics daily (r = 0.216; p = 0.098), which also typically increases BDNF levels [24].
In contrast, we found that five studies showed a significant reduction in BDNF levels after IF. Similarly, a study found a significant reduction in BDNF levels while demonstrating significant improvement in a patient with focal seizures post-Ramadan. Thus, it was proposed that RIF plays a role in inducing the transcription of BDNF which stimulates the production and survival of new hippocampal neurons, maintains the synaptic structure, and thus promotes more sustained neuronal resistance to stress [40]. Fawzi et al. [25] found that the change in total energy and BMI during Ramadan fasting were significant and independent variables associated with the increase in serum BDNF levels by 44%; however, they could not demonstrate any benefits in schizophrenia patients as a lower BDNF level may worsen the psychiatric status, such as a relapse of bipolar disorder. Moreover, previous studies also reported a significant decrease in BDNF levels upon IF intervention [30,35,38]; however, the results indicated that other factors may contribute to BDNF alterations like body composition parameters, hormonal status, sex, and exercise. On the other hand, a study reported that intermittent energy restriction induces a positive mood when the cognitive function in overweight individuals was assessed and this effect was due to increased self-confidence and ongoing motivational calls in their 6-month weight loss journey rather than the alteration in BDNF concentrations [35]. In addition, other studies suggested that RIF may positively affect cognitive function by improving the individual’s mood along with a significant change in the BDNF level at the end of the Ramadan fasting month [28,30]. The reported partial improvement in BDNF levels after observing RIF could be also ascribed to the effect of RIF in reducing body weight [41], adiposity [42], visceral adiposity [43], metabolic syndrome components [44], cardiometabolic risk factors [45], proinflammatory cytokines and oxidative stress markers [46,47], and IGF-1 [43]; all of these have been implicated in the pathogenesis of mental health problems and decreased BDNF levels.
4. Quality Assessment
The systematic review included a diverse range of study types, each contributing unique insights. The randomized controlled trials (RCTs), comprising Jamshed et al. [15], Carlson et al. [31], Harvie et al. [35], Catenacci et al. [36], Bastani et al. [28], Schübel et al. [37], Glud et al. [38], Wallace et al. [33], and Bartholomew et al. [27], offered rigorous experimental data. Complementing these were non-RCTs like Kessler et al. [32] and Abdulsada et al. [26]; prospective studies by Fawzi et al. [25] and Riat et al. [30]; a cross-sectional study by Guimarães et al. [24]; a prospective controlled trial by Ghashang et al. [29]; and a study using a repeated measures cross-over design by Gibbons et al. [34].
The quality assessment of the studies using the Cochrane tool reveals varied results. Adequate sequence generation was observed in Jamshed et al. [15], mitigating selection bias. Allocation concealment was robust in Carlson et al. [31], enhancing the study’s internal validity. The blinding of participants, a critical aspect of reducing performance bias, was well-implemented by Catenacci et al. (2016) [36]. Attrition bias was minimized due to the comprehensive handling of incomplete outcome data by Bastani et al. [28]. However, the potential for detection bias was present, as outcome assessment blinding was not consistent across all studies, which was particularly noted in Harvie et al. (2011) [35]. The review also highlighted selective reporting and other biases in a subset of studies, including Schübel et al. [37], warranting cautious interpretation of these results (Figure 2a,b).
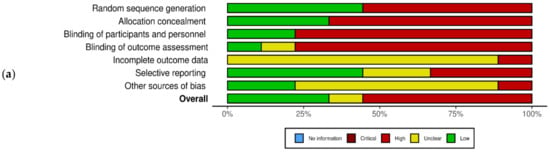
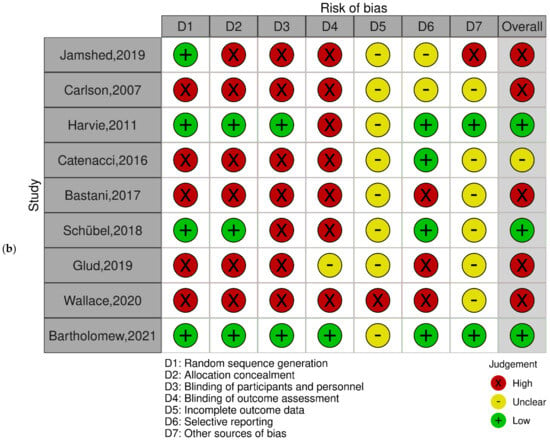
Figure 2.
Quality assessment of the experimental studies included in the systematic review using the Cochrane tool. (a) Risk of bias with each risk of bias item for each included study; (b) risk of bias graph with each risk of bias item presented as percentages across all included studies. Green: low risk of bias; yellow: unclear risk of bias; red: high risk of bias (n = 9) [15,27,28,31,33,35,36,37,38].
As per the Cochrane Risk of Bias Tool for Randomized Controlled Trials, the included studies had several sources of bias. We observed several forms of bias across the studies including selection bias, which was noted in the way participants were allocated to the intervention and control groups, potentially affecting the comparability of these groups; measurement bias, which pertained to inconsistencies in how outcomes were measured and recorded across different studies, which could influence the results; dietary adherence Bias, specific to studies involving dietary interventions, which arose from variations in participants’ adherence to dietary guidelines; time-frame bias, which was observed in the duration of the studies, which varied and might have impacted the outcomes; limited data points leading to bias, which refers to the scarcity of data points in some studies restricting our ability to draw comprehensive conclusions; lack of baseline measurement bias, which, in some studies, the absence of baseline measurements hindered the assessment of changes over time; and omission of relevant variables bias, which was noted in studies that failed to account for or report relevant variables that could influence the outcomes.
5. Discussion
This systematic review tried to assess the efficacy of interventions with different IF regimens on BDNF levels and their association with cognitive functions in humans. Interventions with different forms of fasting regimens such as CR, ADF, TRE, and RIF were targeted. The current results revealed mixed effects of CR and IF regimens on BDNF levels, with no clear picture being drawn concerning the effect of these dietary interventions on the targeted outcome, a matter that dictates the need for well-controlled, long-term experimental studies to elucidate the impact of IF and CR regimens on BDNF levels.
BDNF function is associated with energy metabolism and synaptic and behavioral plasticity, which influence the cognitive functions of learning and maintaining memory capacity in humans. Higher BDNF concentrations in the hippocampus have been associated with both improved cognitive function [48] and metabolic health [49]. Recently, many animal (rodent) studies have revealed that IF positively stimulates the production of BDNF in the hippocampus, cerebral cortex, and striatum and reduces cognitive deficits by enhancing neurotrophic support and suppressing the expression of pro-inflammatory cytokines [18,50,51].
In terms of human studies, the increase in BDNF concentrations due to IF interventions has attracted considerable attention. In this systematic review, we analyzed and evaluated 16 human intervention studies from the literature to investigate the effect of CR and different IF regimens on BDNF concentration and cognitive function. During the evaluation, several concerns resulted in complex results and we could not determine the effect of IF on BDNF concentrations and cognitive function due to the varied application of different methodologies in the studies. First, the collection of the peripheral BDNF concentration varied between studies which related to whether the BDNF concentration was measured from serum or plasma samples; in addition, the centrifuge protocol, clotting period, and temperature can affect the measurement of plasma or serum concentrations in the studies which made it difficult to generalize the findings of peripheral BDNF concentrations between the 16 studies.
In animal studies, it was determined that there is a higher concentration of BDNF in sera than in plasma [52]. Moreover, reports indicated a correlation between fasting and an increase in BDNF levels in overweight or obese subjects, which was associated with changes in body composition or fat percentages [38,53,54,55]. However, in this review, some of the study results varied and this is because other major factors impacted the BDNF concentration related to IF. For example, changes in energy balance and gene expression during long hours of fasting such as Ramadan affect the regulation of hormones, such as cortisol [56] and insulin-like growth factor (IGF-1) [43], which alter the concentration of BDNF [57], as well as a sex-based biological parameter, which affects serum BDNF levels [26].
Further, RIF has been associated with the overexpression of a set of genes (TFAM, SOD2, and Nrf2) which have been implicated in improving neuroplasticity and decreasing neuroinflammation [58]. These genes showed significantly increased expression at the end of the fasting month, increasing by 90.5%, 54.1%, and 411.5% for the three genes, respectively [59]. In addition, the expression of the fat mass and obesity-associated (FTO) gene has been found to affect hippocampal function and regulate BDNF processing, which helps to further explain the intricate relationship between RIF and BDNF. A recent gene expression study revealed that RIF was associated with an approximately 30% reduction in the levels of FTO gene expression in overweight and obese people observing the fasting month [60], which helps to explain the controversial effects of RIF and CR on BDNF.
A study reported a 25% higher serum BDNF level in women compared to men, confirming that circulating BDNF levels are sex-dependent [38]. Previous reports also showed that women tend to have a higher expression of BDNF in several brain regions [38,61] and they have higher circulating BDNF levels in the last phase of their menstrual cycle compared to the first phase [61]. These findings suggested that gonadal hormones could influence the estrogen-specific effect on circulating BDNF levels more in women than in men. Furthermore, the expression of BDNF at the protein and mRNA levels is responsive to exercise and has a positive influence on cognition function [62]. However, BDNF can only be altered depending on the intensity of exercise, age, sex, body mass, diet, and fitness level of the individual [63,64,65]. Additionally, IF in a specific population such as schizophrenia patients provided inconsistent results in BDNF concentration due to the type of diet, the effect of drug treatments, phenotypes, the intensity of symptoms, and the duration of schizophrenia [17]. Notably, no significant outcome was found in the association between IF and BDNF concentration. There is a positive confirmed link between long-term healthy lifestyle interventions, including a healthy diet, calorie restriction, physical activity, and quality of sleep, and beneficial effects on BDNF in the brain and that the elevation of BDNF can improve neurodegeneration in the nervous system [20,66].
Recently, IF has emerged as a multifaceted approach that influences long-chain fatty acid oxidation through the intricate interplay of gut microbiota changes and BDNF production [67,68,69]. This triad not only offers insights into the metabolic benefits of IF but also sheds light on the complex connections between the gut, the brain, and metabolic health. Further research is warranted to elucidate the precise mechanisms underlying these interactions, paving the way for personalized interventions that harness the potential of IF for optimizing metabolic function and overall well-being.
The observed differences in the effect of IF on BDNF could be also explained by the sex-specific differences in lipid metabolism [70]. In one clinical trial, sex was found to be one of the biological determinants that shaped the effect of fasting on circulating BDNF levels. Such a difference was also a mirror for the differences in the effect of observing RIF on the two sexes in healthy and disease conditions, as revealed by previous reviews [71,72].
Astroglia, a type of glial cell in the brain, play a crucial and multifaceted role in the metabolism of fatty acids, contributing significantly to overall brain function [73]. These star-shaped cells are not merely passive supporters; they actively participate in the intricate biochemical processes that sustain neural health [74]. Astroglia are instrumental in the uptake, storage, and utilization of fatty acids, serving as key intermediaries in the intricate lipid metabolism within the brain [75]. Through their sophisticated network of processes, astroglia contributes to energy homeostasis, neurotransmitter synthesis, and neuroprotection [76]. The close interaction between astroglia and neurons highlights the dynamic interplay between different cell types in the brain, emphasizing the intricate balance required for optimal cognitive function and overall neurological well-being [73,76]. Gaining knowledge about the involvement of astroglia in the metabolism of fatty acids offers valuable information on potential therapeutic approaches for different neurological disorders. It also enhances our understanding of the impact of IF on brain health, the suggested neuroprotective effects of IF, and the mediating role of BDNF in brain health and cognitive function.
In the context of studying the effect of IF on BDNF, the complex process of synaptic mitochondria efficiently oxidizing long-chain fatty acids gains particular relevance [77]. During fasting periods, the brain’s reliance on alternative energy sources, such as the oxidation of long-chain fatty acids by synaptic mitochondria, may enhance the production of ketone bodies and trigger a metabolic state that supports increased BDNF expression [50,78]. The nuanced interplay between fatty acid metabolism and BDNF in the context of IF highlights the intricate molecular mechanisms underlying the potential cognitive benefits associated with this dietary intervention [79], offering a promising avenue for further exploration in neuroscientific research.
Our study highlights several directions for future research. Reliable protocols for the assessment of peripheral BDNF levels need to be developed to enable a proper evaluation of the peripheral concentration of BDNF in future studies. More RCTs in humans are crucial to investigating the effectiveness of IF on cognitive function in obese or healthy individuals. In addition, the duration and type of IF intervention and incorporation of hormones or biomarker outcomes could help to estimate the effect of peripheral BDNF concentrations. It should be noted that this review has several limitations, including the presence of only 16 RCTs with a variety of methodologies. Secondly, the inclusion criteria varied, with some studies including healthy individuals and others excluding those with metabolic syndrome or neurological disorders. Thirdly, the type of fasting and diet in the IF interventions varied. Nevertheless, our study is the first systematic review to examine IF’s effect on BDNF concentrations and cognitive functions in humans. To identify the long-term effects of IF on BDNF, high-quality research with large-scale randomized controlled trials is required.
6. Conclusions
There are controversial results from human studies regarding the IF and CR effects on BDNF levels. The long-term effects of IF on BDNF levels have yet to be investigated. Due to the dissimilarity of the studies’ outcomes and contradictory findings, this review cannot generalize the results based on human trial interventions related to IF and BDNF levels. To understand the impact of IF regimens on BDNF levels and cognitive functions, large-scale, controlled clinical studies are greatly needed.
Author Contributions
Conceptualization, M.E.F., R.A., L.M. and H.A.; methodology, M.E.F., L.M. and R.A.; software, M.A.K.; validation, M.E.F. and H.A.; formal analysis, L.M. and M.A.K.; investigation, M.E.F., H.M.K., H.M.K., F.Z. and H.M.K.; resources, M.E.F. and M.A.K.; data curation, L.M. and R.A.; writing—original draft preparation, M.E.F., L.M., R.A. and H.A.; writing—review and editing, F.Z., H.M.K., R.A. and H.A.; visualization, L.M. and M.A.K.; supervision, M.E.F.; project administration, M.E.F.; funding acquisition, M.E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126–127, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Bertucci, J.I.; Hatef, A.; Unniappan, S. Feeding and food availability modulate brain-derived neurotrophic factor, an orexigen with metabolic roles in zebrafish. Sci. Rep. 2020, 10, 10727. [Google Scholar] [CrossRef] [PubMed]
- Katuri, R.B.; Gaur, G.S.; Sahoo, J.P.; Bobby, Z.; Shanmugavel, K. Association of Circulating Brain-Derived Neurotrophic Factor with Cognition among Adult Obese Population. J. Obes. Metab. Syndr. 2021, 30, 163–172. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Yao, H.; Imamura, Y.; Hashimoto, M.; Monji, A. Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: The Sefuri study. Sci. Rep. 2020, 10, 16442. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, S.; Karimi, I.; Jafari, F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metab. Brain Dis. 2017, 32, 651–665. [Google Scholar] [CrossRef]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Malek, L.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Phillips, M.C. Fasting as a therapy in neurological disease. Nutrients 2019, 11, 2501. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Anderson, J.L.; May, H.T.; Le, V.T.; Bair, T.L.; Bennett, S.T.; Knowlton, K.U.; Muhlestein, J.B. Intermittent fasting and changes in clinical risk scores: Secondary analysis of a randomized controlled trial. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200209. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen, M.F.K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2021, 1, Cd013496. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Albrahim, T.; Alangry, R.; Alotaibi, R.; Almandil, L.; Alburikan, S. Effects of Regular Exercise and Intermittent Fasting on Neurotransmitters, Inflammation, Oxidative Stress, and Brain-Derived Neurotrophic Factor in Cortex of Ovariectomized Rats. Nutrients 2023, 15, 4270. [Google Scholar] [CrossRef] [PubMed]
- Gudden, J.; Arias Vasquez, A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef] [PubMed]
- Elesawy, B.H.; Raafat, B.M.; Muqbali, A.A.; Abbas, A.M.; Sakr, H.F. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sci. 2021, 11, 242. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Seidler, K.; Barrow, M. Intermittent fasting and cognitive performance—Targeting BDNF as potential strategy to optimise brain health. Front. Neuroendocr. 2022, 65, 100971. [Google Scholar] [CrossRef]
- Cherif, A.; Roelands, B.; Meeusen, R.; Chamari, K. Effects of Intermittent Fasting, Caloric Restriction, and Ramadan Intermittent Fasting on Cognitive Performance at Rest and During Exercise in Adults. Sport. Med. 2016, 46, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.R.; Jacka, F.N.; Gama, C.S.; Berk, M.; Leitão-Azevedo, C.L.; Belmonte de Abreu, M.G.; Lobato, M.I.; Andreazza, A.C.; Ceresér, K.M.; Kapczinski, F.; et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, M.H.; Fawzi, M.M.; Said, N.S.; Fawzi, M.M.; Fouad, A.A.; Abdel-Moety, H. Effect of Ramadan fasting on anthropometric, metabolic, inflammatory and psychopathology status of Egyptian male patients with schizophrenia. Psychiatry Res. 2015, 225, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Abdulsada, M.M.; Wilhelm, Z.R.; Opekun, A.R.; Devaraj, S.; Jalal, P.K.; Mindikoglu, A.L. The effect of four-week intermittent fasting from dawn to sunset on circulating brain-derived neurotrophic factor levels in subjects with metabolic syndrome and healthy subjects. Metab. Open 2021, 9, 100070. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, C.L.; Muhlestein, J.B.; May, H.T.; Le, V.T.; Galenko, O.; Garrett, K.D.; Brunker, C.; Hopkins, R.O.; Carlquist, J.F.; Knowlton, K.U.; et al. Randomized controlled trial of once-per-week intermittent fasting for health improvement: The WONDERFUL trial. Eur. Heart J. Open 2021, 1, oeab026. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Rajabi, S.; Kianimarkani, F. The Effects of Fasting During Ramadan on the Concentration of Serotonin, Dopamine, Brain-Derived Neurotrophic Factor and Nerve Growth Factor. Neurol. Int. 2017, 9, 7043. [Google Scholar] [CrossRef]
- Khoshandam Ghashang, S.; Hamdan, I.; Lichtinghagen, R.; Gutenbrunner, C.; Nugraha, B. Alterations of Brain-Derived Neurotrophic Factor and Creatinine During Ramadan Fasting: A Prospective, Controlled Clinical Trial. Iran. Red. Crescent Med. J. 2019; in Press. [Google Scholar] [CrossRef]
- Riat, A.; Suwandi, A.; Ghashang, S.K.; Buettner, M.; Eljurnazi, L.; Grassl, G.A.; Gutenbrunner, C.; Nugraha, B. Ramadan Fasting in Germany (17–18 h/Day): Effect on Cortisol and Brain-Derived Neurotrophic Factor in Association with Mood and Body Composition Parameters. Front. Nutr. 2021, 8, 697920. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.S.; Stange, R.; Schlenkermann, M.; Jeitler, M.; Michalsen, A.; Selle, A.; Raucci, F.; Steckhan, N. A nonrandomized controlled clinical pilot trial on 8 wk of intermittent fasting (24 h/wk). Nutrition 2018, 46, 143–152.e142. [Google Scholar] [CrossRef]
- Wallace, A.W. The Impact of Six Weeks of Intermittent Fasting, with and without Aerobic Exercise, on Serum BDNF in Young Adult Males; California State University, Long Beach: Long Beach, CA, USA, 2020. [Google Scholar]
- Gibbons, T.D.; Cotter, J.D.; Ainslie, P.N.; Abraham, W.C.; Mockett, B.G.; Campbell, H.A.; Jones, E.M.; Jenkins, E.J.; Thomas, K.N. Fasting for 20 h does not affect exercise-induced increases in circulating BDNF in humans. J. Physiol. 2023, 601, 2121–2137. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Glud, M.; Christiansen, T.; Larsen, L.H.; Richelsen, B.; Bruun, J.M. Changes in Circulating BDNF in relation to Sex, Diet, and Exercise: A 12-Week Randomized Controlled Study in Overweight and Obese Participants. J. Obes. 2019, 2019, 4537274. [Google Scholar] [CrossRef]
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The Role of Altered BDNF/TrkB Signaling in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2019, 13, 368. [Google Scholar] [CrossRef]
- Magdy, R.; Kishk, N.A.; Abokrysha, N.T.; Ramzy, G.M.; Rizk, H.I.; Hussein, M. Predictors of successful Ramadan fasting in Muslim patients with epilepsy: A prospective study. Seizure 2020, 80, 67–70. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alsibai, J.; Clark, C.C.; Faris, M.E. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 2020, 59, 2291–2316. [Google Scholar] [CrossRef]
- Fernando, H.; Zibellini, J.; Harris, R.; Seimon, R.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.; Madkour, M.I.; Obaideen, A.K.; Dalah, E.Z.; Hasan, H.A.; Radwan, H.M.; Jahrami, H.A.; Hamdy, O.; Mohammad, M.G. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res. Clin. Pract. 2019, 153, 166–175. [Google Scholar] [CrossRef]
- Faris, M.; Jahrami, H.A.; Alsibai, J.; Obaideen, A.A. Impact of Ramadan Diurnal Intermittent Fasting on Metabolic Syndrome Components in Healthy, Non-Athletic Muslim People Aged over 15 Years: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2019, 123, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.A.; Faris, M.E.; Janahi, A.; Janahi, M.; Abdelrahim, D.N.; Madkour, M.I.; Sater, M.S.; Hassan, A.B.; Bahammam, A.S. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2273–2301. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.E.; Jahrami, H.A.; Obaideen, A.A.; Madkour, M.I. Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: Systematic review and meta-analysis. J. Nutr. Intermed. Metab. 2019, 15, 18–26. [Google Scholar] [CrossRef]
- Faris, M.; Kacimi, S.; Al-Kurd, R.a.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of nutritional interventions on BDNF concentrations in humans: A systematic review. Nutr. Neurosci. 2022, 25, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, K.; Otu, F.F.; Agyekum, J.A.; Dzudzor, B. Brain-derived neurotrophic factor is associated with cardiometabolic risk factors in HIV patients on combination antiretroviral therapy in Ghana. Egypt. J. Intern. Med. 2023, 35, 74. [Google Scholar] [CrossRef]
- Brocchi, A.; Rebelos, E.; Dardano, A.; Mantuano, M.; Daniele, G. Effects of Intermittent Fasting on Brain Metabolism. Nutrients 2022, 14, 1275. [Google Scholar] [CrossRef]
- Mayor, E. Neurotrophic effects of intermittent fasting, calorie restriction and exercise: A review and annotated bibliography. Front. Aging 2023, 4, 1161814. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef]
- Lee, I.T.; Wang, J.S.; Fu, C.P.; Lin, S.Y.; Sheu, W.H. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome: A prospective study. Medicine 2016, 95, e5260. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy intake, meal frequency, and health: A neurobiological perspective. Annu. Rev. Nutr. 2005, 25, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020, 52, 147–161. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Madkour, M.; Jahrami, H.; Salahat, D.; Alhasan, F.; BaHammam, A.; Al-Islam Faris, M. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: A prospective observational study. PLoS ONE 2020, 15, e0237922. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.R.; Safavi, E.; Rooholamini, M.; Jaafari, F.; Darvishi, S.; Rahbar, A. Effects of Intermittent Fasting during Ramadan on Insulin-like Growth Factor-1, Interleukin 2, and Lipid Profile in Healthy Muslims. Int. J. Prev. Med. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.I.; Malhab, L.J.B.; Abdel-Rahman, W.M.; Abdelrahim, D.N.; Saber-Ayad, M.; Faris, M.E. Ramadan Diurnal Intermittent Fasting Is Associated with Attenuated FTO Gene Expression in Subjects with Overweight and Obesity: A Prospective Cohort Study. Front. Nutr. 2022, 8, 741811. [Google Scholar] [CrossRef]
- Begliuomini, S.; Casarosa, E.; Pluchino, N.; Lenzi, E.; Centofanti, M.; Freschi, L.; Pieri, M.; Genazzani, A.D.; Luisi, S.; Genazzani, A.R. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum. Reprod. 2007, 22, 995–1002. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Babiarz, M.; Laskowski, R.; Grzywacz, T. Effects of Strength Training on BDNF in Healthy Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 13795. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E.; de Assis, G.G.; Clemente, F.M.; Feito, Y.; Stastny, P.; Zuwała-Jagiełło, J.; Bibrowicz, B.; Wolański, P. Effect of four different forms of high intensity training on BDNF response to Wingate and Graded Exercise Test. Sci. Rep. 2021, 11, 8599. [Google Scholar] [CrossRef] [PubMed]
- Máderová, D.; Krumpolec, P.; Slobodová, L.; Schön, M.; Tirpáková, V.; Kovaničová, Z.; Klepochová, R.; Vajda, M.; Šutovský, S.; Cvečka, J.; et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides 2019, 78, 101961. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Padinjakara, N.; Lautenschlager, N.T. Effects of intermittent fasting on cognitive health and Alzheimer’s disease. Nutr. Rev. 2023, 81, 1225–1233. [Google Scholar] [CrossRef]
- Carneiro, L.; Pellerin, L. Nutritional Impact on Metabolic Homeostasis and Brain Health. Front. Neurosci. 2022, 15, 767405. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, X.; Li, Y.; Luo, A.; Zhao, Y.; Luo, X.; Li, S. Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota. Nutrients 2023, 15, 4915. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Cheng, C.-W.; Cao, A.Q.; Tripathi, S.; Mana, M.D.; Bauer-Rowe, K.E.; Abu-Remaileh, M.; Clavain, L.; Erdemir, A.; Lewis, C.A. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 2018, 22, 769–778.e4. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T. Sex-Specific Differences in Lipid and Glucose Metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef]
- Abdelrahim, D.N.; Rachida, R.; Krami, A.M.; Nadia, A.; Faris, M.E. Sex as a biological determinant in anthropometric, biochemical, and dietary changes during Ramadan intermittent fasting in healthy people: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102762. [Google Scholar] [CrossRef]
- Roky, R.; Aadil, N.; Krami, A.M.; Benaji, B.; Errabih, I.; Abdelrahim, D.N.; Faris, M.E. Sex as a Biological Factor in the Changes in Disease Patients During Ramadan Intermittent Fasting: A Systematic Review. Front. Nutr. 2022, 9, 908674. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological conditions of the neurovascular unit. Neuropathology 2020, 40, 121–137. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Magistretti, P.J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295. [Google Scholar] [CrossRef]
- Markham, A.; Bains, R.; Franklin, P.; Spedding, M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: How important is BDNF? Br. J. Pharmacol. 2014, 171, 2206–2229. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).