Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
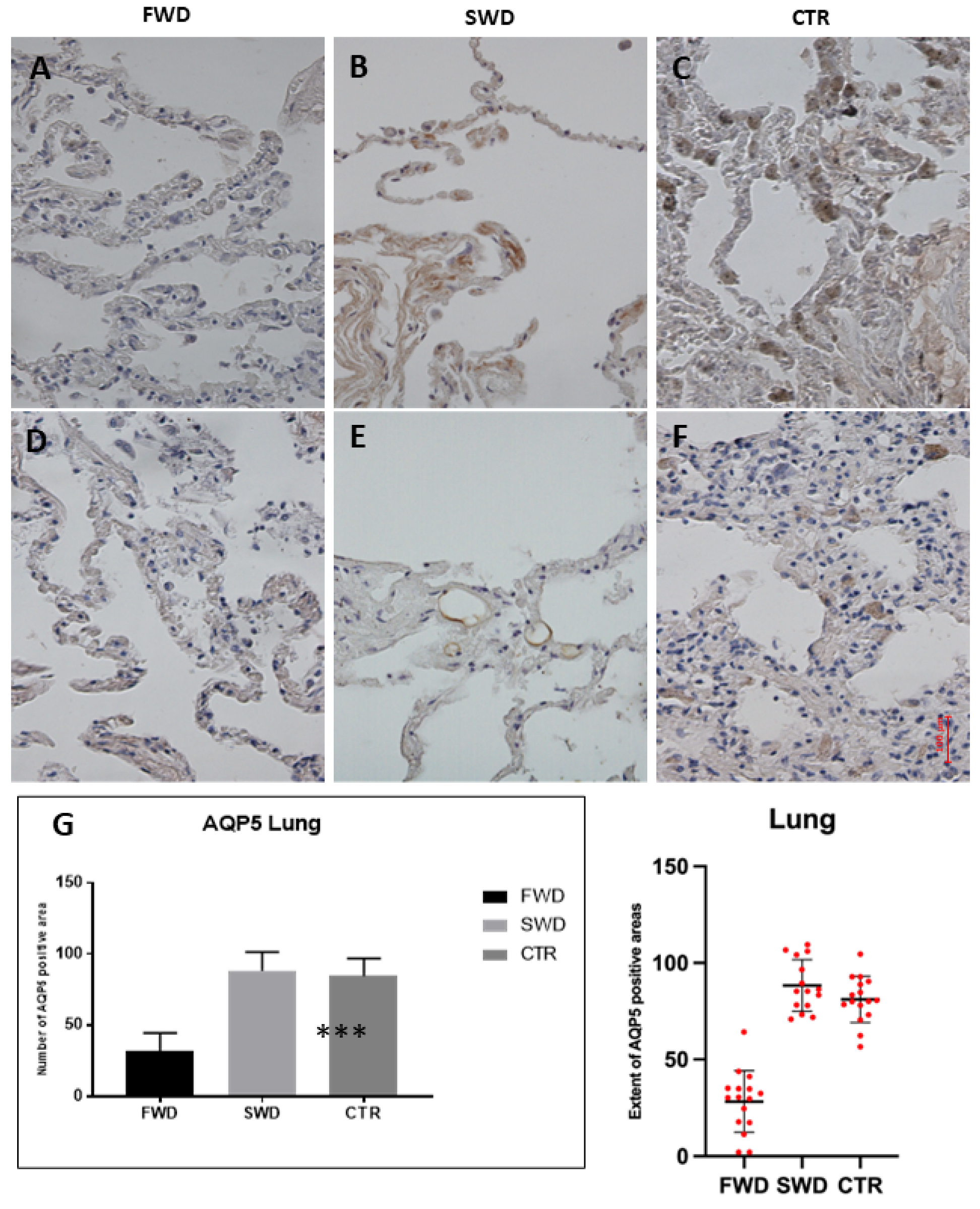
3.1. Immunohistochemical Analysis of AQP5 in the Lung
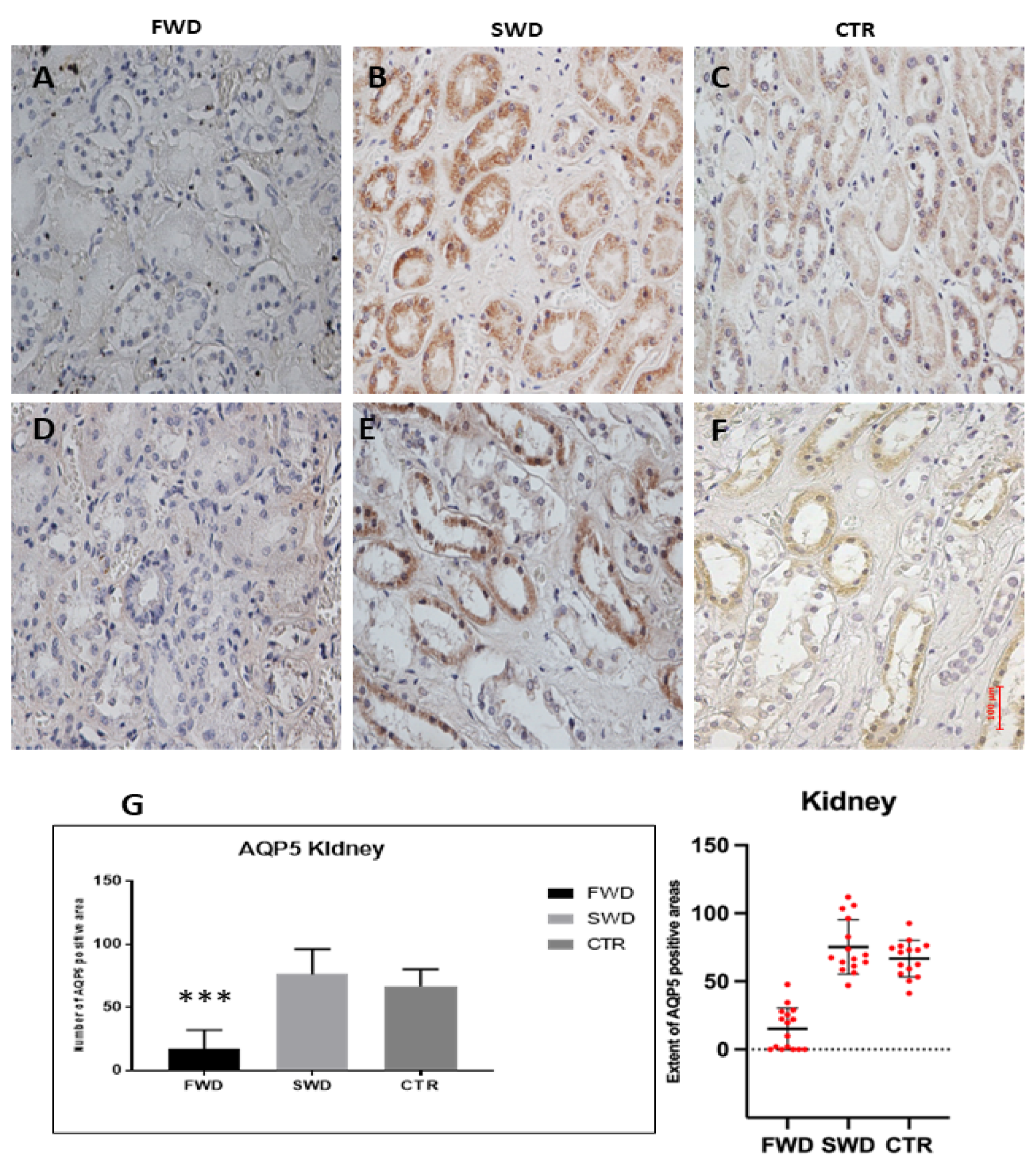
3.2. Immunohistochemical Analysis of AQP5 in the Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Beeck, E.F.; Branche, C.M.; Szpilman, D.; Modell, J.H.; Bierens, J.J. A new definition of drowning: Towards documentation and prevention of a global public health problem. Bull. World Health Organ. 2005, 83, 853–856. [Google Scholar] [PubMed]
- Haw, C.; Hawton, K. Suicide and Self-Harm by Drowning: A Review of the Literature. Arch. Suicide Res. 2016, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Saukko, P.; Knight, B. Knight’s Forensic Pathology, 3rd ed.; Hodder Arnold: London, UK, 2004; pp. 395–411. [Google Scholar]
- Lunetta, P.; Penttilä, A.; Sajantila, A. Circumstances and macropathologic findings in 1590 consecutive cases of bodies found in water. Am J Forensic Med.Pathol. 2002, 23, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Hendey, N.I. The diagnostic value of diatoms in cases of drowning. Med. Sci. Law 1973, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Peabody, A.J. Diatoms and drowning—A review. Med. Sci. Law 1980, 20, 254–261. [Google Scholar] [CrossRef]
- Auer, A. Qualitative diatom analysis as a tool to diagnose drowning. Am. J. Forensic Med. Pathol. 1991, 12, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Pollanen, M.S.; Cheung, C.; Chiasson, D.A. The diagnostic value of the diatom test for drowning 1. Utility: A retrospective analysis of 771 cases of drowning in Ontario, Canada. J. Forensic Sci. 1997, 42, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Piette, M.H.; De Letter, E.A. Drowning: Still a difficult autopsy diagnosis. Forensic Sci. Int. 2006, 163, 1–9. [Google Scholar] [CrossRef]
- Papadodima, A.S.; Athanaselis, A.S.; Skliros, E.; Spiliopoulou, C.A. Forensic investigation of submersion deaths. Int. J. Clin. Pract. 2010, 64, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.; Fechner, G.; Puschel, K. Ultrastructural pathology of the alveolar apparatus in experimental drowning. Z. Rechtsmed. 1983, 91, 47–60. [Google Scholar] [CrossRef]
- Brinkmann, B.; Fechner, G.; Puschel, K. Lung histology in experimental drowning. Z. Rechtsmed. 1983, 89, 267–277. [Google Scholar] [PubMed]
- Zhu, B.L.; Quan, L.; Li, D.R.; Taniguchi, M.; Kamikodai, Y.; Tsuda, K.; Fujita, M.Q.; Nishi, K.; Tsuji, T.; Maeda, H. Postmortem lung weight in drownings: A comparison with acute asphyxiation and cardiac death. Leg. Med. 2003, 5, 20–26. [Google Scholar] [CrossRef]
- de la Grandmaison, G.L.; Leterreux, M.; Lasseuguette, K.; Alvarez, J.C.; de Mazancourt, P.; Durigon, M. Study of the diagnostic value of iron in fresh water drowning. Forensic Sci. Int. 2006, 157, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Azparren, J.E.; Fernandez-Rodriguez, A.; Vallejo, G. Diagnosing death by drowning in fresh water using blood strontium as an indicator. Forensic Sci. Int. 2003, 137, 55–59. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishida, K.; Taniguchi, M.; Quan, L.; Oritani, S.; Tsuda, K.; Kamikodai, Y.; Fujita, M.Q.; Maeda, H. Possible postmortem serum markers for differentiation between fresh-, saltwater drowning and acute cardiac death: A preliminary investigation. Leg. Med. 2003, 5 (Suppl. 1), S298–S301. [Google Scholar] [CrossRef]
- Kakizaki, E.; Kozawa, S.; Matsuda, H.; Muraoka, E.; Uchiyama, T.; Sakai, M.; Yukawa, N. Freshwater bacterioplankton cultured from liver, kidney and lungs of a decomposed cadaver retrieved from a sandy seashore: Possibility of drowning in a river and then floating out to sea. Leg. Med. 2010, 12, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.; Hernandez, M.A.; Karger, B.; Ortmann, C. Pulmonary myelomonocyte subtypes in drowning and other causes of death. Int. J. Leg. Med. 1997, 110, 295–298. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishida, K.; Quan, L.; Li, D.R.; Taniguchi, M.; Fujita, M.Q.; Maeda, H.; Tsuji, T. Pulmonary immunohistochemistry and serum levels of a surfactant-associated protein A in fatal drowning. Leg. Med. 2002, 4, 1–6. [Google Scholar] [CrossRef]
- An, J.L.; Ishida, Y.; Kimura, A.; Kondo, T. Immunohistochemical examination of intracerebral aquaporin-4 expression and its application for differential diagnosis between freshwater and saltwater drowning. Int. J. Leg. Med. 2011, 125, 59–65. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- An, J.L.; Ishida, Y.; Kimura, A.; Kondo, T. Forensic application of intrarenal aquaporin-2 expression for differential diagnosis between freshwater and saltwater drowning. Int. J. Leg. Med. 2010, 124, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ishida, Y.; Mizunuma, S.; Kimura, A.; Kondo, T. Differential diagnosis between freshwater drowning and saltwater drowning based on intrapulmonary aquaporin-5 expression. Int. J. Leg. Med. 2009, 123, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ha, E.J.; Cho, H.W.; Kim, H.R.; Lee, D.; Eom, Y.B. Potential forensic application of receptor for advanced glycation end products (RAGE) and aquaporin 5 (AQP5) as novel biomarkers for diagnosis of drowning. J. Forensic Leg. Med. 2019, 62, 56–62. [Google Scholar] [CrossRef]
- Barranco, R.; Castiglioni, C.; Ventura, F.; Fracasso, T. Immunohistochemical expression of P-selectin, SP-A, HSP70, aquaporin, and fibronectin in saltwater drowning and freshwater drowning. Int. J. Leg. Med. 2019, 133, 1461–1467. [Google Scholar] [CrossRef]
- Procino, G.; Mastrofrancesco, L.; Sallustio, F.; Costantino, V.; Barbieri, C.; Pisani, F.; Schena, F.P.; Svelto, M.; Valenti, G. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell. Physiol. Biochem. 2011, 28, 683–692. [Google Scholar] [CrossRef]
- Verkman, A.S.; Michael, A.; Matthay, M.A.; Song, Y. Aquaporin water channels and lung physiology. Am. J. Physiol. Cell. Mol. Physiol. 2000, 278, 867–879. [Google Scholar] [CrossRef]
- Ma, T.; Fukuda, N.; Song, Y.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-5 knockout mice. J. Clin. Investig. 2000, 105, 93–100. [Google Scholar] [CrossRef]
- Cohen, D.M.; Wasserman, J.C.; Gullans, S.R. Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am. J. Physiol. 1991, 261, 594–601. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular Response to Hyperosmotic Stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Liu, X.; Bandyopadhyay, B.C.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A role for AQP5 in activation of TRPV4 by hypotonicity: Concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006, 281, 15485–15495. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhang, X.; Zhou, Q.; Li, J.M.; Berger, S.; Borok, Z.; Zhou, B.; Xiao, Z.; Yin, H.; et al. AQP5 is a new transcriptional target of Dot1a and a regulator of Aqp2. PLoS ONE 2013, 8, e53342. [Google Scholar] [CrossRef] [PubMed]
- Pinchi, E.; Frati, A.; Cipolloni, L.; Aromatario, M.; Gatto, V.; La Russa, R.; Pesce, A.; Santurro, A.; Fraschetti, F.; Frati, P.; et al. Clinical-pathological study on β-APP, IL-1β, GFAP, NFL, Spectrin II, 8OHdG, TUNEL, miR-21, miR-16, miR-92 expressions to verify DAI-diagnosis, grade and prognosis. Sci. Rep. 2018, 8, 2387. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Maiese, A.; Passaro, G.; Tosoni, A.; Mirijello, A.; Simone, S.D.; Baldari, B.; Cipolloni, L.; La Russa, R. Neutropenic Enterocolitis and Sepsis: Towards the Definition of a Pathologic Profile. Medicina 2021, 57, 638. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Ferrara, M.; La Russa, R.; Pollice, G.; Gurgoglione, G.; Frisoni, P.; Alfieri, L.; De Simone, S.; Neri, M.; Cipolloni, L. Wound Vitality in Decomposed Bodies: New Frontiers through Immunohistochemistry. Front. Med. 2021, 8, 802841. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frisoni, P.; Diani, L.; De Simone, S.; Bosco, M.A.; Cipolloni, L.; Neri, M. Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study. Medicina 2022, 58, 1458. https://doi.org/10.3390/medicina58101458
Frisoni P, Diani L, De Simone S, Bosco MA, Cipolloni L, Neri M. Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study. Medicina. 2022; 58(10):1458. https://doi.org/10.3390/medicina58101458
Chicago/Turabian StyleFrisoni, Paolo, Luca Diani, Stefania De Simone, Maria Antonella Bosco, Luigi Cipolloni, and Margherita Neri. 2022. "Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study" Medicina 58, no. 10: 1458. https://doi.org/10.3390/medicina58101458
APA StyleFrisoni, P., Diani, L., De Simone, S., Bosco, M. A., Cipolloni, L., & Neri, M. (2022). Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study. Medicina, 58(10), 1458. https://doi.org/10.3390/medicina58101458







