Abstract
Regenerative techniques are increasingly applied in endodontic surgery, but different materials may have varying impacts on soft and hard tissue healing. This systematic review aims to evaluate the effectiveness of autologous platelet concentrates (APCs) in clinical and radiographic healing after endodontic surgery. The data for this systematic review were processed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for improving the reporting of systematic reviews and meta-analyses. A literature search was conducted until October 2023 on PubMed, Scopus, and Cochrane Databases. Randomized controlled trials and controlled clinical trials addressing the use of APCs in patients who presented persistent periapical lesions and needed periapical surgery were included. Dual publications, narrative reviews, systematic reviews, case series, questionnaires, animal studies, case reports, letters to the editor, in vitro studies, and abstracts were excluded. In total, the search resulted in 14 papers. Clinical and radiographical findings were reported, showing that when APCs were used, patients exhibited less pain and swelling and a greater reduction of apical radiolucency after 12 months follow-up on average. However, the moderate/high risk of bias of included studies and their high heterogeneity, do not allow one to draw definitive conclusions on the effectiveness of APC after endodontic surgery.
1. Introduction
The necrosis of pulp tissue not adequately treated can lead to periapical periodontitis, which is the complex of inflammatory pathologies of the periapical tissues of the tooth (alveolar bone and periodontal ligament) [,]. Endodontic surgery consists of the reduction or elimination of persistent periapical pathology when primary orthograde endodontics or retreatment have failed or are not feasible []. Historically, conventional endodontic surgery, which involved larger surgical access and less sophisticated instrumentation, often presented challenges in achieving predictable outcomes. Nevertheless, with the advent of microsurgical techniques and the integration of cutting-edge technologies, the field of endodontics has evolved significantly []. However, considering that endodontic surgery is linked to a less predictable prognosis compared to orthograde endodontic treatment [] and even a single tooth can be strategic in the whole oral prosthetic rehabilitation, the possibility of accelerating bone regeneration in periapical surgical defects could be of great interest to the clinician to proceed earlier with permanent rehabilitation.
The following several methods have been used to promote bone regeneration and soft tissue healing in periapical defects as an adjunct to endodontic surgery: barrier membranes, bone grafting materials, bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), and enamel matrix proteins (EMD) []. In particular, non-resorbable expanded polytetrafluoroethylene (e-PTFE) and bioabsorbable collagen have been commonly used as they can prevent the apical migration of epithelial cells and facilitate the repopulation of the bony defect by osteogenic cells; furthermore, bone grafts can preserve the necessary space for new bone formation, supply essential osteogenic cells to promote bone growth (osteogenic effect), stimulate host cells to regenerate lost bone tissue (osteoinductive effect), and act as supportive frameworks (osteoconductive effect). Some reviews have supported the use of regenerative techniques in endodontic surgery [,], but others have reached negative conclusions [,]. So, the effectiveness of their application is still questionable and remains a subject of ongoing investigation and debate.
In recent years, autologous platelet concentrates (APCs) have been introduced as an autologous grafting material in several different fields of dentistry [,].
APCs are autologous blood products used in several medical and dental fields to increase soft and hard tissue healing rate [].
Several technical procedures have been developed to obtain different platelet concentrates with variable yield of platelets and cellular components [].
APCs can be classified into platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) based on distinct preparation processes. PRP, as the first-generation platelet concentrate, is plasma with a high platelet concentration obtained through specific centrifugation of fresh whole blood. PRF, on the other hand, as the second-generation platelet concentrate, is characterized by strong fibrin polymerization obtained during the centrifugation process. This procedure requires blood collection without an anticoagulant and immediate centrifugation for the formation of a fibrin clot, which includes not only platelets but also leukocytes [,].
APCs can be thought of as a reservoir of growth factors as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), etc., which have been involved in cell proliferation, chemotaxis, and extracellular matrix production/angiogenesis [,], fibroblast growth factors (FGF) 1 and 2 and vascular endothelial growth factor (VEGF) which play critical roles in the hemostasis, proliferative, and remodeling phases of wound healing [,]. Platelet degranulation also leads to the release of cytokine and chemokines, such as interleukin (IL)-1β, C-C motif ligand 5 (CCL5), IL-8, and macro- phage inflammatory protein (MIP)-1α, which contribute to the healing process []. Moreover, the use of APCs as an adjunct in oral surgery was reported to add beneficial effects in terms of pain relief and an improvement of postoperative quality of life [].
The application of APCs in endodontic surgery has already been described in recent clinical cases and in a randomized clinical trial in the specific field of treatment of apicomarginal defects []. However, their application in this field is still questionable and the benefits they provide to both surgeon and patient have been reported to be moderate and remain controversial. Moreover, few systematic reviews, including all types of APCs and different methods of analysis, were published in the literature.
Thus, the aim of this systematic review was to evaluate the effectiveness of APCs in terms of clinical outcomes and radiographic healing in patients undergoing apical surgery and evaluate whether the design of the primary studies may affect the results. The null hypotheses in most of the articles included in the above review predicted that periapical surgical defects filled with APCs would require the same healing time as sites treated with conventional surgical techniques and that patients would experience the same postoperative discomfort with or without application of the APC.
2. Methods
2.1. Focused Question
The data for this systematic review were processed following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines []. According to the PICO criteria (P: population, I: intervention, C: comparison, O: outcome) statement, this review aimed to answer the following question: “Do autologous platelet concentrates provide benefits in terms of reduced postoperative discomfort and pain (Clinical Outcomes) and accelerated radiographic healing (Radiographic Outcomes) in patients (Population) undergoing endodontic procedures (Intervention)?” Physiologic healing of the surgical site provided by the blood clot or biomaterials was used as a comparison/control. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with number CRD42023401240.
2.2. Search Strategy
To prepare the study protocol, a pilot search was performed on the PubMed search platform, followed by a systematic evaluation of potentially suitable studies for inclusion in the study. At the end of the pilot search, data extraction forms were drafted. The literature search was conducted by consulting three electronic databases (PubMed, Scopus, and The Cochrane Library) until October 2023, using keyword combinations and MeSH terms, according to the database rules (Table 1).

Table 1.
Search strategy.
The manual search also included a search from the following journals: Giornale Italiano di Endodonzia, Journal of Endodontics, Journal of Clinical Periodontology, International Journal of Periodontics and Restorative Dentistry, Clinical Oral Investigation, Clinical Oral Implant Research, International Surgery, Implant Dentistry, Quintessence International, Journal of Prosthodontic, International Journal of Prosthodontics, European Journal of Oral Implantology, Journal of Oral and Maxillofacial Surgery. In addition, an attempt was made to search the grey literature by searching for potentially suitable studies among conference abstracts published on WoS and Scopus and scientific dental conference databases. Two authors (AA, RG) searched the articles independently and resolved disagreements by discussing their search results.
2.3. Inclusion and Exclusion Criteria
Studies were selected based on the following inclusion criteria:
- The study was randomized controlled trials (RCTs) or clinical controlled trials (CCTs);
- Patients presented with persistent periapical lesions and needed periapical surgery;
- APCs were utilized in the intervention group(s);
- Physiologic healing or regenerative materials alone or combination of APCs and regenerative materials instead of APC were utilized in the control group;
- Reported clinical or radiographical outcomes or both.
Studies were excluded based on the following exclusion criteria:
- Dual publications, narrative reviews, systematic reviews, case series, questionnaires, animal studies, case reports, letters to the editor, in vitro studies, abstracts;
- Outcomes of interest were not extractable;
- Articles written in any language other than English;
- Full text not available.
2.4. Selecting and Extracting Data from Studies
The selection process is reported in Figure 1 (PRISMA flow diagram). The titles and abstracts, when available, of all articles identified through electronic searches were independently analyzed by two authors (AA, RG). For studies that appeared to meet the inclusion criteria, or for those for which there was insufficient data in the title and abstract, the entire article was consulted. Full articles obtained from all search methods, electronic and otherwise, were independently evaluated by two authors (AA, RG) to determine whether the studies met the inclusion criteria. Discrepancies were resolved by discussion, and if resolution was not possible, a third review author was consulted (AV). The main reasons for the exclusions of title, abstract, and full text were the following: study design (case report/case series), animal studies, regenerative technique, hemostatic agents instead of APCs, and full text not available. All studies that met the inclusion criteria were then subjected to risk-of-bias assessment and data extraction. Data were extracted by two review authors independently (AV, RG), using specially drafted data extraction forms, and any discrepancies were resolved with discussion. The following data were recorded for each of the following articles, as shown in Table 2 (characteristics of the included studies): author of the study, year of publication and country in which the research was carried out, type of study, sample size, age and sex of the patients involved in the study, diagnosis, type of intervention performed, control group analyzed in each study (when present), follow-up, diagnostic methodology, and clinical and radiographic results.
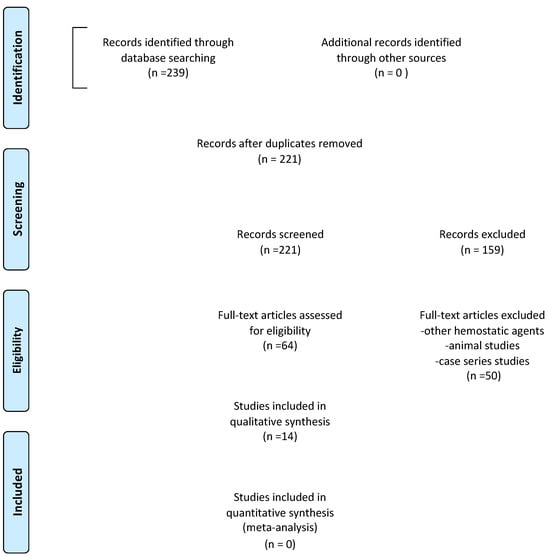
Figure 1.
Prisma flow diagram.

Table 2.
Study characteristics: CCT, controlled clinical trial; RCT, randomized clinical trial; PRF, platelet-rich fibrin; BG, bioactive glass; PRGF, plasma rich in growth factor.
2.5. Methodological Quality of Included Reviews
Two authors (AA and RG) independently assessed the studies in terms of inclusion criteria, relevance, eligibility, and risk of bias following the recommendations of the Joanna Briggs Institute Critical Appraisal tool (JBI) []. Any disagreement was solved by consensus between reviewers and statisticians (PD). The JBI does not provide a range of scores that indicate the overall quality but considering the relative importance of each domain and its potential impact on the study results and interpreting the domain scores in the context of the study, the studies were classified as having a low risk of bias if most domains score “Yes”, a moderate risk if some domains are rated “No/Unclear”, and a high risk if multiple domains have significant bias or are rated “No”.
3. Results
3.1. Search Results
From the initial search, 239 studies were identified from electronic databases (PubMed, Scopus, and The Cochrane Library), while no studies were selected through other sources. After removing duplicates, the titles, and abstracts of 221 articles were analyzed. Of those selected, 159 articles were deemed unsuitable by title and abstract and were therefore excluded. The remaining 64 articles were deemed eligible according to the pre-established eligibility criteria. On examining the full text of these articles, case reports, case series, systematic reviews, and narrative reviews were excluded, and finally 14 articles were then included in the review. A total of 14 studies were included in this systematic review, including 9 randomized controlled trials [,,,,,,,,] and 5 clinical controlled trials [,,,,]. Some of them exclusively evaluated the clinical outcomes as postoperative discomfort, patient quality of life, infection, pain, postoperative swelling, and presence/absence of bleeding [,,,]. Others only analyzed radiographic healing of periapical bone tissue [,,,]. Only six studies evaluated both outcomes [,,,,,]. A meta-analysis was not possible due to the heterogeneity of statistical measures and outcomes used, and the poor statistical methodology quality of some studies.
3.2. Summary of Clinical Findings
In the study of Del Fabbro et al. [], the addition of the liquid form and clot of PRGF after endodontic surgery gave a significant reduction in pain and swelling, fewer postoperative analgesics taken, and improved functional activities (mouth opening, chewing, speaking, sleeping, daily routines, and work) compared with patients in the control group. Similar results were obtained by Taschieri et al. [] in terms of less pain and swelling when PRGF was used in situations of Schneiderian membrane perforation that occurred during endodontic surgery. In Soto-Peñaloza et al. [], 50 apical lesions of the maxillary upper jaw were treated with and without A-PRF as an adjunctive treatment and showed that pain perception and quality of life (functional limitations and other symptoms) were significantly lower in the A-PRF test group. In contrast, Meschi et al. [] showed no statistically significant difference (p ≤ 0.05) between the test and control groups in terms of VAS, occurrence of pain symptoms, impairment of daily activities, and medication use, daily in the 7 days following endodontic surgery, using L-PRF clot. In the study by Singh et al. [], pain, mobility, swelling, and the vitality of adjacent teeth after apical endodontic surgery were evaluated between the following three groups: hydroxyapatite granules, CERAMENT bone, and PRF. PRF reduced pain, swelling, tooth mobility, and bleeding compared with hydroxyapatite and CERAMENT. Similarly, Angerame et al. [] showed that patients in the PRF-treated group (test group) experienced less pain in the postoperative 2–6 h and developed less edema, which was always limited and intraoral. Finally, Dhiman et al. [] and Goyal et al. [] analyzed periodontal parameters, including pocket depth (PD), clinical attachment level (CAL), and gingival marginal position (GMP). Dhiman et al. [] revealed that only pocket depth showed a statistically significant reduction in the test group treated with PRF. On the other hand, the study by Goyal et al. [] found that PRP showed a similar reduction in periodontal pocket depth, clinical attachment level, and gingival margin position in comparison with collagen membrane and PRP + collagen sponge group.
In Yahata et al. [], regarding the VAS scores, there was no significant difference between the CGF and control groups preoperatively and at all postoperative appointments.
Finally, in Thakur et al. [], regarding the parameters used for predicting quality of life, patients in the PRF Medium group reported significantly less swelling on the first, second, and third days postoperatively and less than average second, third, and fourth days postoperatively. There were significant differences in postoperative pain intensity (based on mean VAS scores) observed on the first, second, third, and fourth days postoperatively, with patients treated with PRF Medium showing consistently less pain.
3.3. Summary of Radiographic Findings
The radiographic healing of periapical bone tissue was detected by using different diagnostic methods. Meschi et al. [], Ahmed et al. [], and Parihk et al. [], used cone beam computed tomography (CBCT). Specifically, Meschi et al. [] found no improvement in bone healing when L-PRF was combined with a root surgical treatment compared to the same treatment without L-PRF; Parihk et al. [] found that the PRP-treated site showed better healing as early as 8 weeks, and CBCT after 1 year showed an increase in periapical bone density in relation to the PRP-treated site. Ahmed et al. [] found a significant volumetric reduction of a periapical lesion after 1 year as well when PRF was used alone. On the other hand, Monga et al. [], Dhiman et al. [], Goyal et al. [], and Angerame et al. [] performed evaluations of radiographic outcomes via a digital X-ray system using periapical radiographs. Specifically, Monga et al. [] conducted a study of 30 patients with periapical radiolucency in maxillary anterior teeth. After 9 months, a significantly higher radiographic healing rate was observed in group PRF+MTA (82.36%). In Dhiman et al. [], no significant differences were observed in the size of periapical lesions at 12-month follow-up between PRF group and spontaneous healing.
In Angerame et al. [], periapical radiographs were taken before and after surgery and at each follow-up visit. The study showed that at recalls 2 and 3 months after surgery, the test group treated with PRF showed significantly better periapical radiographic healing scores than the control group. Thereafter, the periapical healing scores of the control and test groups were similar, and statistical analysis showed no significant differences.
In Yahata et al. [], the total success rate assessed using periapical radiography at 12 months was 91.7%. Although no significant difference was observed in the success rates between the CGF and control groups evaluated by periapical radiography and CBCT, the lesion volume reduction rate was 75.6% in the CGF group and 61.0% in the control group, with a significantly higher reduction rate in the former.
Finally, in Thakur et al. [], significant improvements were seen in the size of the periapical lesion (SPL), and the volume of the periapical lesion (VPL) at the 12-month follow-up when compared with baseline in both groups. Specifically, buccal bone formation was observed in 26% of cases in the PRF Medium group and in 20% of cases in the PRF High group, with no significant difference between the groups.
3.4. Risk of Bias Assessment
The results of the bias risk assessment for randomized controlled trials (RCTs) and controlled clinical studies included in the review are shown in Table 3 and Table 4, respectively. For RCTs, five studies had a low risk of overall bias, three moderate, and only one high. The most perplexing domains were as follows: “Were participants blind to treatment assignment?” and “Were those delivering treatment blind to treatment assignment?” For CCTs, three had a moderate risk of overall bias and two high.

Table 3.
Assessment of quality and risk of bias for randomized controlled trials (RCT) included in the systematic review. Each domain was satisfied (yes), not satisfied (no), unclear, or not assessable (N/A) according to the Joanna Briggs Institute Critical Appraisal tool.

Table 4.
Assessment of quality and risk of bias for quasi-experimental studies included in the systematic review. Each domain was satisfied (yes), not satisfied (no), unclear, or not applicable (N/A) according to the Joanna Briggs Institute Critical Appraisal tool.
4. Discussion
The present systematic review aimed to assess the effectiveness of APCs in clinical and radiographic healing after endodontic surgery. The findings revealed that when APCs were used, patients showed a significant reduction in postoperative pain and swelling and a greater reduction in apical radiolucency after 12 months of follow-up on average. In this review, it was observed that Del Fabbro, Soto-Penaloza, and Taschieri [,,] reported more significant improvements in clinical outcome measures, including VAS (visual analogue scale) pain scores, functional outcomes, and QoL (quality of life) scores, which were not corroborated by the findings of the study by Meschi et al. []. This discrepancy may be attributed to the utilization of distinct autologous platelet concentrates (PRGF and A-PRF in the first three studies and L-PRF in the last one) and variations in the centrifugation and preparation methods employed, which could potentially influence the biological properties and molecular characteristics of the platelet concentrate. The proposed mechanisms underlying the alleviation of pain and reduction of inflammation induced by platelet products are linked to the local reduction of inflammatory factors, such as phospholipase A2 (PLA2), interleukin-1α (IL-1α), IL-1β, IL-6, IL-8, tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2). Furthermore, the release of growth factors and cytokines from platelet α granules, which have local anti-inflammatory, anti-apoptotic, and analgesic effects (e.g., via cytokines like IL-4 or IL-10), as well as their involvement in extracellular matrix production (ECM) and neural regeneration, are believed to be the primary mechanisms responsible for the benefits of APCs [].
In general, while PRGF, A-PRF, and L-PRF have the potential to contribute to pain relief through their growth factors, anti-inflammatory properties, and tissue regeneration abilities, the differences in their composition and preparation methods can result in variations in their antinociceptive effects. The choice of platelet concentrate may depend on the specific clinical situation and the desired therapeutic outcome.
In addition, pain, swelling, and, in general, the extent of postoperative discomfort, are influenced by other several factors, including the complexity of the procedure, the time of surgery, the tissue trauma, the patient’s overall health, and the quality of postoperative care. In this regard, microsurgery in endodontics has revolutionized the field, offering precise and minimally invasive techniques that could be more tolerable for patients than the conventional ones and enhance the healing and success rates of complex endodontic procedures with respect to the past. Moreover, the use of piezosurgery reported by Ahmed et al. [] during the procedure, may have an adjunctive role in reducing postoperative swelling and pain []; thus, the role of APCs in reducing pain, inflammation, and swelling may be partially masked if a microsurgical approach was used.
Otherwise, contradictory results were observed in this review about the effect of APCs on reduction in apical radiolucency. The osteogenic potential of autologous platelet concentrates has garnered significant attention in the field of regenerative medicine, particularly in oral and maxillofacial surgery, orthopedics, and implantology, especially in post-extraction socket healing [,], the osseointegration of dental implants, sinus lift procedures, and the healing of periodontal bone defects [,].
The osteogenic potential of these products has been widely demonstrated. Platelet concentrates contain growth factors like bone morphogenetic proteins (BMPs), Platelet-Derived Growth Factor (PDGF), and Transforming Growth Factor-beta (TGF-β), which can activate and accelerate the activity of osteoblasts. These factors play a vital role in bone regeneration and repair. Moreover, they improve the migration of various cells involved in bone regeneration, including osteoblasts, osteoclasts (cells responsible for bone resorption), and mesenchymal stem cells (MSCs), which are essential for the remodeling of bone tissue and the establishment of a well-vascularized bone matrix [,,].
Nonetheless, the clinical effectiveness of APCs in bone regeneration procedures remains a subject of debate due to varying outcomes documented in various clinical applications [,,,], which is also substantiated by the results of this review. Bone healing is a complex and highly orchestrated biological phenomenon that involves a sequence of events that lead to the regeneration and restoration of damaged or lost bone tissue. In this review, the following several methods and tools in different period time are used to measure periapical bone healing: CBCT or periapical radiographs at 3 months [], 9 months [], or 1-year.
Although periapical radiographs are one of the most used methods to evaluate bone healing, the CBCT has reported as a reliable method for monitoring reduced osseous lesion size and volume due to its three-dimensional measurement. Moreover, CBCT may be a valuable tool in conducting follow-ups in endodontics, even if the amount of ionizing radiation to which the patient is exposed is greater compared to single periapical radiographs [,]. In many cases, a combination of several assessment methods may be employed to provide a comprehensive evaluation of bone healing progress.
The influence of different follow-up intervals on bone healing can provide valuable insights into the healing process. In the first weeks of follow-up, radiographic imaging can reveal the development of callus formation, which is a key indicator of ongoing bone healing. In the long-term follow-up (months to a year). radiographic imaging assesses the quality and density of the healed matured bone. The different times of follow-up may influence the effectiveness of treatment. Moreover, in some cases, the use of biomaterials in adjunct to APCs may affect the reliability of radiographic evaluation []. The decision to use APCs alone or in combination with biomaterials is context-specific and depends on factors such as the type of tissue being treated, the size of the lesion, and the desired therapeutic goals. Since most included studies have demonstrated that graft materials showed no additional benefit when compared to APCs alone [,], a self-derived source of regenerative agents is advantageous in that it reduces the risk of immune rejection or adverse reactions. Moreover, where the body’s natural regenerative mechanisms are sufficient, like in four-wall-sided defects of apical lesions, APCs alone may be suitable. However, when more extensive tissue repair and regeneration are required, the strategic combination of APCs with biomaterials may probably offer a superior approach [].
Indeed, contradictory results about soft and hard tissue healing between different APCs may be explained according to their composition and preparation methods. For example, PRF has been shown to be significantly better in promoting soft tissue healing and faster regeneration of bone after third molar extraction in comparison to PRP, and this could be attributed to simpler preparation protocols of PRF over PRP and the ability of PRF to release growth factors in a controlled way [].
4.1. Risk-of-Bias Judgement of Eligible Studies
For RCTs, five studies had a low risk of overall bias, three moderate, and only one high. The domain that posed the greatest challenge was ensuring that patients remained unaware of their treatment allocation, and also whether those responsible for administering the treatment were similarly blinded to the treatment assignments. This difficulty arose not due to a methodological error but was a result of practical constraints since the venipuncture made for APCs preparation identified the intervention, and ethical reasons precluded the drawing of blood in both groups. Another risk to be highlighted is the blinding of outcome assessors when evaluating periapical radiography or CBCT, especially when a radiopaque bone graft serves as a control. In such instances, the presence of the bone graft may potentially obscure the assessment of bone healing and lead to an underestimation of lesion size reduction and bone density compared to the control group.
4.2. Limitations
Although the findings obtained from the present review suggest that the use of APCs during the endodontic surgical procedure is related to lower levels of pain, swelling, and swelling in the early post-surgical period, as well as to a reduction in apical radiolucency in the first 12 months of follow-up, it is necessary to reiterate its limitations. Substantial variation in clinical predictability and efficacy could be related to several variables, such as study design, defect type and location, type of surgical approach, APC preparation protocol, and patient response. In the included studies, the following different APCs prepared by different protocols were found: Angerame et al. [] made use of PRF (2500 rpm for 10 min), Del Fabbro M et al. [] instead made use of PRGF (3200 rpm for 8 min), Goyal et al. [] still used PRP (2400 rpm for 10 min followed by 3600 rpm for 15 min) while Soto-Penaloza [] made use of A-PRF (1300 rpm for 8 min). This did not allow for a uniform and homogeneous evaluation of the data, even though all APCs individually demonstrated improved clinical outcomes. This heterogeneity was also found in the surgical procedure used in terms of the type of surgical access, execution of the bone breach, cutting of the root apex, preparation of the apical cavity, and material used for retrograde root filling. Unfortunately, the high heterogeneity of the studies in terms of APCs and surgical protocol used does not allow for drawing definitive conclusions and fails to provide valid clinical guidelines for the use of APCs in surgical endodontics.
4.3. Prospective
Employing regenerative techniques with APCs in the clinical practice of endodontic surgery holds the potential to enhance the recovery of periapical lesions. In the realm of clinical research, forthcoming trials should carefully consider the influence of lesion type and size on the effectiveness of these products. However, it is recommended not to use bone graft materials in combination with APCs, as they did not show any significant difference and offered only extra financial cost to the patient. Additionally, researchers may explore different combinations of APCs to optimize their impact on wound healing after endodontic surgery and conduct studies with more homogeneous techniques and outcome measurements that can be compared and subjected to meta-analysis.
5. Conclusions
Within its limits, the present systematic review showed the effectiveness of APCs in reducing the pain and swelling in the early post-surgical period, as well as an improvement in daily activities and quality of life in patients undergoing endodontic surgery. However, the high heterogeneity of the studies in terms of APCs and surgical protocol used does not allow for drawing definitive conclusions. Additional research with expanded sample sizes, extended follow-up periods, and standardized protocols is necessary to gain a more comprehensive understanding of the role of autologous platelet concentrates in endodontic surgery.
Author Contributions
Conceptualization, R.G. and G.S.; methodology, P.D.; software, P.D.; validation, R.G., M.C. and G.S.; formal analysis, M.C.; investigation, A.A.; resources, A.V.; data curation, A.E.d.L. and A.A.; writing—original draft preparation, A.A. and A.E.d.L.; writing—review and editing, R.G., A.A. and A.V.; visualization, A.V.; supervision, R.G.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Setzer, M.C.; Kohli, M.R.; Shah, S.B. Outcome of endodontic surgery: A meta-analysis of the literature. Part 2: Comparison of endodontic microsurgical techniques with and without the use of higher magnification. J. Endod. 2012, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Pisano, M.; Caggiano, M.; Bhasin, P.; Lo Giudice, R.; Abdellatif, D. Root Canal Cleaning after Different Irrigation Techniques: An Ex Vivo Analysis. Medicina 2022, 58, 193. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Gulabivala, K. Factors that influence the outcomes of surgical endodontic treatment. Int. Endod. J. 2023, 56 (Suppl. S2), 116–139. [Google Scholar] [CrossRef] [PubMed]
- Floratos, S.; Kim, S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent. Clin. N. Am. 2017, 61, 81–91. [Google Scholar] [CrossRef]
- Elemam, R.F.; Pretty, I. Comparison of the success rate of endodontic treatment and implant treatment. ISRN Dent. 2011, 2011, 640509. [Google Scholar] [CrossRef]
- Liu, T.J.; Zhou, J.N.; Guo, L.H. Impact of different regenerative techniques and materials on the healing outcome of endodontic surgery: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 536–555. [Google Scholar] [CrossRef]
- Sanchez-Torres, A.; Sanchez-Garces, M.A.; Gay-Escoda, C. Materials and prognostic factors of bone regeneration in periapical surgery: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e419–e425. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, X.; Yang, J.; Jiang, H.; Yan, P. The effect of regeneration techniques on periapical surgery with different protocols for different lesion types: A meta-analysis. J. Oral. Maxillofac. Surg. 2016, 74, 239–246. [Google Scholar] [CrossRef]
- von Arx, T.; Alsaeed, M. The use of regenerative techniques in apical surgery: A literature review. Saudi Dent. J. 2011, 23, 113–127. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Elkabbany, A.; Del Fabbro, M.; von Arx, T. Guided tissue regeneration using a barrier mem- brane in endodontic surgery. Swiss Dent. J. 2016, 126, 13–25. [Google Scholar]
- Rohilla, R.; Tewari, S.; Nayyar, A.S. Efficacy of Guided Tissue Regeneration (GTR) membranes in the healing of apico-marginal defects: A prospective, controlled clinical trial. Int. J. Orofac. Res. 2017, 2, 11–17. [Google Scholar] [CrossRef]
- Kurmanalina, M.; Uraz, R.; Skaģers, A.; Locs, J.; Taganiyazova, A.; Omargali, A. Radiological evaluation of endodontic treatment of chronic apical periodontitis using biphasic calcium phosphate biomaterial. Eurasian J. Anal. Chem. 2018, 13, em54. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel applications of platelet concentrates in tissue regeneration (Review). Exp. Ther. Med. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Huang, Z.; Zheng, X. Impact of autologous platelet concentrates on wound area reduction: A meta-analysis of randomized controlled trials. Int. Wound J. 2023. [Google Scholar] [CrossRef]
- Pensato, R.; Al-Amer, R.; La Padula, S. Protocol for Obtaining Platelet-Rich Plasma (PRP), Platelet-Poor Plasma (PPP), and Thrombin for Autologous use. Aesthetic Plast. Surg. 2023. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral. Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Gasparro, R.; Qorri, E.; Valletta, A.; Masucci, M.; Sammartino, P.; Amato, A.; Marenzi, G. Non-transfusional hemocomponents: From biology to the clinic—A literature review. Bioengineering 2018, 5, 27. [Google Scholar] [CrossRef]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral. Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.R.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-rich plasma counteracts detrimental effect of high-glucose concentrations on mesenchymal stem cells from Bichat fat pad. J. Tissue Eng. Regen. Med. 2020, 14, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Cairns, M. Autologous Platelet Concentrates to improve post extraction outcomes. Evid. Based Dent. 2018, 19, 118–119. [Google Scholar] [CrossRef]
- Yan, L.; Lin, J.; Yang, L.; He, S.; Tan, X.; Huang, D. Clinical Effect Evaluation of Concentrated Growth Factor in Endodontic Microsurgery: A Cross-Sectional Study. J. Endod. 2023, 49, 836–845. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 10, 28–55. [Google Scholar]
- Ahmed, G.M.; Saif, N.; Nageh, M.; Elbaz, A. CBCT volumetric evaluation of bone healing after endodontic microsurgery using platelet-rich fibrin (PRF). ENDO 2018, 12, 241–248. [Google Scholar]
- Angerame, D.; De Biasi, M.; Kastrioti, I.; Franco, V.; Castaldo, A.; Maglione, M. Application of platelet-rich fibrin in endodontic surgery: A pilot study. Giornale Italiano di Endodonzia 2015, 29, 51–57. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Ceresoli, V.; Lolato, A.; Taschieri, S. Effect of platelet concentrates on quality of life after periradicular surgery: A randomized clinical study. J. Endod. 2012, 38, 733–739. [Google Scholar] [CrossRef]
- Dhiman, M.; Kumar, S.; Duhan, J.; Sangwan, P.; Tewari, S. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J. Endod. 2015, 41, 985–991. [Google Scholar] [CrossRef]
- Goyal, B.; Tewari, S.; Duhan, J.; Sehgal, P.K. Comparative evaluation of platelet-rich plasma and guided tissue regeneration membrane in the healing of apicomarginal defects: A clinical study. J. Endod. 2011, 37, 773–780. [Google Scholar] [CrossRef]
- Meschi, N.; Fieuws, S.; Vanhoenacker, A.; Strijbos, O.; Van der Veken, D.; Politis, C.; Lambrechts, P. Root-end surgery with leucocyte- and platelet-rich fibrin and an occlusive membrane: A randomized controlled clinical trial on patients’ quality of life. Clin. Oral. Investig. 2018, 22, 2401–2411. [Google Scholar] [CrossRef]
- Meschi, N.; Vanhoenacker, A.; Strijbos, O.; Camargo Dos Santos, B.; Rubbers, E.; Peeters, V.; Curvers, F.; Van Mierlo, M.; Geukens, A.; Fieuws, S.; et al. Multi-modular bone healing assessment in a randomized controlled clinical trial of root-end surgery with the use of leukocyte- and platelet-rich fibrin and an occlusive membrane. Clin. Oral. Investig. 2020, 24, 4439–4453. [Google Scholar] [CrossRef]
- Monga, P.; Grover, R.; Mahajan, P.; Keshav, V.; Singh, N.; Singh, G. A comparative clinical study to evaluate the healing of large periapical lesions using platelet-rich fibrin and hydroxyapatite. Endodontology 2016, 28, 27–31. [Google Scholar] [CrossRef]
- Parikh, B.; Navin, S.; Vaishali, P. A comparative evaluation of healing with a computed tomography scan of bilateral periapical lesions treated with and without the use of platelet-rich plasma. Indian J. Dent. Res. 2011, 22, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ismail, P.M.S.; Kambli, V.; Kumar, R.; Singh, K.D.; Kochhar, A.S.; Babaji, P. Evaluation of Hydroxyapatite Granules, CERAMENT™, and Platelet-rich Fibrin in the Management of Endodontic Apical Surgery. J. Contemp. Dent. Pract. 2020, 21, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Soto-Peñaloza, D.; Peñarrocha-Diago, M.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; Tarazona-Alvarez, B.; Peñarrocha-Oltra, D. Pain and quality of life after endodontic surgery with or without advanced platelet-rich fibrin membrane application: A randomized clinical trial. Clin. Oral. Investig. 2020, 24, 1727–1738. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Tsesis, I.; Del Fabbro, M. Impact of the use of plasma rich in growth factors (PRGF) on the quality of life of patients treated with endodontic surgery when a perforation of sinus membrane occurred. A comparative study. Oral. Maxillofac. Surg. 2014, 18, 43–52. [Google Scholar] [CrossRef]
- Thakur, V.; Mittal, S.; Tewari, S.; Kamboj, M.; Duhan, J.; Sangwan, P.; Kumar, V.; Gupta, A. Comparative histological evaluation of two PRF formulations (PRF High and PRF Medium) on quality of life and healing outcome of apicomarginal defects: A randomized clinical trial. J. Cranio-Maxillofac. Surg. 2023, 51, 166–177. [Google Scholar] [CrossRef]
- Yahata, Y.; Handa, K.; Ohkura, N.; Okamoto, M.; Ohshima, J.; Itoh, S.; Kawashima, N.; Tanaka, T.; Sato, N.; Noiri, Y.; et al. Autologous concentrated growth factor mediated accelerated bone healing in root-end microsurgery: A multicenter randomized clinical trial. Regen. Ther. 2023, 24, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. JBI Man. Evid. Synth. 2020, 5, 217–269. [Google Scholar]
- Al-Hamed, F.S.; Mahri, M.; Al-Waeli, H.; Torres, J.; Badran, Z.; Tamimi, F. Regenerative Effect of Platelet Concentrates in Oral and Craniofacial Regeneration. Front. Cardiovasc. Med. 2019, 6, 126. [Google Scholar] [CrossRef]
- Pavlíková, G.; Foltán, R.; Horká, M.; Hanzelka, T.; Borunská, H.; Sedý, J. Piezosurgery in oral and maxillofacial surgery. Int. J. Oral. Maxillofac. Surg. 2011, 40, 451–457. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ní Ríordáin, R. Autologous platelet concentrates in oral surgery: Protocols, properties, and clinical applications. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of Postextraction Sockets Preserved with Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J. Oral. Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Gasparro, R.; Adamo, D.; Masucci, M.; Sammartino, G.; Mignogna, M.D. Use of injectable platelet-rich fibrin in the treatment of plasma cell mucositis of the oral cavity refractory to corticosteroid therapy: A case report. Dermatol. Ther. 2019, 32, e13062. [Google Scholar] [CrossRef]
- Gasparro, R.; Sammartino, G.; Mariniello, M.; di Lauro, E.A.; Spagnuolo, G.; Marenzi, G. Treatment of periodontal pockets at the distal aspect of mandibular second molar after surgical removal of impacted third molar and application of L-PRF: A split-mouth randomized clinical trial. Quintessence Int. 2020, 51, 204–211. [Google Scholar]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1998, 85, 638–642. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.; Hafner, G. Growth factor levels in the platelet rich plasma produced by 2 different methods: Curasan PRP kit versus PCCS PRP system. Int. J. Oral. Maxillofac. Implant. 2002, 17, 184–190. [Google Scholar]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Nikolidakis, D.; Mulder, J.; Jansen, J.A.; Creugers, N.H. Effect of platelet- rich plasma on bone regeneration in dentistry: A systematic review. Clin. Oral. Implants Res. 2008, 19, 539–545. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Markou, N.; Pepelassi, E.; Nikolidakis, D. The adjunctive use of platelet- rich plasma in the therapy of periodontal intraosseous defects: A systematic review. J. Periodontal Res. 2010, 45, 428–443. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S. Is autologous platelet concentrate beneficial for post-extraction socket healing? a systematic review. Int. J. Oral. Maxillofac. Surg. 2011, 40, 891–900. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral. Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Ahlowalia, M.S.; Patel, S.; Anwar, H.M.; Cama, G.; Austin, R.S.; Wilson, R.; Mannocci, F. Accuracy of CBCT for volumetric measurement of simulated periapical lesions. Int. Endod. J. 2013, 46, 538–546. [Google Scholar] [CrossRef]
- Kanagasingam, S.; Lim, C.X.; Yong, C.P.; Mannocci, F.; Patel, S. Diagnostic accuracy of periapical radiography and cone beam computed tomography in detecting apical periodontitis using histopathological findings as a reference standard. Int. Endod. J. 2017, 50, 417–426. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Kolerman, R.; Mangani, L.; Ivanova, V.; Zlatev, S. Autologous Platelet Concentrates (APCs) for Hard Tissue Regeneration in Oral Implantology, Sinus Floor Elevation, Peri-Implantitis, Socket Preservation, and Medication-Related Osteonecrosis of the Jaw (MRONJ): A Literature Review. Biology 2022, 11, 1254. [Google Scholar] [CrossRef]
- Işık, G.; Özden Yüce, M.; Koçak-Topbaş, N.; Günbay, T. Guided bone regeneration simultaneous with implant placement using bovine-derived xenograft with and without liquid platelet-rich fibrin: A randomized controlled clinical trial. Clin. Oral. Investig. 2021, 25, 5563–5575. [Google Scholar] [CrossRef]
- Yelamali, T.; Saikrishna, D. Role of platelet rich fibrin and platelet rich plasma in wound healing of extracted third molar sockets: A comparative study. J. Maxillofac. Oral. Surg. 2015, 14, 410–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).