Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Smooth titanium with no residual stress (S);
- Smooth titanium with residual stress (S + RS);
- Rough titanium without residual stress (R);
- Rough titanium with residual stress (R + RS).
2.2. Roughness, Wettability, and Surface Energy
2.3. Residual Stresses
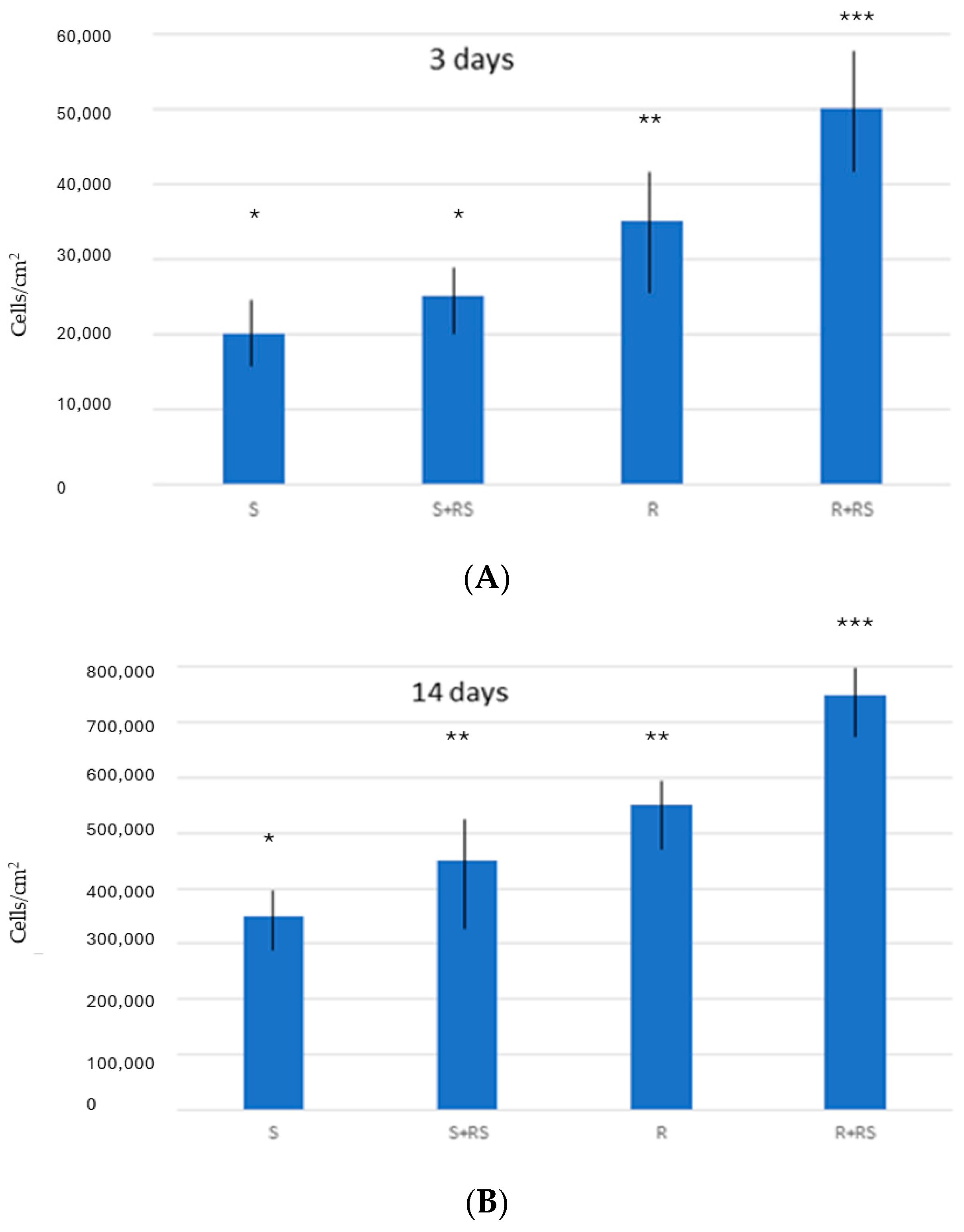
2.4. Osteoblast Culture
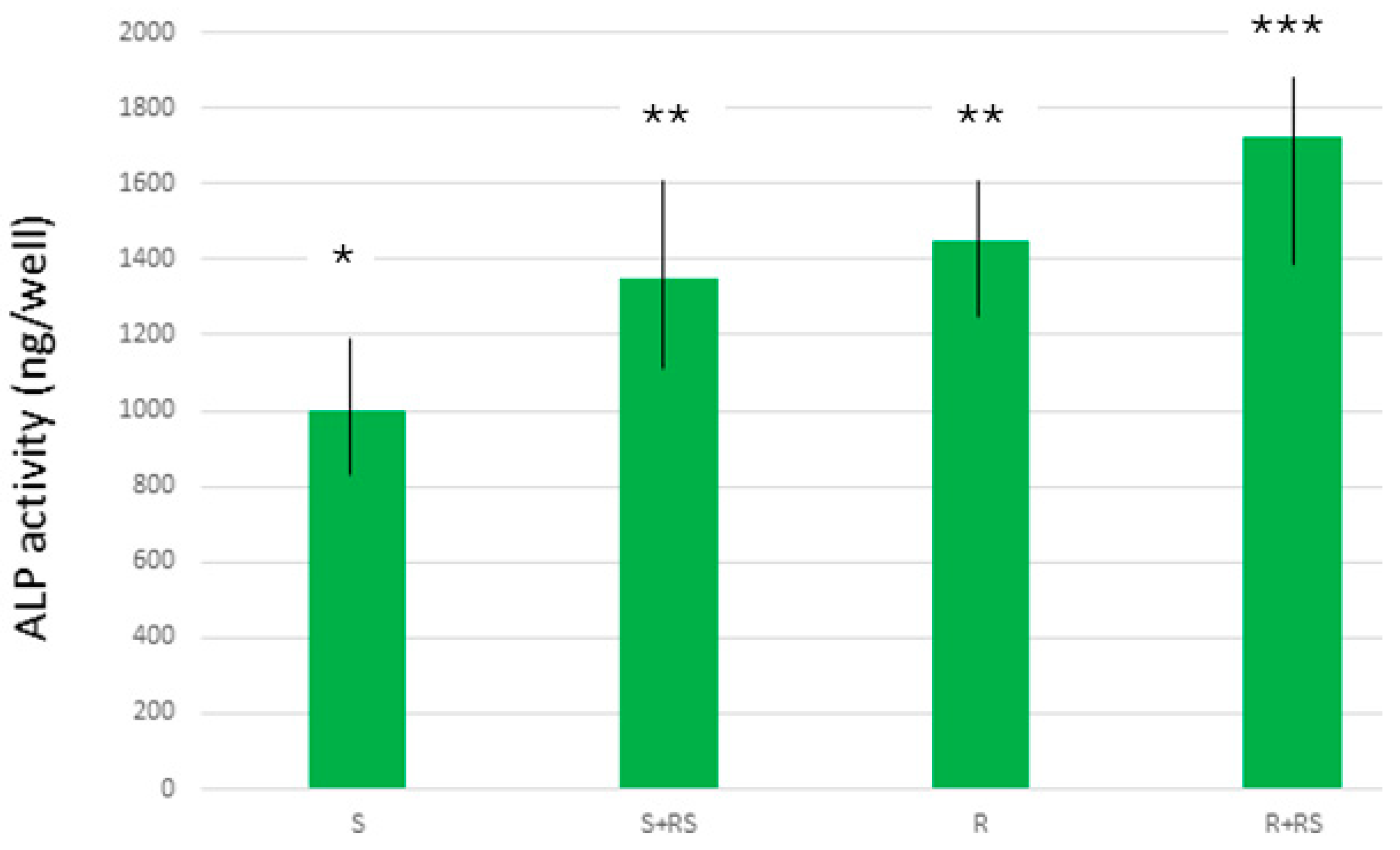
2.5. Mineralization of the Osteoblasts
2.6. Bacterial Strains and Growth Conditions
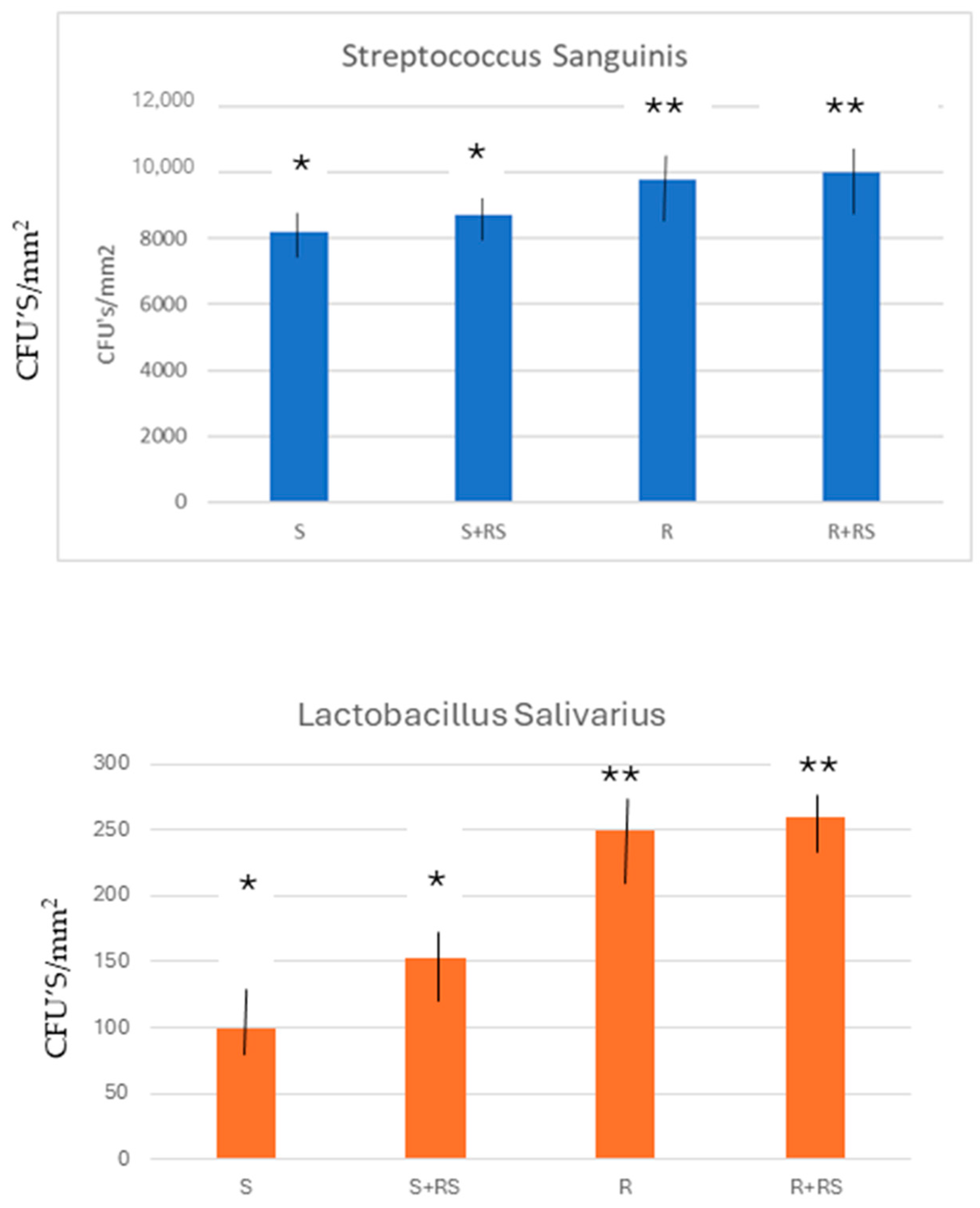
2.7. Evaluation of the Adhesion of Bacteria
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanes, R.J.; Bernard, J.P.; Blanes, Z.M.; Belser, U.C. A 10-year prospective study of ITI dental implants placed in the posterior region. I: Clinical and radiographic results. Clin. Oral Implant. Res. 2007, 18, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Brânemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of edentulous jaw. Experience from a 10-year period. Scand. J. Plast Reconstr. Surg. 1977, 16, 1–132. [Google Scholar]
- Branemark, P.I. Tissue-Integrated Prostheses. Osseointegration in Clinical Dentistry; Quintessence Publishing Co., Inc.: Berlin, Germany, 1985. [Google Scholar]
- Brånemark, P.I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses I. Experimental studies. Scand. J. Plast Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Pohler, O.; Sutter, F. Gewebsreaktion auf ein Titan-Hohlzylinder Implantatmit Titan-Spritzschicht oberflächlich. Schweiz. Monatsschrift Zahnheilkd. 1976, 86, 713–727. [Google Scholar]
- Schroeder, A.; Stich, H.; Straumann, F.; Sutter, F. Leber die Anlagerung von Osteo Zement an einen belasteten Implantatkörper. Schweiz. Monatsschrift Zahnheilkd. 1978, 88, 1051–1058. [Google Scholar]
- Schroeder, A.; van der Zypery, E.; Stitch, H.; Sutter, F. The reactions of bone, connective tissue, and epithelium to endos- teal implants with titanium-sprayed surfaces. J. Maxillofac. Surg. 1981, 9, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Albrektsson, T. Integration of screw implants in the rabbit. A 1-year follow-up of removal of titanium implants. Int. J. Oral Maxillofac. Implant. 1987, 2, 69–75. [Google Scholar]
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.; Lang, N.P. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multicenter study with 2359 implants. Clin. Oral Implant. Res. 1997, 8, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implant. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Planell, J.A.; Engel, E. Human-osteoblast proliferation and differentiation on grit-blasted and bioactive titanium for dental applications. J. Mater. Sci. Mater. Med. 2002, 13, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Lázaro, P.; Rios, J.V.; Lluch, S.; Marqués, M.; Guillem-Martí, J.; Gil, F.J. Influence of acid-etching after grit-blasted on osseointegration of titanium dental implants: In Vitro and in vivo studies. J. Mater. Sci. Mater. Med. 2013, 24, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Aparicioa, C.; Padrósb, A.; Gil, F.-J. In Vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J. Mech. Behav. Biomed. Mater. 2011, 4, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M. Titanium for Dental Implants (I). En “Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications”; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Sicilia, A.; Sicilia, P.; Mallo, C.; Cuesta, S.; Sanz, M. A retrospective study on the crestal bone loss associated with different implant surfaces in chronic periodontitis patients. Clin. Oral Implant. Res. 2018, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial Organization of Osteoblast Fi-bronectin-Matrix on Titanium Surface—Effects of Roughness, Chemical Heterogeneity, and Surface Free Energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Monsalve-Guil, L.; Jiménez-Guerra, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pequeroles, M.; Perez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration be-havior. In Vivo study in rabbits. J. Oral Implantol. 2016, 42, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Flemming, T.; Cosyn, J.; De Bruyn, H.; Eisner, B.M.; Hultin, M.; Isidor, F.; Lang, N.P.; Lund, B.; Meyle, J.; et al. The patient undergoing implant therapy. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin. Oral Implant. Res. 2015, 26, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.-L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Lin, G.H.; Suarez, F.; MacEachern, M.; Wang, H.L. Surgical management of peri-implantitis: A systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014, 85, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2021, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pieuchot, L.; Marteau, J.; Guignandon, A.; Dos Santos, T.; Brigaud, I.; Chauvy, P.-F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat. Commun. 2018, 9, 3995. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Park, S.J.; Kim, D.S.; Doh, J. Sinusoidal wavy surfaces for curvature-guided migration of T lymphocytes. Biomaterials 2015, 51, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Charest, J.L.; Eliason, M.T.; García, A.J.; King, W.P. Combined microscale mechanical topography and chemical patterns on polymer cell culture substrates. Biomaterials 2006, 27, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Mitchel, J.A.; Hoffman-Kim, D. Cellular scale anisotropic topography guides Schwann cell motility. PLoS ONE 2011, 6, e24316. [Google Scholar] [CrossRef] [PubMed]
- Bade, N.D.; Xu, T.; Kamien, R.D.; Assoian RK Stebe, K.J. Gaussian curvature directs stress fiber orientation and cell migration. Biophys. J. 2018, 114, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, G.T.; Song, H.; Mao, H.-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Petrie, R.J.; Koo, H.; Yamada, K.M. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 2014, 345, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial Properties of Triethoxysilylpropyl Succinic Anhydride Silane (TESPSA) on Titanium Dental Implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.-J.; Aparicio, C. Adsorption of fibronectin, fibrinogen, and albumin on TiO2: Time-Resolved Kinetics, Structural Changes, and Competition Study. Biointerphases 2012, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Marti, J.; Cinca, N.; Punset, M.; Cano, I.G.; Gil, F.J.; Guilemany, J.M.; Dosta, S. Porous titanium-hydroxyapatite composite coating obtained on titanium by cold gas spray with high bond strength for biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Espinar, E.; Llamas, J.M.; Sevilla, P. Fatigue life of bioactive titanium dental implants treated by means of Grit Blasting and Thermo-Chemical treatment. Clin. Implant Dent. Relat. Res 2014, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Aragoneses, J.; Valverde, N.L.; Fernandez-Dominguez, M.; Mena-Alvarez, J.; Rodriguez, C.; Gil, J.; Aragoneses, J.M. Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors. Materials 2022, 15, 4036. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar] [CrossRef]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Manolagas, S.C. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020, 16, e1008714. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 2013, 26, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 16, 1042–1047. [Google Scholar] [CrossRef]
- Karsenty, G. Update on the biology of osteocalcin. Endocr. Pract. 2017, 23, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Karsenty, G. Regulation of energy metabolism by bone-derived hormones. In Principles of Bone Biology; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 303–318. [Google Scholar]
- Wellendorph, P.; Johansen, L.D.; A Jensen, A.; Casanova, E.; Gassmann, M.; Deprez, P.; Clément-Lacroix, P.; Bettler, B.; Bräuner-Osborne, H. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J. Mol. Endocrinol. 2008, 42, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.V.; Gasparini, S.J.; Tu, J.; Zhou, H.; Seibel, M.J.; Bräuner-Osborne, H. Metabolic and skeletal homeostasis are maintained in full locus GPRC6A knockout mice. Sci. Rep. 2019, 9, 5995. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Karsenty, G. The osteoblast: An insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 2010, 26, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Román, J.; Padilla, S.; Doadrio, J.C.; Gil, F.J. Bioactivity and mechanical properties of SiO2–CaO–P2O5 glass-ceramics. J. Mater. Chem. 2005, 15, 1353–1359. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.-C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010, 142, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Chekroun, A.; Pujo-Menjouet, L.; Falcoz, S.; Tsuen, K.; Yang, K.Y.-H.; Berteau, J.-P. Theoretical evidence of osteoblast self-inhibition after activation of the genetic regulatory network controlling mineralization. J. Theor. Biol. 2022, 537, 111005. [Google Scholar] [CrossRef] [PubMed]
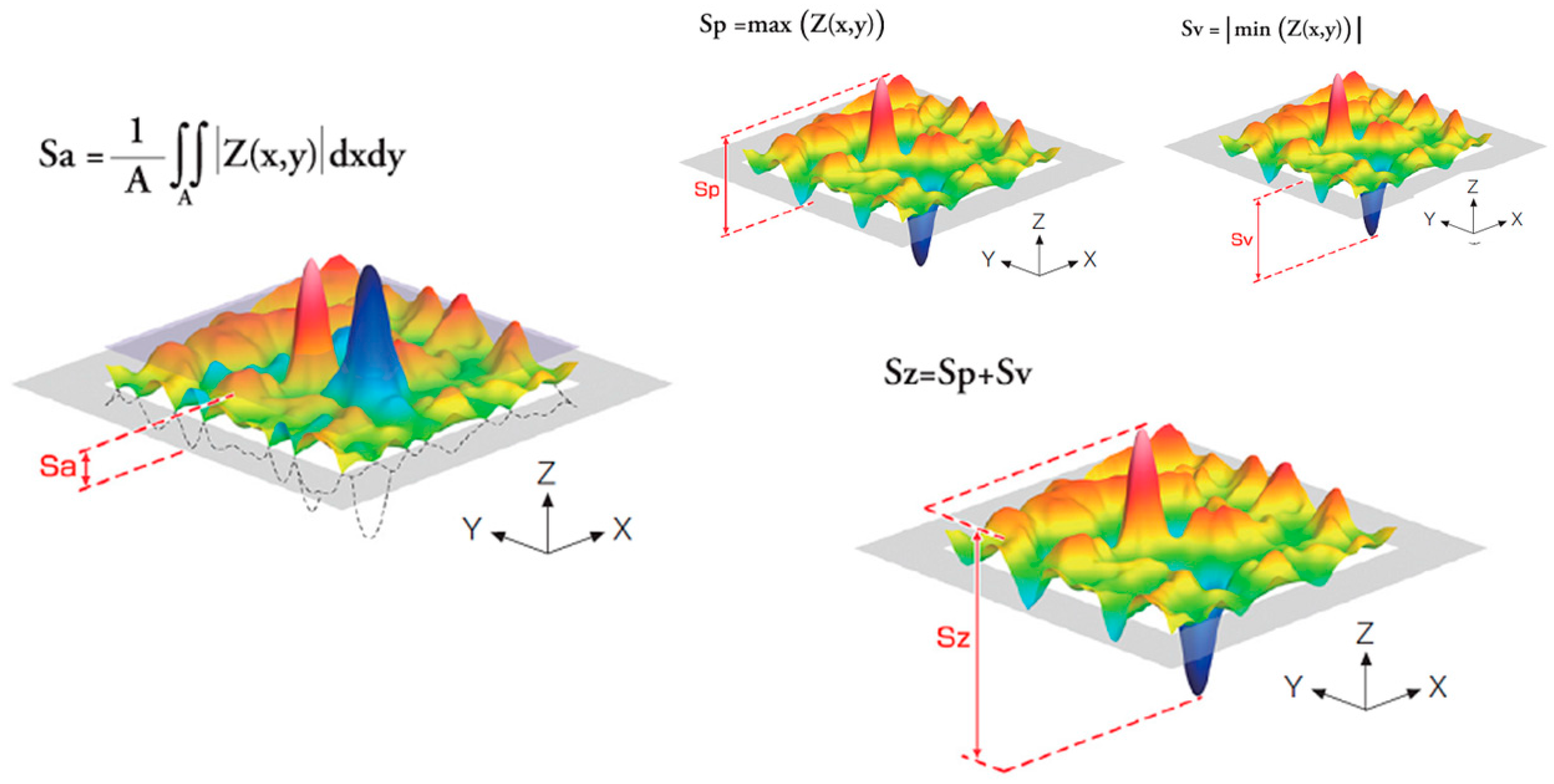
| Sa (µm) | Sz (µm) | Index Area | CA (°) | DC (mJ/m2) | PC (mJ/m2) | Total SFE (mJ/m2) | σresidual (MPa) | |
|---|---|---|---|---|---|---|---|---|
| S | 0.21 ± 0.02 * | 0.34 ± 0.02 * | 1.09 ± 0.01 * | 77 ± 5 * | 24.8 ± 1.2 * | 10.2 ± 2.0 * | 35.0 ± 3.2 * | −10 ± 2 * |
| S + RS | 0.24 ± 0.10 * | 0.41 ± 0.11 * | 1.08 ± 0.06 * | 58 ± 3 ** | 27.2 ± 1.2 ** | 18.3 ± 1.8 ** | 45.5 ± 2.2 ** | −189 ± 20 ** |
| R | 2.04 ± 0.15 ** | 4.67 ± 1.07 ** | 1.66 ± 0.04 ** | 69 ± 4 * | 27.7 ± 1.3 ** | 12.5 ± 2.1 * | 40.2 ± 1.2 *** | −8 ± 3 * |
| R + RS | 1.99 ± 0.18 ** | 4.89 ± 1.67 ** | 1.76 ± 0.04 ** | 53 ± 2 ** | 29.0 ± 2.2 ** | 20.4 ± 1.9 ** | 49.4 ± 1.8 ** | −201 ± 12 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.; Maia, P.; Rios-Santos, J.V.; Herrero-Climent, M.; Rios-Carrasco, B.; Aparicio, C.; Gil, J. Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization. Materials 2024, 17, 1626. https://doi.org/10.3390/ma17071626
Pereira R, Maia P, Rios-Santos JV, Herrero-Climent M, Rios-Carrasco B, Aparicio C, Gil J. Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization. Materials. 2024; 17(7):1626. https://doi.org/10.3390/ma17071626
Chicago/Turabian StylePereira, Rita, Paulo Maia, Jose Vicente Rios-Santos, Mariano Herrero-Climent, Blanca Rios-Carrasco, Conrado Aparicio, and Javier Gil. 2024. "Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization" Materials 17, no. 7: 1626. https://doi.org/10.3390/ma17071626
APA StylePereira, R., Maia, P., Rios-Santos, J. V., Herrero-Climent, M., Rios-Carrasco, B., Aparicio, C., & Gil, J. (2024). Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization. Materials, 17(7), 1626. https://doi.org/10.3390/ma17071626











