Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Properties
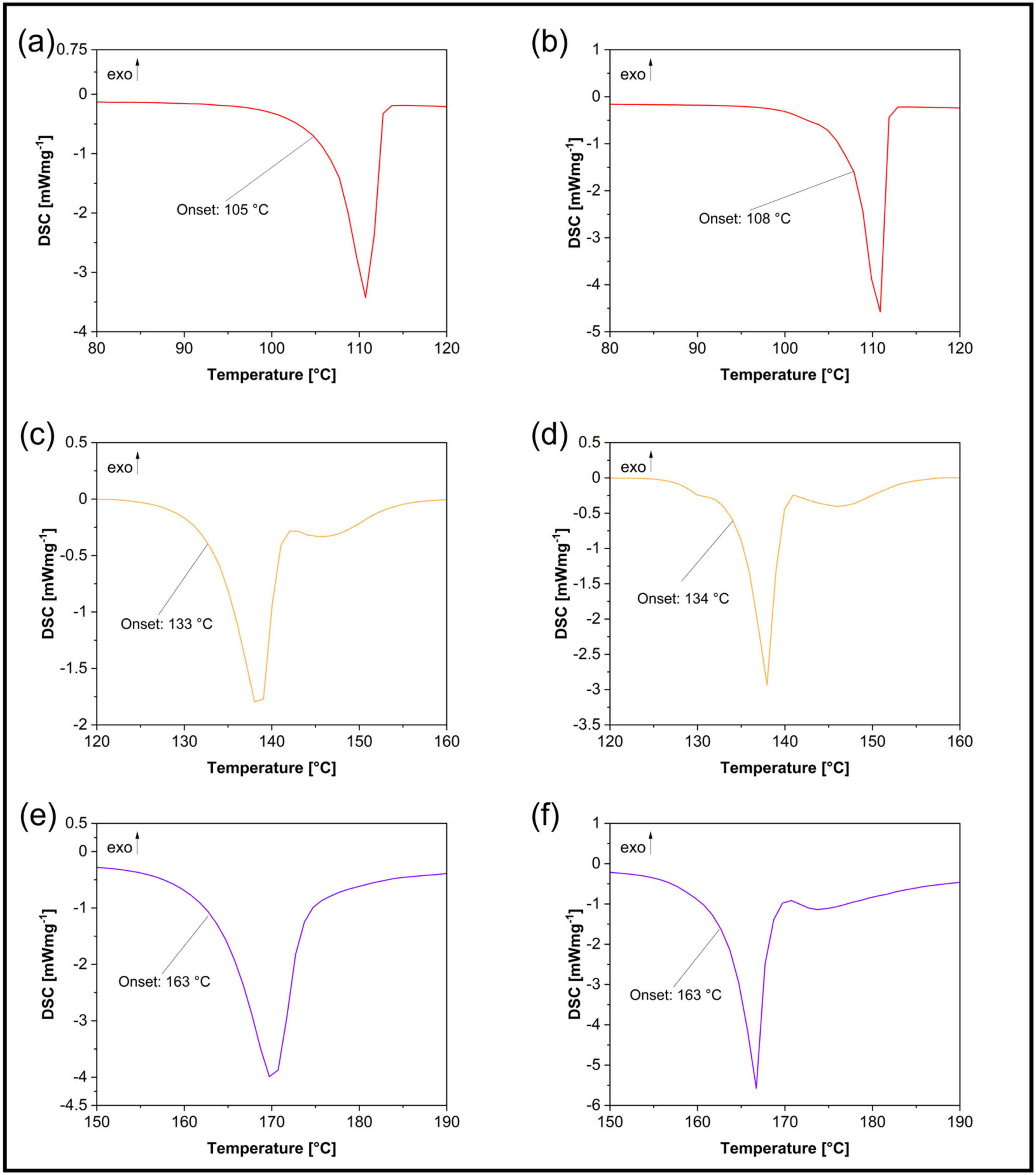
3.2. Thermodynamic Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; de Melo, T.R.F.; dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2015, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Antraygues, K.; Maingot, M.; Schellhorn, B.; Trebosc, V.; Gitzinger, M.; Deprez, B.; Defert, O.; Dale, G.E.; Bourotte, M.; Lociuro, S.; et al. Design and synthesis of water-soluble prodrugs of rifabutin for intraveneous administration. Eur. J. Med. Chem. 2022, 238, 114515. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Wang, C.; Zhang, L.; Zhang, X.; Liu, S.; Xu, Y.; Wang, H.; Dai, Q.; Liu, C.; et al. Discovery of Antibacterial Contezolid Acefosamil: Innovative O-Acyl Phosphoramidate Prodrug for IV and Oral Therapies. ACS Med. Chem. Lett. 2022, 13, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Sanches, B.M.A.; Ferreira, E.I. Is prodrug design an approach to increase water solubility? Int. J. Pharm. 2019, 568, 118498. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, L.; Tang, Y.; Zhu, H.; Wang, H.; Xiao, H.; Wang, F.; Tao, W. Preparation of cisplatin delivery calcium phosphate nanoparticles using poly(Pt(IV) prodrug) as the payload. Mater. Today Commun. 2022, 33, 104283. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Pandey, N.; Bohra, B.S.; Tiwari, H.; Pal, M.; Negi, P.B.; Dandapat, A.; Mehta, S.; Sahoo, N.G. Development of biodegradable chitosan/graphene oxide nanocomposite via spray drying method for drug loading and delivery application. J. Drug Deliv. Sci. Technol. 2022, 74, 103555. [Google Scholar] [CrossRef]
- Nora, G.-I.; Venkatasubramanian, R.; Strindberg, S.; Siqueira-Jørgensen, S.D.; Pagano, L.; Romanski, F.S.; Swarnakar, N.K.; Rades, T.; Müllertz, A. Combining lipid based drug delivery and amorphous solid dispersions for improved oral drug absorption of a poorly water-soluble drug. J. Control. Release 2022, 349, 206–212. [Google Scholar] [CrossRef]
- Khan, A.A.; Akhtar, S.; Yadav, Y.; Akhtar, A.; Alelwani, W.; Bannunah, A.M.; Mahmood, S. Lopinavir-Loaded Self-Nanoemulsifying Drug Delivery System for Enhanced Solubility: Development, Characterisation and Caco-2 Cell Uptake. Curr. Drug Deliv. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef]
- Petitprez, J.; Legrand, F.-X.; Tams, C.; Pipkin, J.D.; Antle, V.; Kfoury, M.; Fourmentin, S. Huge solubility increase of poorly water-soluble pharmaceuticals by sulfobutylether-β-cyclodextrin complexation in a low-melting mixture. Environ. Chem. Lett. 2022, 20, 1561–1568. [Google Scholar] [CrossRef]
- Loftsson, T. Drug solubilization by complexation. Int. J. Pharm. 2017, 531, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singla, P.; Kaur, I. Labrasol mediated enhanced solubilization of natural hydrophobic drugs in Pluronic micelles: Physicochemical and in vitro release studies. J. Mol. Liq. 2022, 361, 119596. [Google Scholar] [CrossRef]
- Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Pawełczyk, A.; Dadej, D.; Łażewska, D.; Jelińska, A. Improved solubility of lornoxicam by inclusion into SBA-15: Comparison of loading methods. Eur. J. Pharm. Sci. 2022, 171, 106133. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; de Diego, H.L. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef]
- Jindal, A.; Prashar, M.; Dureja, J.; Dhingra, N.; Chadha, K.; Karan, M.; Chadha, R. Pharmaceutical Cocrystals of Famotidine: Structural and Biopharmaceutical Evaluation. J. Pharm. Sci. 2022, 111, 2788–2798. [Google Scholar] [CrossRef]
- Lenschow, I.C.S.; Bazzo, G.C.; Zétola, M.; Stulzer, H.K.; Soares, L.; Pezzini, B.R. Ball-milled valsartan and its combination with mannitol: The case of drug polyamorphism. J. Therm. Anal. Calorim. 2022, 147, 8765–8777. [Google Scholar] [CrossRef]
- Vemuri, V.D.; Lankalapalli, S.; Chandra Reddy Guntaka, P. Posaconazole-amino acid cocrystals for improving solubility and oral bioavailability while maintaining antifungal activity and low In vivo toxicity. J. Drug Deliv. Sci. Technol. 2022, 74, 103491. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Li, D.; Bai, E.; Zhang, H.; Duan, Y.; Huang, Y. Platensimycin-berberine chloride co-amorphous drug system: Sustained release and prolonged half-life. Eur. J. Pharm. Biopharm. 2022, 179, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Nechipadappu, S.K.; Swain, D. Combined synthetic and solubility aspects of orotate salt of bilastine. J. Mol. Struct. 2023, 1271, 134148. [Google Scholar] [CrossRef]
- Liu, L.; An, Q.; Zhang, Y.; Sun, W.; Li, J.; Feng, Y.; Geng, Y.; Cheng, G. Improving the solubility, hygroscopicity and permeability of enrofloxacin by forming 1:2 pharmaceutical salt cocrystal with neutral and anionic co-existing p-nitrobenzoic acid. J. Drug Deliv. Sci. Technol. 2022, 76, 103732. [Google Scholar] [CrossRef]
- Neelam, U.K.; Daveedu, B.; Ambabhai, V.N.; Siripragada, M.R.; Kumar, S.R.; Balasubramanian, S. Physicochemical aspects and comparative analysis of Voxelotor and its salt and cocrystal. J. Mol. Struct. 2023, 1271, 134024. [Google Scholar] [CrossRef]
- Bethune, S.J.; Huang, N.; Jayasankar, A.; Rodríguez-Hornedo, N. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Cryst. Growth Des. 2009, 9, 3976–3988. [Google Scholar] [CrossRef]
- Banik, M.; Gopi, S.P.; Ganguly, S.; Desiraju, G.R. Cocrystal and Salt Forms of Furosemide: Solubility and Diffusion Variations. Cryst. Growth Des. 2016, 16, 5418–5428. [Google Scholar] [CrossRef]
- Kruger, C. The relevance of international assessments to GRAS determinations. Regul. Toxicol. Pharmacol. 2016, 79 (Suppl. S2), S119–S123. [Google Scholar] [CrossRef]
- Martins, F.T.; Paparidis, N.; Doriguetto, A.C.; Ellena, J. Crystal Engineering of an Anti-HIV Drug Based on the Recognition of Assembling Molecular Frameworks. Cryst. Growth Des. 2009, 9, 5283–5292. [Google Scholar] [CrossRef]
- Mittapalli, S.; Mannava, M.K.C.; Khandavilli, U.B.R.; Allu, S.; Nangia, A. Soluble Salts and Cocrystals of Clotrimazole. Cryst. Growth Des. 2015, 15, 2493–2504. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, B.; Jia, L.; Wang, Y.; Wang, M.; Yang, H.; Qiao, Y.; Gong, J.; Tang, W. Tuning Physicochemical Properties of Antipsychotic Drug Aripiprazole with Multicomponent Crystal Strategy Based on Structure and Property Relationship. Cryst. Growth Des. 2020, 20, 3747–3761. [Google Scholar] [CrossRef]
- Voronin, A.P.; Surov, A.O.; Churakov, A.V.; Parashchuk, O.D.; Rykounov, A.A.; Vener, M.V. Combined X-ray Crystallographic, IR/Raman Spectroscopic, and Periodic DFT Investigations of New Multicomponent Crystalline Forms of Anthelmintic Drugs: A Case Study of Carbendazim Maleate. Molecules 2020, 25, 2386. [Google Scholar] [CrossRef] [PubMed]
- Djaló, M.; Cunha, A.E.S.; Luís, J.P.; Quaresma, S.; Fernandes, A.; André, V.; Duarte, M.T. Sparfloxacin Multicomponent Crystals: Targeting the Solubility of Problematic Antibiotics. Cryst. Growth Des. 2021, 21, 995–1005. [Google Scholar] [CrossRef]
- Sun, N.; Avdeef, A. Biorelevant pK(a) (37 °C) predicted from the 2D structure of the molecule and its pK(a) at 25 °C. J. Pharm. Biomed. Anal. 2011, 56, 173–182. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The salt-cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Bowery, N.G.; Smart, T.G. GABA and glycine as neurotransmitters: A brief history. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S109–S119. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Enna, S.J.; McCarson, K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin. Pharmacokinet. 2010, 49, 661–669. [Google Scholar] [CrossRef]
- Bramness, J.G.; Sandvik, P.; Engeland, A.; Skurtveit, S. Does Pregabalin (Lyrica®) help patients reduce their use of benzodiazepines? A comparison with gabapentin using the Norwegian Prescription Database. Basic Clin. Pharmacol. Toxicol. 2010, 107, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Pecikoza, U.; Micov, A.; Vučković, S.; Stepanović-Petrović, R. Antiepileptic drugs as analgesics/adjuvants in inflammatory pain: Current preclinical evidence. Pharmacol. Ther. 2018, 192, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, R.; Poeggel, K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport 2002, 13, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Marti-Prats, L.; Belin-Rauscent, A.; Fouyssac, M.; Puaud, M.; Cocker, P.J.; Everitt, B.J.; Belin, D. Baclofen decreases compulsive alcohol drinking in rats characterized by reduced levels of GAT-3 in the central amygdala. Addict. Biol. 2021, 26, e13011. [Google Scholar] [CrossRef]
- Kent, C.N.; Park, C.; Lindsley, C.W. Classics in Chemical Neuroscience: Baclofen. ACS Chem. Neurosci. 2020, 11, 1740–1755. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Balbás, I.J.; Valentín-Guevara, B.; López-Mendoza, E.; Linzaga-Elizalde, I.; Ordoñez, M.; Román-Bravo, P. Efficient Synthesis of β-Aryl-γ-lactams and Their Resolution with (S)-Naproxen: Preparation of (R)- and (S)-Baclofen. Molecules 2015, 20, 22028–22043. [Google Scholar] [CrossRef] [PubMed]
- Lapin, I. Phenibut (beta-phenyl-GABA): A tranquilizer and nootropic drug. CNS Drug Rev. 2001, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Comparative pharmacological activity of optical isomers of phenibut. Eur. J. Pharmacol. 2008, 583, 128–134. [Google Scholar] [CrossRef]
- Peterson, M.; Oliveira, M. Preparation of Organic Acid Salts of Gabapentin. WO2004091278, 8 April 2004. [Google Scholar]
- Plata Salaman, C.R.; Tesson, N.; Trilla Castano, M.; Cardenas Romana, L. Crystalline Forms of Pregabalin and Co-Formers in the Treatment of Pain. EP2527319, 24 May 2011. [Google Scholar]
- Gendron, F.-X.; Mahieux, J.; Sanselme, M.; Coquerel, G. Resolution of Baclofenium Hydrogenomaleate By Using Preferential Crystallization. A First Case of Complete Solid Solution at High Temperature and a Large Miscibility Gap in the Solid State. Cryst. Growth Des. 2019, 19, 4793–4801. [Google Scholar] [CrossRef]
- Báthori, N.B.; Kilinkissa, O.E.Y. Are gamma amino acids promising tools of crystal engineering?—Multicomponent crystals of baclofen. Cryst. Eng. Comm. 2015, 17, 8264–8272. [Google Scholar] [CrossRef]
- Bloom, R.A.; Sadrieh, N. Environmental Assessment Finding of No Significant Impact NDA 21-446/S-028 Lyrica (Pregabalin) Capsules. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/021446Orig1s028EA.pdf (accessed on 20 October 2022).
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 5–88. [Google Scholar]
- Komisarek, D.; Haj Hassani Sohi, T.; Vasylyeva, V. Co-crystals of zwitterionic GABA API’s pregabalin and phenibut: Properties and application. CrystEngComm 2022, 24, 8390–8398. [Google Scholar] [CrossRef]
- Herbst, M.; Komisarek, D.; Strothmann, T.; Vasylyeva, V. A Lection in Humbleness: Crystallization of Chiral and Zwitterionic APIs Baclofen and Phenibut. Crystals 2022, 12, 1393. [Google Scholar] [CrossRef]
- Budny-Godlewski, K.; Leszczyński, M.K.; Tulewicz, A.; Justyniak, I.; Pinkowicz, D.; Sieklucka, B.; Kruczała, K.; Sojka, Z.; Lewiński, J. A Case Study on the Desired Selectivity in Solid-State Mechano- and Slow-Chemistry, Melt, and Solution Methodologies. ChemSusChem 2021, 14, 3887–3894. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de La O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, G.; Zhang, K.; Yao, M.; Jin, Y.; Zhang, P.; Wu, S.; Gong, J. Green Mechanochemical Strategy for the Discovery and Selective Preparation of Polymorphs of Active Pharmaceutical Ingredient γ-Aminobutyric Acid (GABA). ACS Sustain. Chem. Eng. 2020, 8, 16781–16790. [Google Scholar] [CrossRef]
- Chikhalia, V.; Forbes, R.T.; Storey, R.A.; Ticehurst, M. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur. J. Pharm. Sci. 2006, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.F. Perspectives on the amorphisation/milling relationship in pharmaceutical materials. Adv. Drug Deliv. Rev. 2016, 100, 51–66. [Google Scholar] [CrossRef]
- Mah, P.T.; Laaksonen, T.; Rades, T.; Aaltonen, J.; Peltonen, L.; Strachan, C.J. Unravelling the relationship between degree of disorder and the dissolution behavior of milled glibenclamide. Mol. Pharm. 2014, 11, 234–242. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, O.; Gupta, A.; Singh, S. Solvent-free Methods for Co-crystal Synthesis: A Review. Curr. Org. Synth. 2019, 16, 385–397. [Google Scholar] [CrossRef]
- Kulla, H.; Haferkamp, S.; Akhmetova, I.; Röllig, M.; Maierhofer, C.; Rademann, K.; Emmerling, F. In Situ Investigations of Mechanochemical One-Pot Syntheses. Angew. Chem. Int. Ed. Engl. 2018, 57, 5930–5933. [Google Scholar] [CrossRef]
- Muresan-Pop, M.; Vulpoi, A.; Simon, V.; Todea, M.; Magyari, K.; Pap, Z.; Simion, A.; Filip, C.; Simon, S. Co-Crystals of Etravirine by Mechanochemical Activation. J. Pharm. Sci. 2022, 111, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef] [PubMed]
- Agilent. CrysAlisPRO; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, Oxfordshire, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Lin, M.; Tesconi, M.; Tischler, M. Use of 1H NMR to facilitate solubility measurement for drug discovery compounds. Int. J. Pharm. 2009, 369, 47–52. [Google Scholar] [CrossRef]
- Zloh, M. NMR spectroscopy in drug discovery and development: Evaluation of physico-chemical properties. ADMET DMPK 2019, 7, 242–251. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Dikmen, G. Determination of the solubility of 2-Methyl-1,3-benzothiazol-5-amine molecule with aqueous ethanol by NMR spectroscopy. J. Mol. Liq. 2018, 272, 851–856. [Google Scholar] [CrossRef]
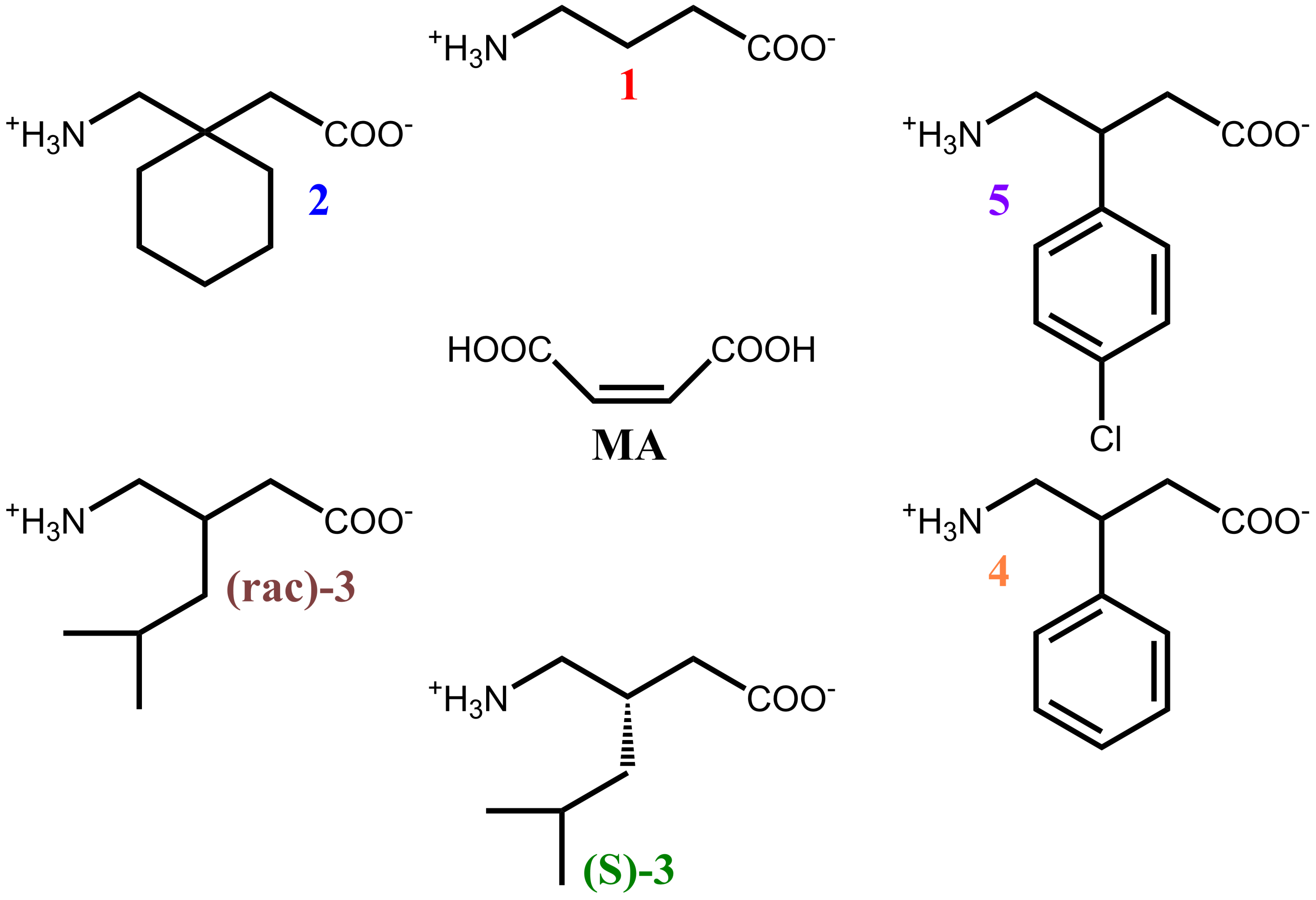
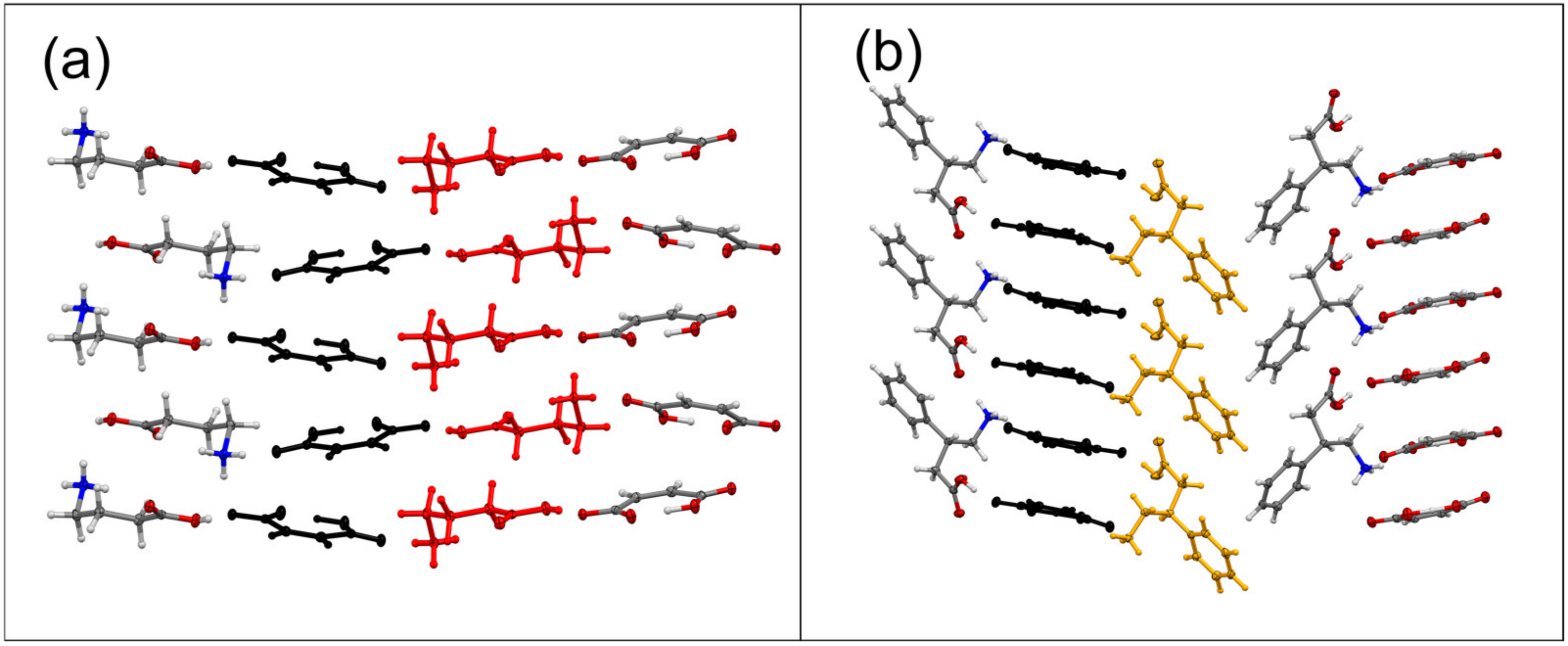
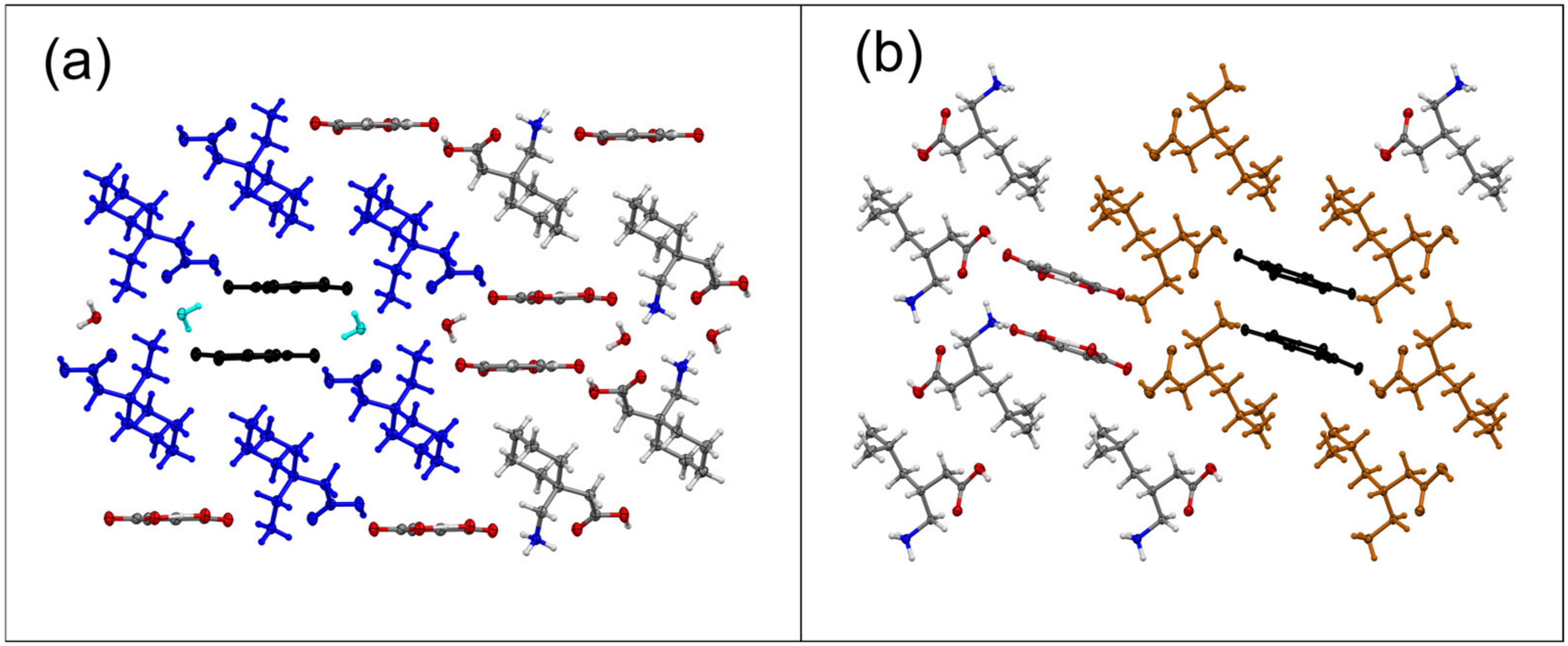


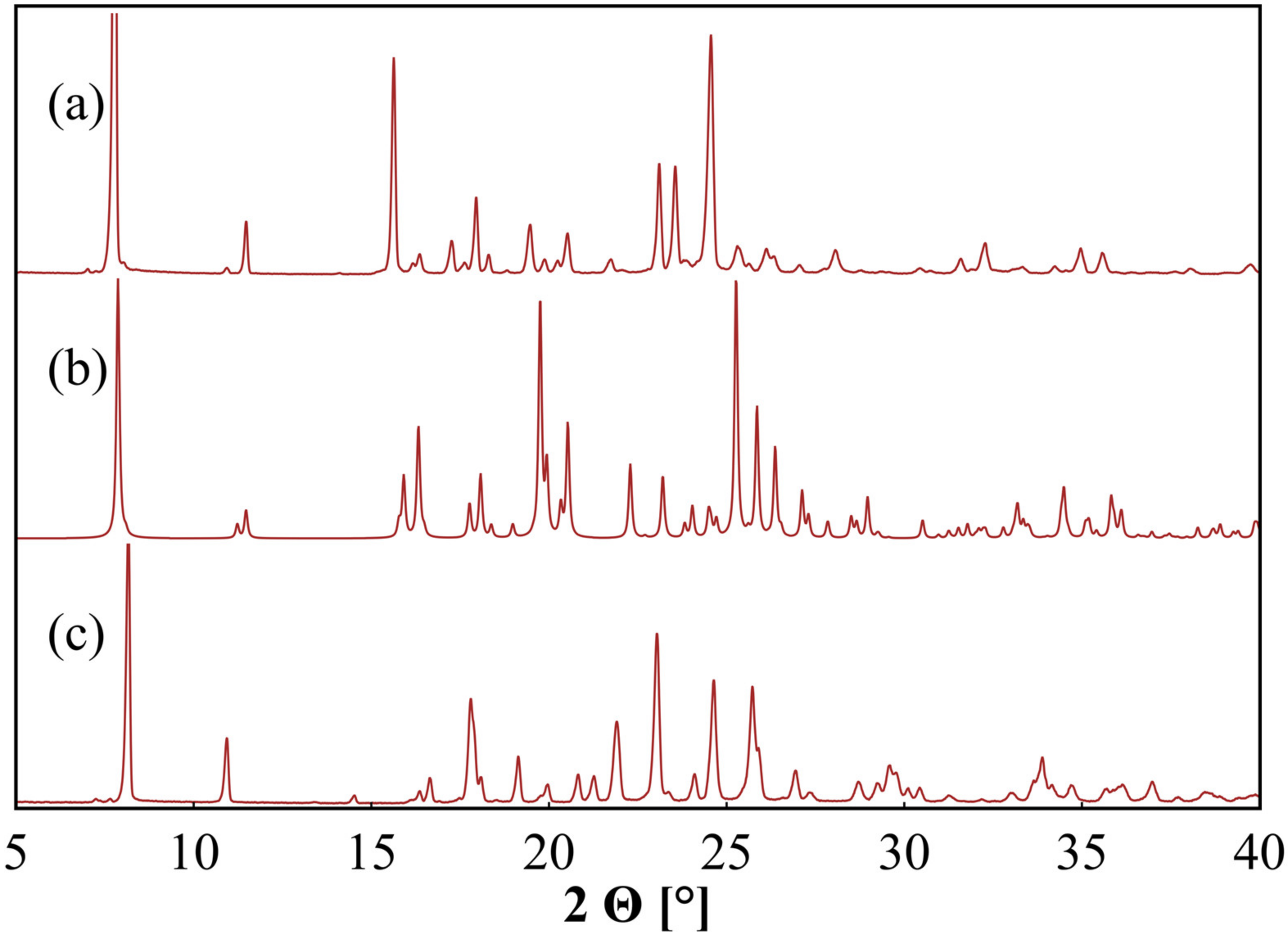

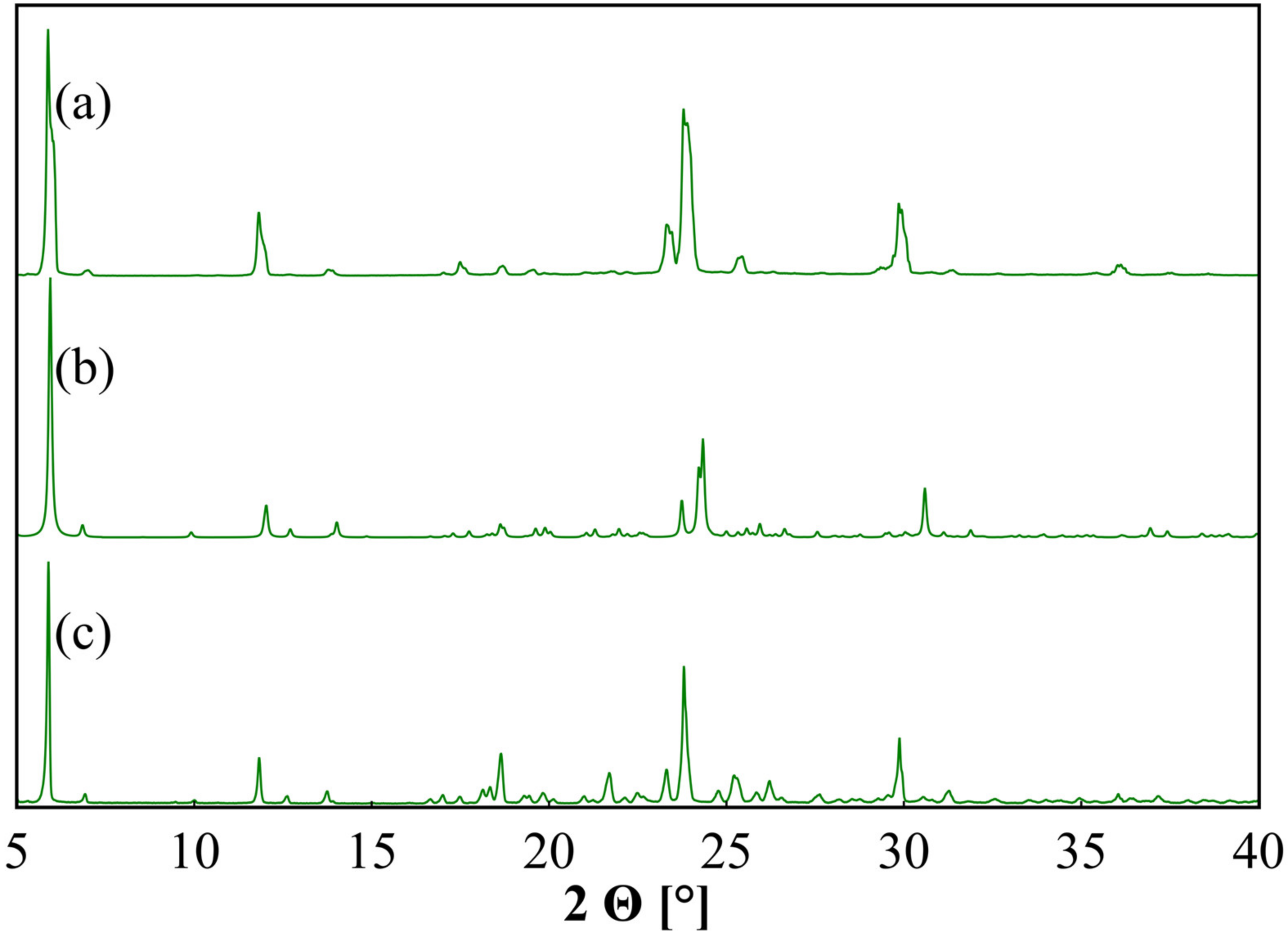




| Sample | Solvent Cryst. | Neat Grind. | Liquid-Assisted Grind. |
|---|---|---|---|
| 1-MA | pure phase | pure phase | not conducted |
| 2-MA | pure 2-MA•H2O | pure phase | mixture with 2-MA•H2O |
| 2-MA•H2O | pure phase | pure 2-MA | mixture with 2-MA |
| (rac)-3-MA-I | more (rac)-3-MA-I | more (rac)-3-MA-II | not conducted |
| (rac)-3-MA-II | more (rac)-3-MA-I | more (rac)-3-MA-II | not conducted |
| (S)-3-MA•H2O | pure phase | no new phase | pure phase |
| 4-MA | impure phase | impure phase | not conducted |
| 5-MA | pure phase | pure phase | not conducted |
| Sample | Melting Enthalpy [Jg−1] | Onset [°C] | End [°C] | Peak [°C] | Peak Width [°C] | Peak Height [mWmg−1] |
|---|---|---|---|---|---|---|
| 1-MA | 176 | 105 | 113 | 111 | 5 | 3.242 |
| 1-MA_M | 178 | 108 | 112 | 111 | 3 | 4.673 |
| 2-MA•H2O | 135 | 65 | 70 | 68 | 3 | 2.864 |
| 2-MA_M (Peak 1) | 14 | 41 | 65 | 61 | 16 | 0.095 |
| 2-MA_M (Peak 2) | 85 | 96 | 103 | 101 | 5 | 1.345 |
| (rac)-3-MA | 125 | 98 | 107 | 104 | 7 | 1.619 |
| (rac)-3-MA_M | 132 | 98 | 104 | 102 | 4 | 2.610 |
| (S)-3-MA•H2O | 53 | 46 | 61 | 57 | 13 | 0.454 |
| (S)-3-MA•H2O_M | 87 | 58 | 61 | 60 | 2 | 3.439 |
| 4-MA | 165 | 133 | 141 | 139 | 6 | 1.850 |
| 4-MA_M | 181 | 134 | 139 | 138 | 4 | 2.945 |
| 5-MA | 376 | 163 | 174 | 170 | 8 | 3.708 |
| 5-MA_M | 362 | 163 | 168 | 167 | 4 | 5.252 |
| Sample | Solubility [gL−1] | Error [%] | Error [gL−1] |
|---|---|---|---|
| MA | 687 | 6 | 44 |
| 1 | 2261 | 1 | 23 |
| 2 | 174 | 4 | 7 |
| (rac)-3•H2O | 33 | 4 | 1.3 |
| (S)-3 | 41 | 2 | 0.7 |
| 4 | 15 | 2 | 0.3 |
| 5 | 3 | 3 | 0.1 |
| 1-MA | 704 | 8 | 60 |
| 1-MA_M | 680 | 7 | 46 |
| 2-MA•H2O | 241 | 3 | 8 |
| 2-MA_M | 218 | 4 | 8 |
| (rac)-3-MA | 719 | 1.4 | 10 |
| (rac)-3-MA_M | 556 | 3 | 19 |
| (S)-3-MA•H2O | 977 | 8 | 79 |
| (S)-3-MA•H2O_M | 809 | 1 | 8 |
| 4-MA | 124 | 3 | 4 |
| 4-MA_M | 128 | 6 | 8 |
| 5-MA | 6 | 4 | 0.3 |
| 5-MA_M | 6 | 10 | 0.6 |
| Category | Solvent Cryst. | Mechanochemical Cryst. |
|---|---|---|
| Quickness | - | + |
| Phase reliability | = | = |
| Product quality | = (slight +) | = (slight -) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komisarek, D.; Taskiran, E.; Vasylyeva, V. Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization? Materials 2023, 16, 2242. https://doi.org/10.3390/ma16062242
Komisarek D, Taskiran E, Vasylyeva V. Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization? Materials. 2023; 16(6):2242. https://doi.org/10.3390/ma16062242
Chicago/Turabian StyleKomisarek, Daniel, Ebru Taskiran, and Vera Vasylyeva. 2023. "Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization?" Materials 16, no. 6: 2242. https://doi.org/10.3390/ma16062242
APA StyleKomisarek, D., Taskiran, E., & Vasylyeva, V. (2023). Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization? Materials, 16(6), 2242. https://doi.org/10.3390/ma16062242






