Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption
Abstract
:1. Introduction
1.1. Background and Rationale
1.2. Objectives
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Details
2.3. Initial Study Selection
3. Results
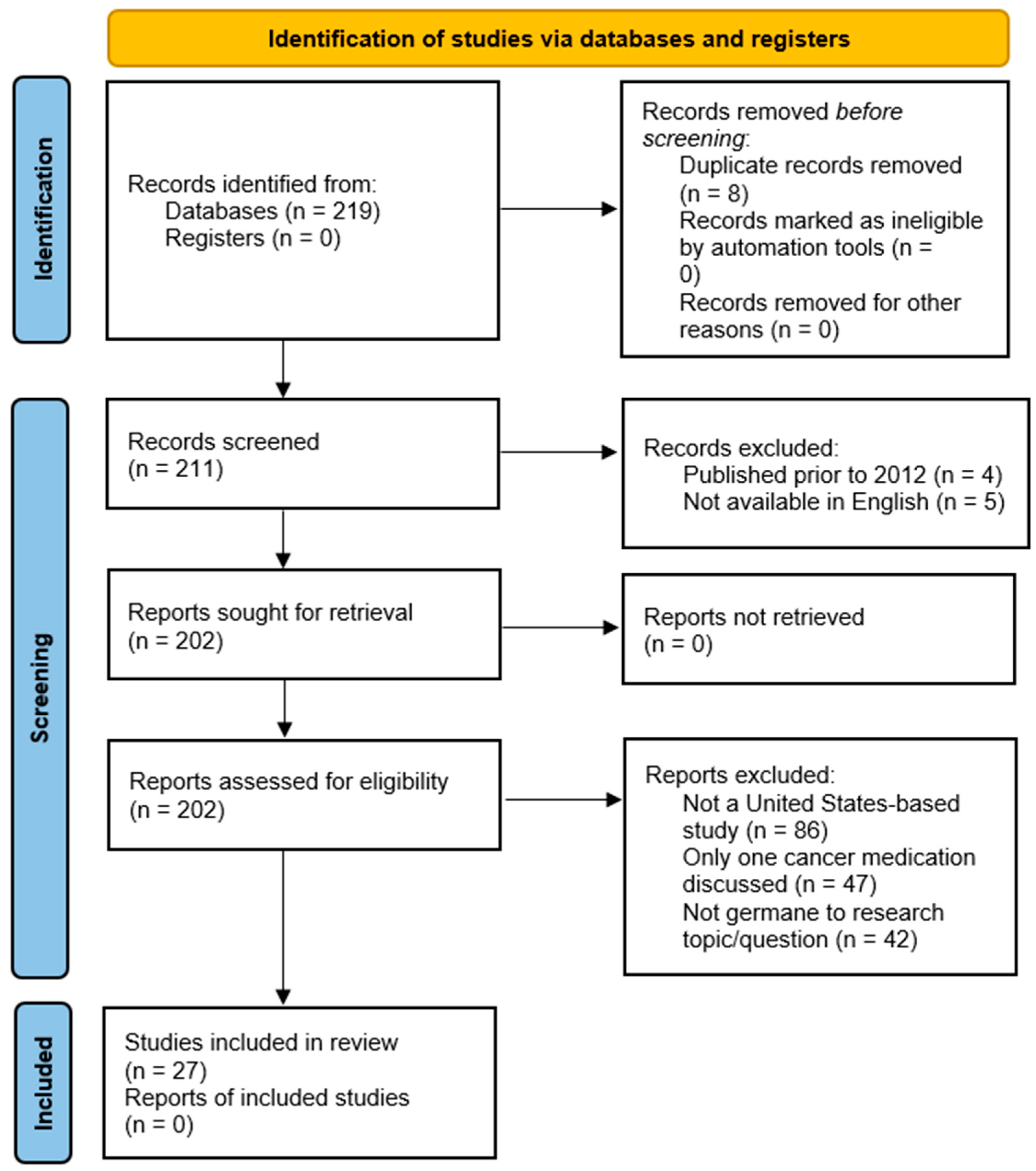
3.1. Study Selection and Exclusion Process
- (a)
- Not a United States provider-based study (86 articles),
- (b)
- Only discussed one specific cancer medication (47 articles),
- (c)
- Not related to the research question (42 articles).
3.2. Study Characteristics
4. Discussion
4.1. Summary of Evidence
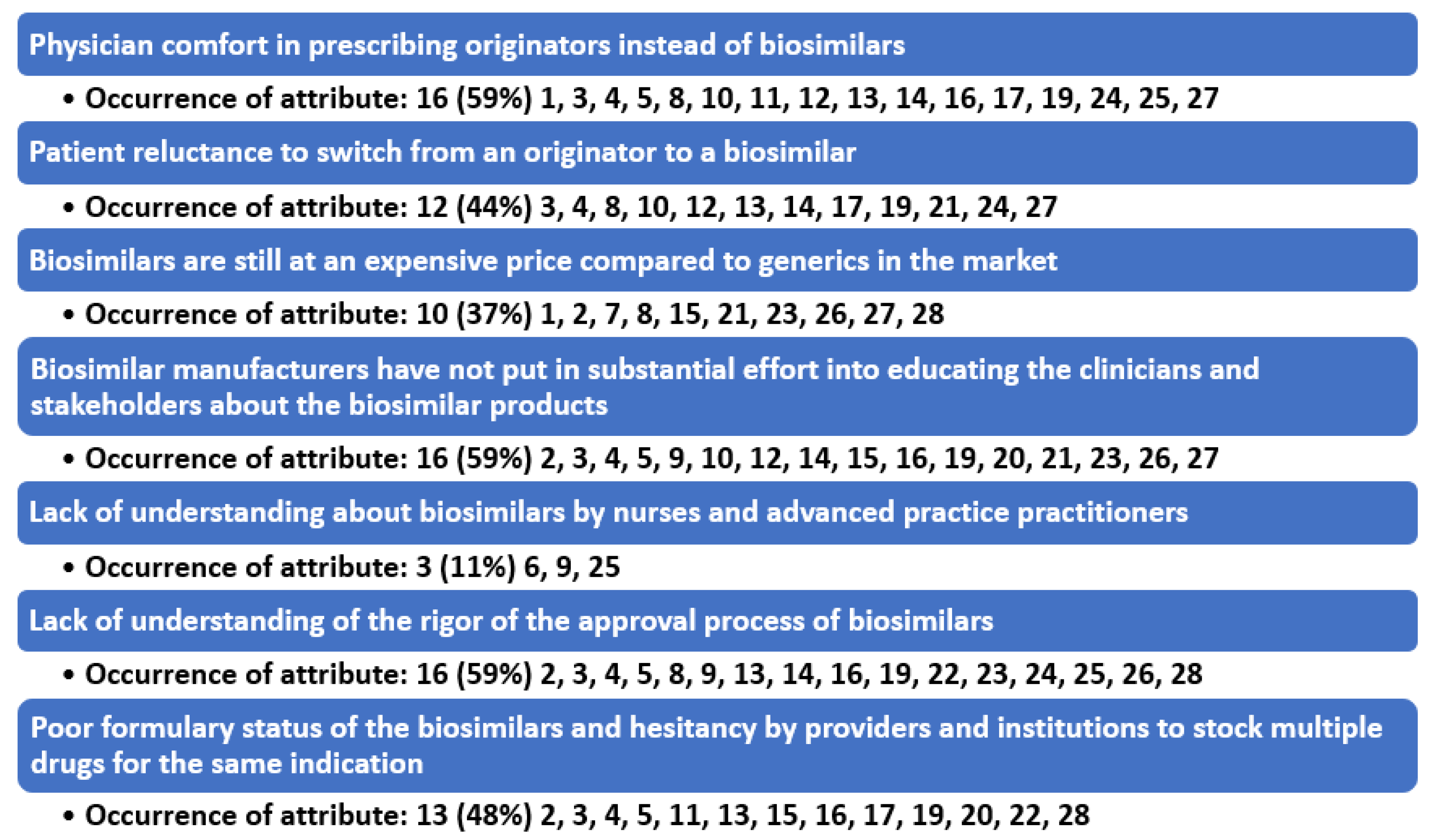
4.2. Barrier to Prescribing #1: Physician Comfort in Prescribing Originators Instead of Biosimilars
4.3. Barrier to Prescribing #2: Patient Reluctance to Switch from a Biologic to a Biosimilar
4.4. Barrier to Prescribing #3: Biosimilars Are Still at a High Price and Physicians and Institutions Can Have More Profitability by Using the Originator Biologic
4.5. Barrier to Prescribing #4: Biosimilar Manufacturers Have Not Put in Substantial Effort into Educating Stakeholders and Many of the Originator Biologic Companies Are Counter Detailing the Biosimilar Products
4.6. Barrier to Prescribing #5: Lack of Understanding about Biosimilars by Nurses and Advanced Practice Practitioners
4.7. Barrier to Prescribing #6: Lack of Understanding of the Rigor of the Approval Process of Biosimilars
4.8. Barrier to Prescribing #7: Formulary Status of the Biosimilars and Hesitancy by Providers and Institutions to Stock Multiple Drugs for the Same Indication
4.9. Implications
4.10. Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nabhan, C.; Valley, A.; Feinberg, B.A. Barriers to Oncology Biosimilars Uptake in the United States. Oncologist 2018, 23, 1261–1265. [Google Scholar] [CrossRef] [Green Version]
- Leighl, N.B.; Nirmalakumar, S.; Ezeife, D.A.; Gyawali, B. An Arm and a Leg: The Rising Cost of Cancer Drugs and Impact on Access. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e1–e12. [Google Scholar] [CrossRef]
- Boccia, R.; Jacobs, I.; Popovian, R.; de Lima Lopes, G., Jr. Can Biosimilars Help Achieve the Goals of US Health Care Reform? Cancer Manag. Res. 2017, 9, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Dolan, C. Opportunities and Challenges in Biosimilar Uptake in Oncology. Am. J. Manag. Care 2018, 24, S237–S243. [Google Scholar]
- Kaida-Yip, F.; Deshpande, K.; Saran, T.; Vyas, D. Biosimilars: Review of Current Applications, Obstacles, and Their Future in Medicine. World J. Clin. Cases 2018, 6, 161. [Google Scholar] [CrossRef]
- Zack, E. Nursing Roles: Clinical Implications Regarding Trends, Administration, and Education for Biosimilars in Oncology Practice. Clin. J. Oncol. Nurs. 2018, 22, 21–26. [Google Scholar] [CrossRef]
- FDA Promotes Efficient Biosimilar Approval. Cancer Discovery, U.S. National Library of Medicine, October 2018. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30154191 (accessed on 20 June 2022).
- Chopra, R.; Lopes, G. Improving Access to Cancer Treatments: The Role of Biosimilars. J. Glob. Oncol. 2017, 3, 596–610. [Google Scholar] [CrossRef]
- Mayden, K.D.; Larson, P.; Geiger, D.; Watson, H. Biosimilars in the United States: Considerations for Oncology Advanced Practitioners. J. Adv. Pract. Oncol. 2015, 6, 108. [Google Scholar]
- Hemmington, A.; Dalbeth, N.; Jarrett, P.; Fraser, A.G.; Broom, R.; Browett, P.; Petrie, K.J. Medical Specialists’ Attitudes to Prescribing Biosimilars. Pharmacoepidemiol. Drug Saf. 2017, 26, 570–577. [Google Scholar] [CrossRef]
- Hirsch, B.R.; Lyman, G.H. Biosimilars: Are they ready for primetime in the United States? J. Natl. Compr. Cancer Netw. 2011, 9, 934–942; quiz 943. [Google Scholar] [CrossRef]
- Abraham, J. Developing Oncology Biosimilars: An Essential Approach for the Future. Semin. Oncol. 2013, 40, S5–S24. [Google Scholar] [CrossRef]
- Jacobs, I.; Ewesuedo, R.; Lula, S.; Zacharchuk, C. Biosimilars for the Treatment of Cancer: A Systematic Review of Published Evidence. BioDrugs 2017, 31, 1–36. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bissig, M.; Curigliano, G.; Coppola, J.; Latymer, M. Talking to Patients about Biosimilars. Future Oncol. 2018, 14, 2403–2414. [Google Scholar] [CrossRef]
- Kar, I.; Kronz, M.; Kolychev, E.; Silverman, P.; Mendiratta, P.; Tomlinson, B.K.; Prunty, J.; Copley, M.; Patel, S.; Caudill, S.; et al. Biosimilar Strategic Implementation at a Large Health System. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2022, 79, 268–275. [Google Scholar] [CrossRef]
- Kolbe, A.R.; Kearsley, A.; Merchant, L.; Temkin, E.; Patel, A.; Xu, J.; Jessup, A. Physician Understanding and Willingness to Prescribe Biosimilars: Findings from a US NationalSurvey. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 363–372. [Google Scholar] [CrossRef]
- McKoy, J.M.; Giles, F.J. Biosimilars: Are They Really Safe? Cancer Treat. Res. 2019, 171, 61–73. [Google Scholar]
- Nixon, N.A.; Hannouf, M.B.; Verma, S. The Evolution of Biosimilars in Oncology, with a Focus on Trastuzumab. Curr. Oncol. 2018, 25, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.; Planchard, D.; Pegram, M.; Gonçalves, J.; Bocquet, F.; Jang, H. Biosimilars in an Era of Rising Oncology Treatment Options. Future Oncol. 2021, 17, 3881–3892. [Google Scholar] [CrossRef]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Lucio, S. The Complexities of Biosimilars and the Regulatory Approval Process. Am. J. Manag. Care 2018, 24, S231–S236. [Google Scholar]
- Simoens, S. How Do Biosimilars Sustain Value, Affordability, and Access to Oncology Care? Expert Rev. Pharm. Outcomes Res. 2021, 21, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Curigliano, G.; Jacobs, I.; Gumbiner, B.; MacDonald, J.; Thomas, D. Clinical Considerations for the Development of Biosimilars in Oncology. MAbs 2015, 7, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariman, J.D. Biosimilars: Exploring the History, Science, and Progress. Clin. J. Oncol. Nurs. 2018, 22, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V. Current Market and Regulatory Landscape of Biosimilars. Am. J. Manag. Care 2018, 24, S451–S456. [Google Scholar]
- Kvien, T.K.; Patel, K.; Strand, V. The Cost Savings of Biosimilars Can Help Increase Patient Access and Lift the Financial Burden of Health Care Systems. Semin. Arthritis Rheum. 2022, 52, 151939. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Dreyling, M.; Gradishar, W.; Andre, M.; Esteva, F.J.; Boulos, S.; González Barca, E.; Curigliano, G. Are Biosimilars the Future of Oncology and Haematology? Drugs 2019, 79, 1609–1624. [Google Scholar] [CrossRef]
- Afzali, A.; Furtner, D.; Melsheimer, R.; Molloy, P.J. The automatic substitution of biosimilars: Definitions of interchangeability are not interchangeable advances in therapy. Adv. Ther. 2021, 38, 2077–2093. [Google Scholar] [CrossRef]
- Gifoni, M.A.; Fernandes, G.S.; Chammas, R. Biosimilar drugs: What would be a reasonable extrapolation? J. Glob. Oncol. 2018, 4. [Google Scholar] [CrossRef]
| Author(s) | Participant(s) | Barriers Leading to Prescribers and Cancer Centers Not Using Biosimilars for Patients |
|---|---|---|
| Leighl, B., et al., 2021 [2] | A team of US community oncologists |
|
| Nabhan, B.A., et al., 2018 [1] | Specialty pharmaceutical distribution for a hospital system |
|
| Boccia, et al., 2017 [3] | National cancer centers in the United States, global medical affairs for Pfizer, and US government relations |
|
| Dolan, C., et al., 2018 [4] | A variety of 376 surveyed United States oncologists |
|
| Kaida-Yip, D., et al., 2018 [5] | Department of Medicine at California Northstate University and University of Texas; Department of Surgery at Michigan State University and Texas Tech University |
|
| Zack, E., 2018 [6] | Oncology nurses |
|
| FDA Promotes Efficient Biosimilar Approval, 2018 [7] | Oncology peer-reviewed journal author |
|
| Chopra, G., et al., 2017 [8] | Clinical oncologists |
|
| Mayden, H., et al., 2015 [9] | Southwest Virginia Cancer Center, Norton, Virginia; Fletcher Allen Healthcare, Burlington, Vermont; Nebraska Cancer Specialists, Omaha, Nebraska; Amgen Inc., Thousand Oaks, California |
|
| Hemmington, A., et al., 2017 [10] | Group of 327 surveyed medical specialists throughout the United States |
|
| Hirsh, B.R., et al., 2011 [11] | Department of Medicine, Duke University and the Duke Cancer Institute, Durham, North Carolina. |
|
| Abraham, J., 2013 [12] | Oncologist Cleveland Clinic |
|
| Jacobs, I., et al., 2017 [13] | Proposed biosimilars were identified in 23 studies (36 publications) in oncology |
|
| Janjigian, Y.Y., et al., 2018 [14] | Clinicians for pharmaceutical companies |
|
| Kar, I., et al., 2022 [15] | Cohort of PharmDs |
|
| Kolbe, A.R., et al., 2021 [16] | 507 surveyed healthcare clinicians from various specialties. |
|
| McCoy, J., et al., 2019 [17] | Comprehensive Cancer Center Physicians from Northwestern University, Chicago, IL, USA |
|
| Nixon, N.A., et al., 2018 [18] | Cancer Center faculty |
|
| Peters, M., et al., 2021 [19] | A team of world community oncologists |
|
| Rosen, R.L., et al., 2017 [20] | Oncologists specializing in colorectal cancer |
|
| Lucio, S., 2018 [21] | PharmD |
|
| Simoens, S., 2021 [22] | Department of Pharmaceuticals and Pharmacological Sciences |
|
| Socinski, M.A., et al., 2015 [23] | Cohort of United States oncologists |
|
| Tariman, J.D. 2018 [24] | Assistant professor at College of Nursing at DePaul University |
|
| Bhatt, V., 2018 [25] | PharmD |
|
| Kvien, V., et al., 2022 [26] | Cohort of clinical professors and oncology medical directors |
|
| Zinzani, P.L., et al., 2019 [27] | Cohort of clinical professors and oncology center directors |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hair, J.; Maryon, T.; Lieneck, C. Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption. Medicina 2022, 58, 1533. https://doi.org/10.3390/medicina58111533
Hair J, Maryon T, Lieneck C. Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption. Medicina. 2022; 58(11):1533. https://doi.org/10.3390/medicina58111533
Chicago/Turabian StyleHair, John, Thomas Maryon, and Cristian Lieneck. 2022. "Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption" Medicina 58, no. 11: 1533. https://doi.org/10.3390/medicina58111533





