Crystallization Phase Regulation of BaO-CaO-SiO2 Glass-Ceramics with High Thermal Expansion Coefficient
Abstract
:1. Introduction
| Compounds | CTE (ppm/°C) | Q × f (GHz) | εr | Reference |
|---|---|---|---|---|
| CaSiO3 | 11.2 | 29,300 | 6.9 | [30,31] |
| Ca2SiO4 | 8.5 | 26,100 | 8.6 | [30,31] |
| BaSiO3 | 12.5 | 6600 | 11.1 | [32,33] |
| Ba2Si3O8 | 11.7 | 29,800 | 8.2 | [32] |
| Ba5Si8O21 | 10.6 | 16,700 | 7.3 | [32] |
| Ba3Si5O13 | 11.8 | 12,500 | 6.9 | [32] |
| BaSi2O5 | 14.0 | 59,500 | 6.7 | [32,33] |
2. Materials and Methods
3. Results and Discussion
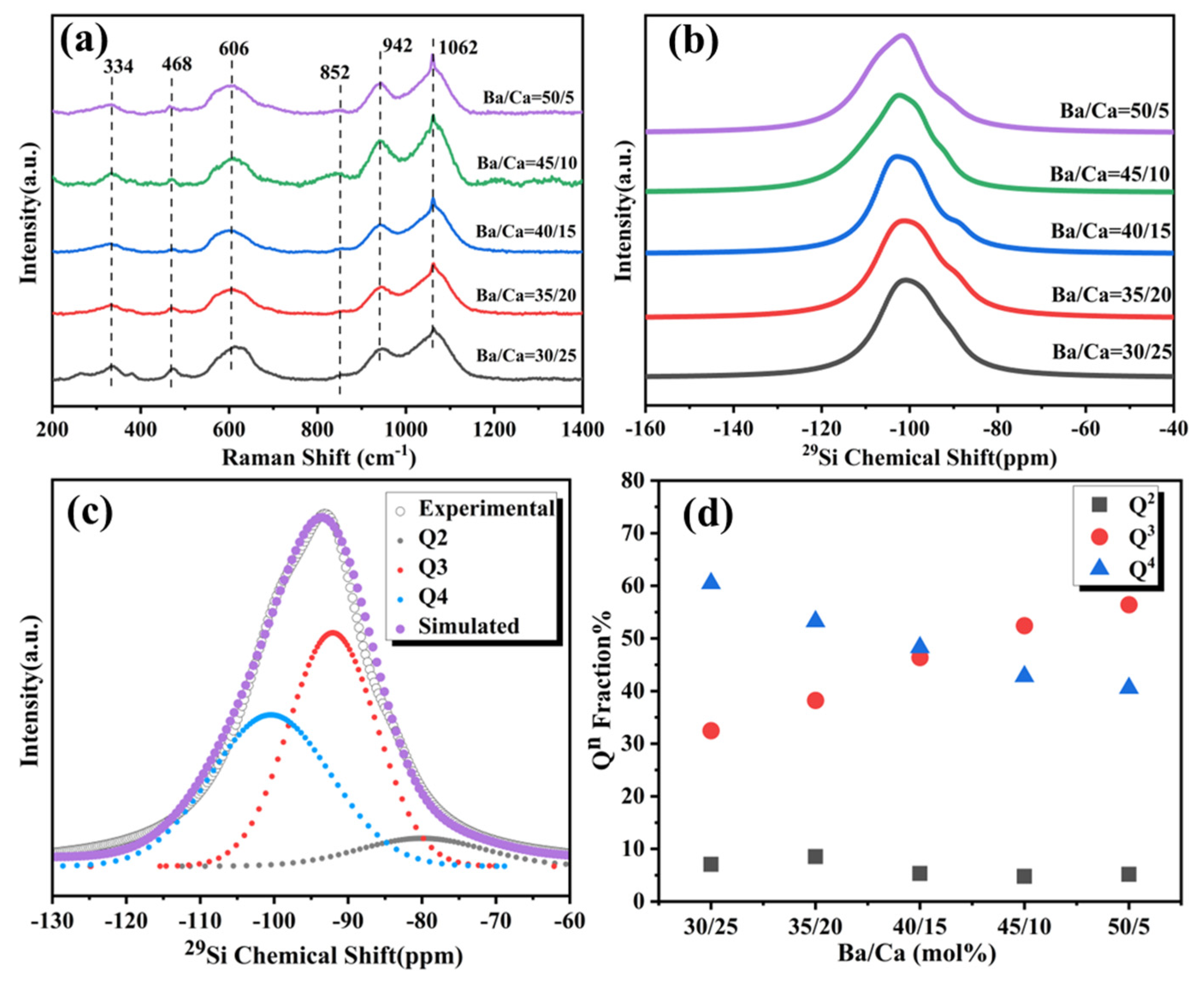
3.1. Effect of Ba/Ca Ratio on Structure and Crystallization of BCS Glasses
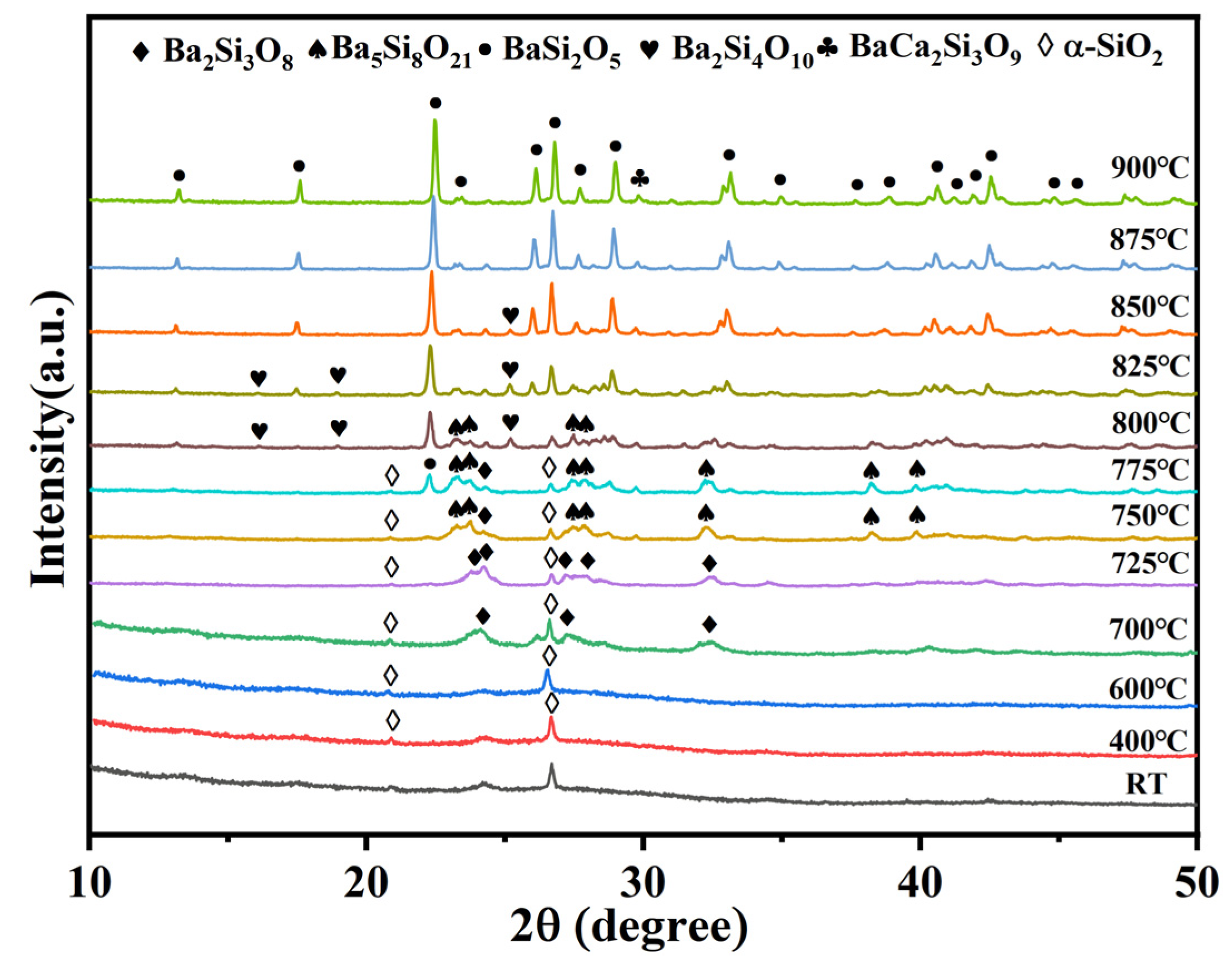
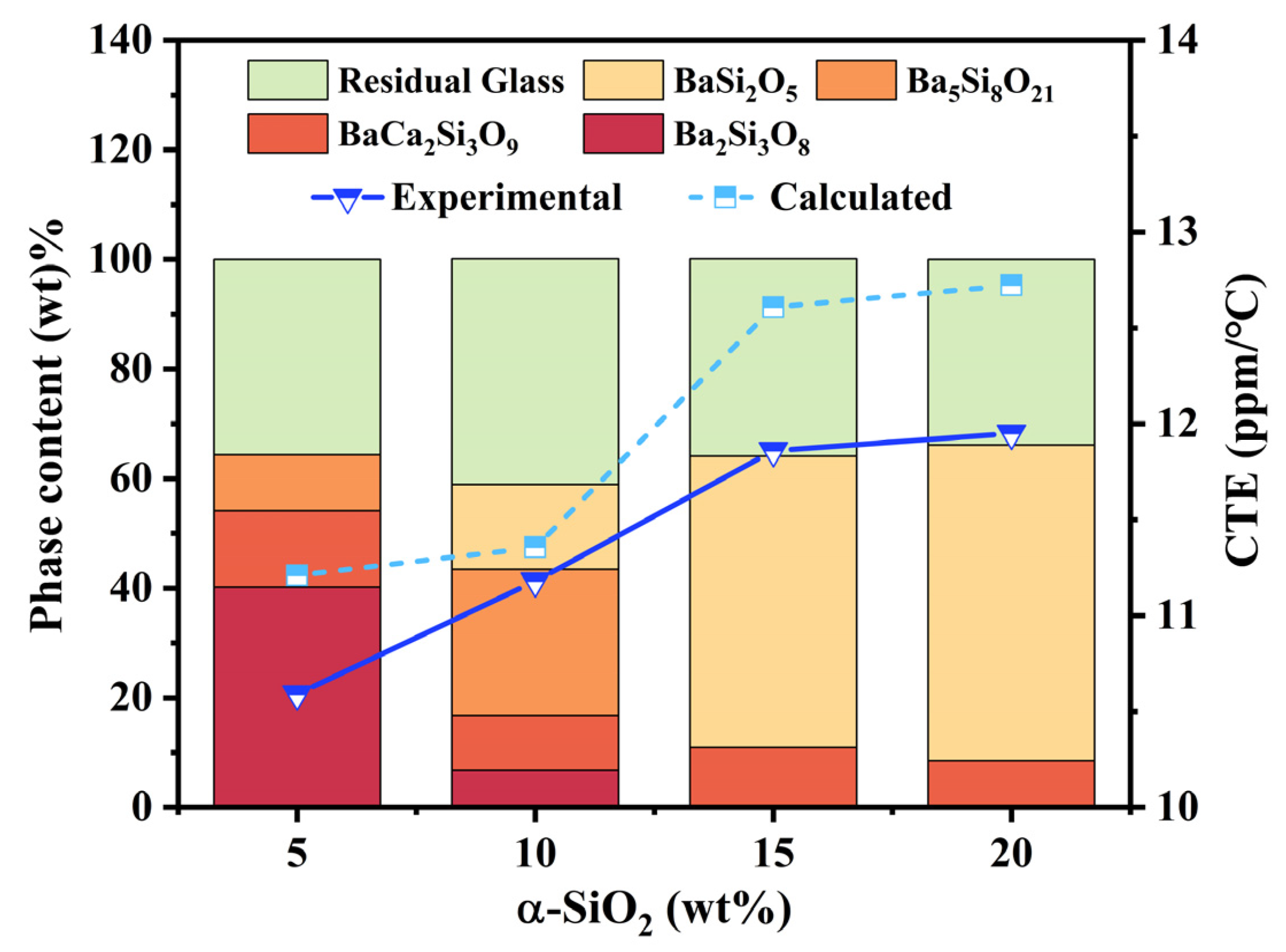
3.2. Phase Regulation of BCS Glass-Ceramics by α-SiO2
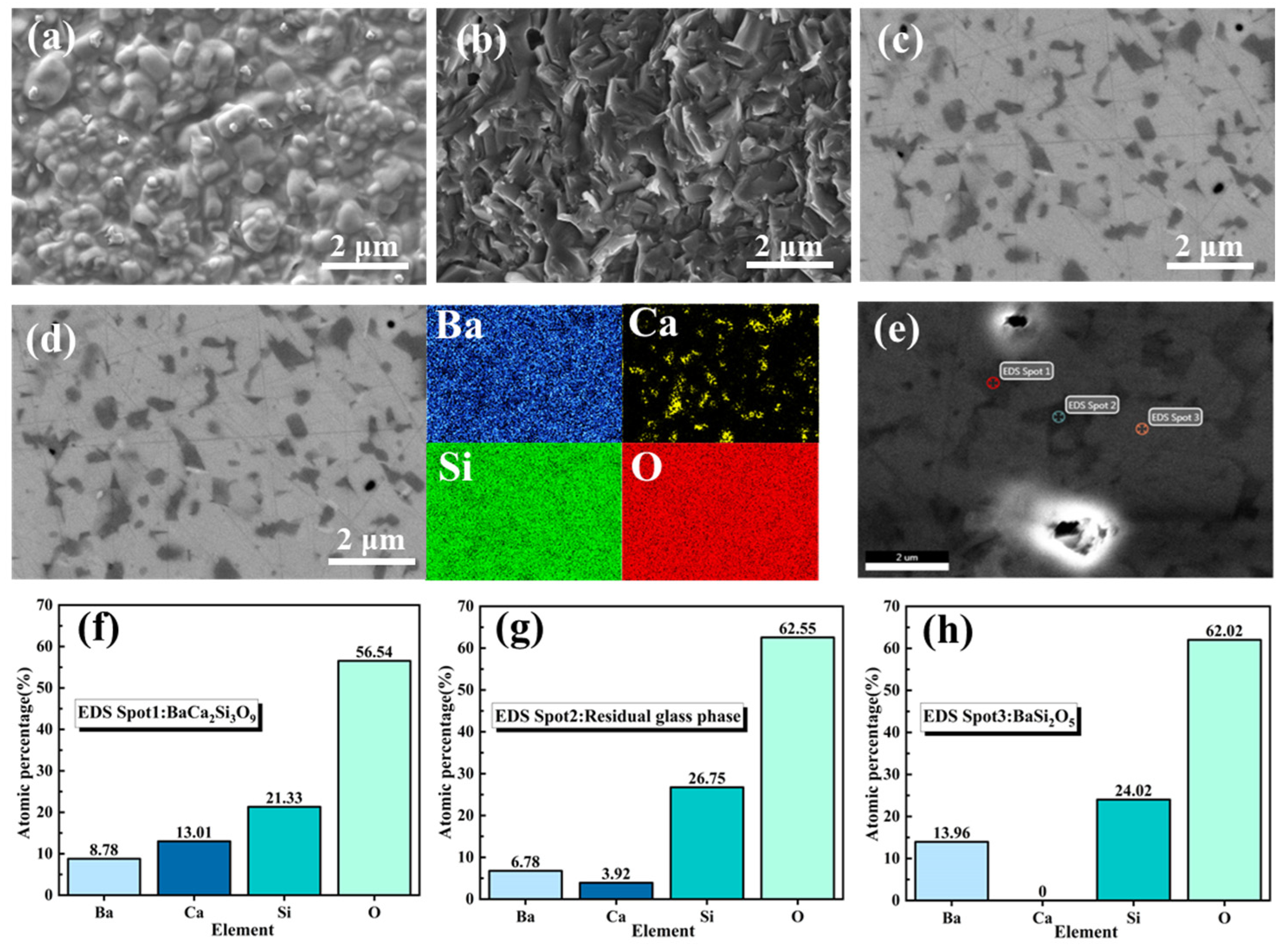
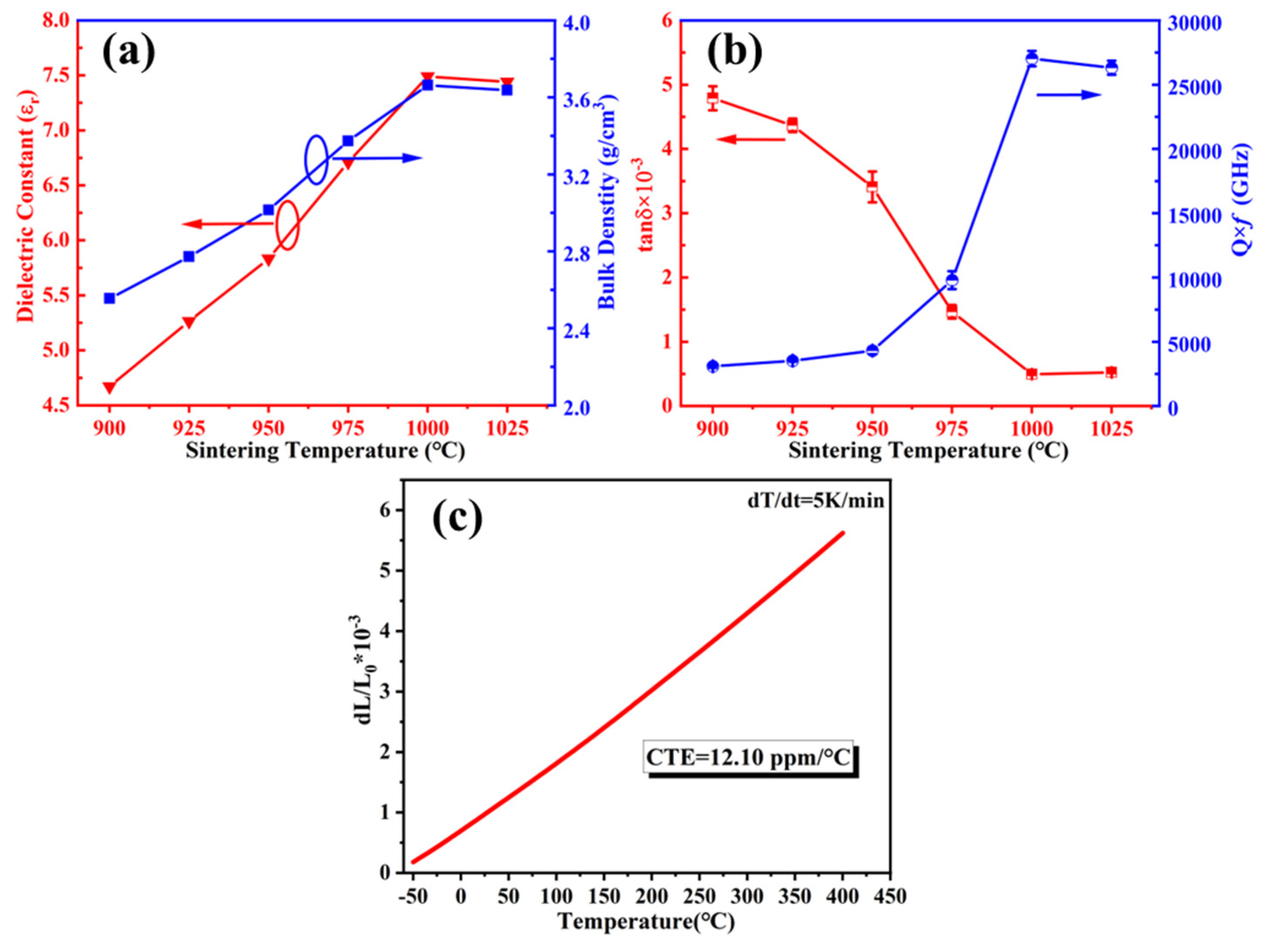
3.3. Properties of the Modified BCS Glass-Ceramics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Q.; Wang, W.; Zhang, H.; Chen, C.; Li, A.; Chen, C.; Yu, H.; Zhang, L.; Tao, H.; Zeng, H.; et al. Creating Single-crystalline β-CaSiO3 for High-performance Electronic Packaging Substrate. Adv. Mater. 2024, 37, 2414156. [Google Scholar] [CrossRef]
- Huang, L.M.; Bian, J.J. Microwave Dielectric Properties of NaMgF3 Ceramic. Mater. Lett. 2023, 350, 134933. [Google Scholar] [CrossRef]
- Kamutzki, F.; Schneider, S.; Barowski, J.; Gurlo, A.; Hanaor, D.A.H. Silicate Dielectric Ceramics for Millimetre Wave Applications. J. Eur. Ceram. Soc. 2021, 41, 3879–3894. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Pang, L.; Hussain, F.; Zhou, T.; Sun, S.; Chen, Z.; Zhou, D. Rational Optimizations of High K Microwave Dielectric Ceramic Bi2(Li0.5Ta1.5)O7 toward LTCC Applications. J. Am. Ceram. Soc. 2024, 108, e20316. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Xu, D.; Shi, Z.; Guo, T.; Hussain, F.; Darwish, M.A.; Zhou, T.; Chen, Y.; Liang, Q.; et al. Temperature Stable (1-x)BaAl2Si2O8-xBa3V2O8 (0.2 ≤ x ≤ 0.5) Microwave Dielectric Composite Ceramics for LTCC Applications. J. Eur. Ceram. Soc. 2025, 45, 117042. [Google Scholar] [CrossRef]
- Capraro, B.; Heidenreich, M.; Töpfer, J. Large Thermal Expansion LTCC System for Cofiring with Integrated Functional Ceramics Layers. Materials 2022, 15, 564. [Google Scholar] [CrossRef]
- Chen, C.; Hou, F.; Liu, F.; She, Q.; Cao, L.; Wan, L. Thermo-Mechanical Reliability Analysis of a RF SiP Module Based on LTCC Substrate. Microelectron. Reliab. 2017, 79, 38–47. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, J.; Li, B.; Li, L. CaF2–AlF3–SiO2 Glass-Ceramic with Low Dielectric Constant for LTCC Application. J. Alloys Compd. 2010, 490, 204–207. [Google Scholar] [CrossRef]
- Induja, I.J.; Surendran, K.P.; Varma, M.R.; Sebastian, M.T. Low κ, Low Loss Alumina-Glass Composite with Low CTE for LTCC Microelectronic Applications. Ceram. Int. 2017, 43, 736–740. [Google Scholar] [CrossRef]
- Wang, W.; Shehbaz, M.; Wang, X.; Du, C.; Xu, D.; Shi, Z.-Q.; Darwish, M.A.; Qiu, H.-S.; Jin, B.-B.; Zhou, T.; et al. Low-Permittivity and Low-Temperature Cofired BaSO4–BaF2 Microwave Dielectric Ceramics for High-Reliability Packaged Electronics. ACS Appl. Mater. Interfaces 2023, 15, 51453–51461. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, W.; Yu, C.; Sun, Q.; Cao, Y.; Li, W.; Jiang, S.; Li, Q.; Zhang, Q.; An, K.; et al. Chemical Heterogeneity Modulated Zero Thermal Expansion Alloy over Super-Wide Temperature Range. Cell Rep. Phys. Sci. 2023, 4, 101254. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, S.; Luo, R.; Cheng, P.; Liu, C.; Han, X.; Qi, S.; Zhang, Y.; Zhu, J.; Xu, J.; et al. Controllable Shape Deformation of an Organic Single Crystal Actuated by Anisotropic Thermal Expansion. Cell Rep. Phys. Sci. 2023, 4, 101451. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Zhou, X.; Li, B. Thermal and Dielectric Properties of the LTCC Composites Based on the Eutectic System BaO–Al2O3–SiO2–B2O3. J. Mater. Sci. Mater. Electron. 2011, 22, 238–243. [Google Scholar] [CrossRef]
- Li, B.; Xu, M.; Tang, B. Effects of ZnO on Crystallization, Microstructures and Properties of BaO–Al2O3–B2O3–SiO2 Glass–Ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 70–76. [Google Scholar] [CrossRef]
- Li, B.; Bian, H.; Jing, K. Effect of MnO Addition on Microstructures and Properties of BaO–Al2O3–B2O3–SiO2 Glass-Ceramics for LTCC Application. Mater. Lett. 2019, 234, 302–305. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Zheng, J. Effect of SiO2 Content on the Sintering Kinetics, Microstructures and Properties of BaO–Al2O3–B2O3–SiO2 Glass-Ceramics for LTCC Application. J. Alloys Compd. 2017, 725, 1091–1097. [Google Scholar] [CrossRef]
- Jin, L.; Guo, J.-F.; Luo, Y.-J.; Zhou, Z.; Chen, S. Tuning High and Low Thermal Expansion Coefficients of Li2O–BaO–Al2O3–B2O3–SiO2/Quartz LTCC Composites by Replacing Quartz Partly with α-Al2O3 or ZrO2. Ceram. Int. 2022, 48, 37353–37361. [Google Scholar] [CrossRef]
- Meinhardt, K.D.; Kim, D.-S.; Chou, Y.-S.; Weil, K.S. Synthesis and Properties of a Barium Aluminosilicate Solid Oxide Fuel Cell Glass-Ceramic Sealant. J. Power Sources 2008, 182, 188–196. [Google Scholar] [CrossRef]
- Xia, G.; He, L.; Yang, D. Preparation and Characterization of CaO-Al2O3-SiO2 Glass/Fused Silica Composites for LTCC Application. J. Alloys Compd. 2012, 531, 70–76. [Google Scholar] [CrossRef]
- Qing, Z. The Effects of B2O3 on the Microstructure and Properties of Lithium Aluminosilicate Glass-Ceramics for LTCC Applications. Mater. Lett. 2018, 212, 126–129. [Google Scholar] [CrossRef]
- Li, B.; Tang, B.; Xu, M. Influences of CaO on Crystallization, Microstructures, and Properties of BaO–Al2O3–B2O3–SiO2 Glass–Ceramics. J. Electron. Mater. 2015, 44, 3849–3854. [Google Scholar] [CrossRef]
- Li, B.; Long, Q.; Duan, D. Effects of ZrO2 on Properties of BaO–Al2O3–B2O3–SiO2 Composites for LTCC Applications. J. Mater. Sci. Mater. Electron. 2016, 27, 2824–2829. [Google Scholar] [CrossRef]
- Li, B.; Long, Q.; Duan, D. Effect of Sintering Temperatures on Properties of BaO–B2O3–SiO2–Al2O3 Glass/Silica Composites for CBGA Package. J. Mater. Sci. Mater. Electron. 2016, 27, 2206–2211. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, X.; Chen, N.; Xu, X.; Lu, Y.; Cheng, J.; Wang, H. Low-temperature-sintered MgO-based Microwave Dielectric Ceramics with Ultralow Loss and High Thermal Conductivity. J. Am. Ceram. Soc. 2023, 106, 1159–1169. [Google Scholar] [CrossRef]
- Schwickert, T.; Sievering, R.; Geasee, P.; Conradt, R. Glass-Ceramic Materials as Sealants for SOFC Applications. Mater. Werkst. 2002, 33, 363–366. [Google Scholar] [CrossRef]
- Shukla, A.; Jung, I.-H.; Decterov, S.A.; Pelton, A.D. Thermodynamic Evaluation and Optimization of the BaO-SiO2 and BaO-CaO-SiO2 Systems. Calphad 2018, 61, 140–147. [Google Scholar] [CrossRef]
- Wisniewski, W.; Thieme, C.; Müller, R.; Reinsch, S.; Groß-Barsnick, S.-M.; Rüssel, C. Oriented Surface Nucleation and Crystal Growth in a 18BaO·22CaO·60SiO2 Mol% Glass Used for SOFC Seals. CrystEngComm 2018, 20, 787–795. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Sharma, A.; Kundu, P.; Mahanty, S.; Basu, R.N. Development and Characterizations of BaO-CaO-Al2O3-SiO2 Glass-Ceramic Sealants for Intermediate Temperature Solid Oxide Fuel Cell Application. J. Non-Cryst. Solids 2008, 354, 4081–4088. [Google Scholar] [CrossRef]
- Li, B.; Bian, H.; Fang, Y. Microstructure, Thermal, Mechanical, and Dielectric Properties of BaO–CaO–Al2O3–B2O3 –SiO2 Glass-Ceramics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 275, 12032. [Google Scholar] [CrossRef]
- Kerstan, M.; Wueller, M.; Ruessel, C. Binary, Ternary and Quaternary Silicates of CaO, BaO and ZnO in High Thermal Expansion Seals for Solid Oxide Fuel Cells Studied by High-Temperature X-Ray Diffraction (HT-XRD). Mater. Res. Bull. 2011, 46, 2456–2463. [Google Scholar] [CrossRef]
- Du, K.; Yin, C.; Zou, Z.; Cheng, M.; Cai, Y.; Yang, J.; Zhang, M.; Lu, W.; Wang, S.; Lei, W. Correlation between Structural Characteristics and Microwave Dielectric Properties of Walstromite BaCa2M3O9 (M = Si, Ge) Ceramics. J. Am. Ceram. Soc. 2023, 106, 5822–5831. [Google Scholar] [CrossRef]
- Lei, W.; Zou, Z.; Chen, Z.; Ullah, B.; Zeb, A.; Lan, X.; Lu, W.; Fan, G.; Wang, X.; Wang, X. Controllable τf Value of Barium Silicate Microwave Dielectric Ceramics with Different Ba/Si Ratios. J. Am. Ceram. Soc. 2018, 101, 25–30. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for Solid Oxide Fuel Cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Evaristo, L.; Silveira, R.; Tissot, M.; Hippler, G.; Moulton, B.; Buchner, S. Effect of High Pressure in Barium Disilicate Glass Investigated by DTA and Raman Spectroscopy. Int. J. Appl. Glass Sci. 2023, 14, 240–246. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A Review of the Structures of Oxide Glasses by Raman Spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Furukawa, T.; Fox, K.E.; White, W.B. Raman Spectroscopic Investigation of the Structure of Silicate Glasses. III. Raman Intensities and Structural Units in Sodium Silicate Glasses. J. Chem. Phys. 1981, 75, 3226–3237. [Google Scholar] [CrossRef]
- Ross, S.; Welsch, A.-M.; Behrens, H. Lithium Conductivity in Glasses of the Li2O–Al2O3–SiO2 System. Phys. Chem. Chem. Phys. 2015, 17, 465–474. [Google Scholar] [CrossRef]
- Sun, T.; Zheng, C.; Zhang, F.; Zhang, J.; Han, J.; Xie, J.; He, J.; Jiang, H. Mixed CaO/MgO Effect on Microstructure, Mechanical Properties and Crystallization Behaviour of Li2O-Al2O3-SiO2-ZrO2-P2O5 Glass. J. Non-Cryst. Solids 2023, 616, 122457. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.K. High-Resolution Solid-State NMR Study of the Effect of Composition on Network Connectivity and Structural Disorder in Multi-Component Glasses in the Diopside and Jadeite Join: Implications for Structure of Andesitic Melts. Geochim. Cosmochim. Acta 2014, 147, 26–42. [Google Scholar] [CrossRef]
- Karlsson, S.; Mathew, R.; Ali, S.; Paemurru, M.; Anton, J.; Stevensson, B.; Edén, M. Mechanical, Thermal, and Structural Investigations of Chemically Strengthened Na2O–CaO–Al2O3–SiO2 Glasses. Front. Mater. 2022, 9, 953759. [Google Scholar] [CrossRef]
- Chen, L.; Long, Y.; Zhou, M.; Wang, H. Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag. Materials 2022, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, L.; Baghshahi, S.; Golikand, A.N.; Hamnabard, Z. Structure, Phase Formation, and Wetting Behavior of BaO–SiO2–B2O3 Based Glass–Ceramics as Sealants for Solid Oxide Fuel Cells. Ionics 2014, 20, 55–64. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. SiO2–Al2O3–CaO Glass-Ceramics: Effects of CaF2 on Crystallization, Microstructure and Properties. Ceram. Int. 2013, 39, 571–578. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Bubnova, R.S.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Filatov, S.K. Thermal Expansion and Structural Complexity of Ba Silicates with Tetrahedrally Coordinated Si Atoms. J. Solid State Chem. 2016, 235, 76–84. [Google Scholar] [CrossRef]
- Komatsu, T. Design and Control of Crystallization in Oxide Glasses. J. Non-Cryst. Solids 2015, 428, 156–175. [Google Scholar] [CrossRef]
- Yang, Y.; Tokunaga, H.; Ono, M.; Hayashi, K.; Mauro, J.C. Thermal Expansion of Silicate Glass-forming Systems at High Temperatures from Topological Pruning of Ring Structures. J. Am. Ceram. Soc. 2020, 103, 4256–4265. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Sampaio, D.V.; Silva, L.D.; Cunha, T.R.; Zanotto, E.D.; Pizani, P.S. The Origin of the Unusual DSC Peaks of Supercooled Barium Disilicate Liquid. CrystEngComm 2019, 21, 2768–2778. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Silva, L.D.; Sabino, S.R.F.; Evaristo, L.L.; Sampaio, D.V.; Buchner, S.; Serbena, F.C.; Pizani, P.S.; Zanotto, E.D. Unusual Crystallization Pathways Revealed in Six Barium Disilicate (BaSi2O5) Glasses. Ceram. Int. 2023, 49, 10852–10863. [Google Scholar] [CrossRef]
- Kerner, E.H. The Elastic and Thermo-Elastic Properties of Composite Media. Proc. Phys. Soc. Lond. Sect. B 1956, 69, 808–813. [Google Scholar] [CrossRef]
- Farid, A.A.; Ahmed, A.S.H.; Rodwell, M.J.W. A 27.5dBm EIRP D-Band Transmitter Module on a Ceramic Interposer. In Proceedings of the 2021 IEEE Radio Frequency Integrated Circuits Symposium (rfic), Atlanta, GA, USA, 7–9 June 2021; pp. 43–46. [Google Scholar]


| Materials | CTE (ppm/°C) | tanδ × 10−4 | εr @ 10 GHz | Reference |
|---|---|---|---|---|
| Dupont 951 | 5.8 | 15 | 7.5 | [50] |
| Kyocera GL771 | 12.3 | 38 | 5.2 | [50] |
| Kyocera GL773 | 11.7 | 25 | 5.8 | [50] |
| Heraeus CT708 | 10.6 | 30 | 6.4 | [6] |
| This work | 12.1 | 4.96 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Liang, Y.; Guan, Q.; Liu, F.; Ma, M.; Li, Y.; Liu, Z. Crystallization Phase Regulation of BaO-CaO-SiO2 Glass-Ceramics with High Thermal Expansion Coefficient. Materials 2025, 18, 1403. https://doi.org/10.3390/ma18071403
Hu H, Liang Y, Guan Q, Liu F, Ma M, Li Y, Liu Z. Crystallization Phase Regulation of BaO-CaO-SiO2 Glass-Ceramics with High Thermal Expansion Coefficient. Materials. 2025; 18(7):1403. https://doi.org/10.3390/ma18071403
Chicago/Turabian StyleHu, Haonan, Yongyuan Liang, Qifan Guan, Feng Liu, Mingsheng Ma, Yongxiang Li, and Zhifu Liu. 2025. "Crystallization Phase Regulation of BaO-CaO-SiO2 Glass-Ceramics with High Thermal Expansion Coefficient" Materials 18, no. 7: 1403. https://doi.org/10.3390/ma18071403
APA StyleHu, H., Liang, Y., Guan, Q., Liu, F., Ma, M., Li, Y., & Liu, Z. (2025). Crystallization Phase Regulation of BaO-CaO-SiO2 Glass-Ceramics with High Thermal Expansion Coefficient. Materials, 18(7), 1403. https://doi.org/10.3390/ma18071403






