Impact of Sulphate Ions Content on Performance of Maleic and Acrylic Superplasticizers in Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
3. Testing Methods
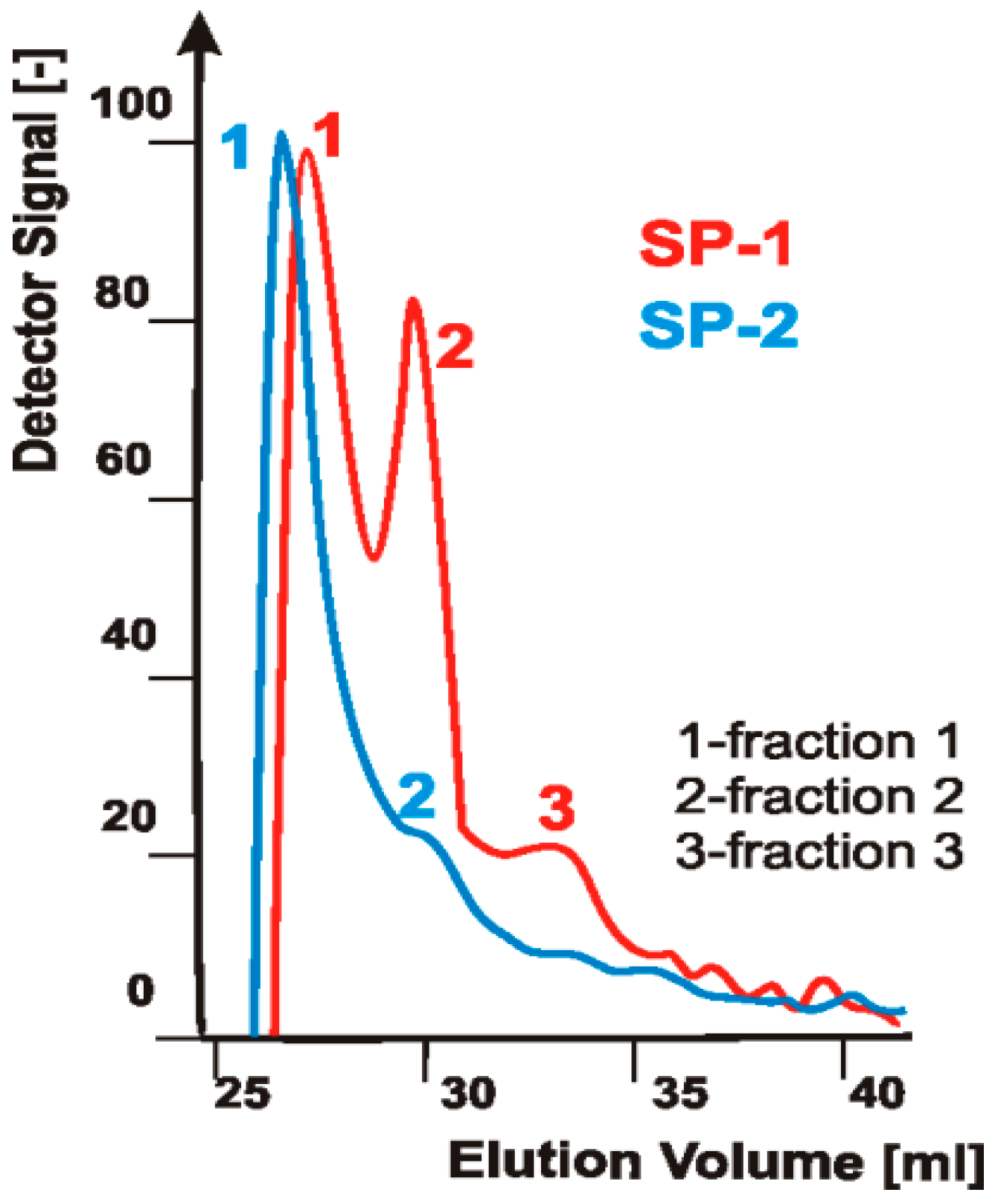
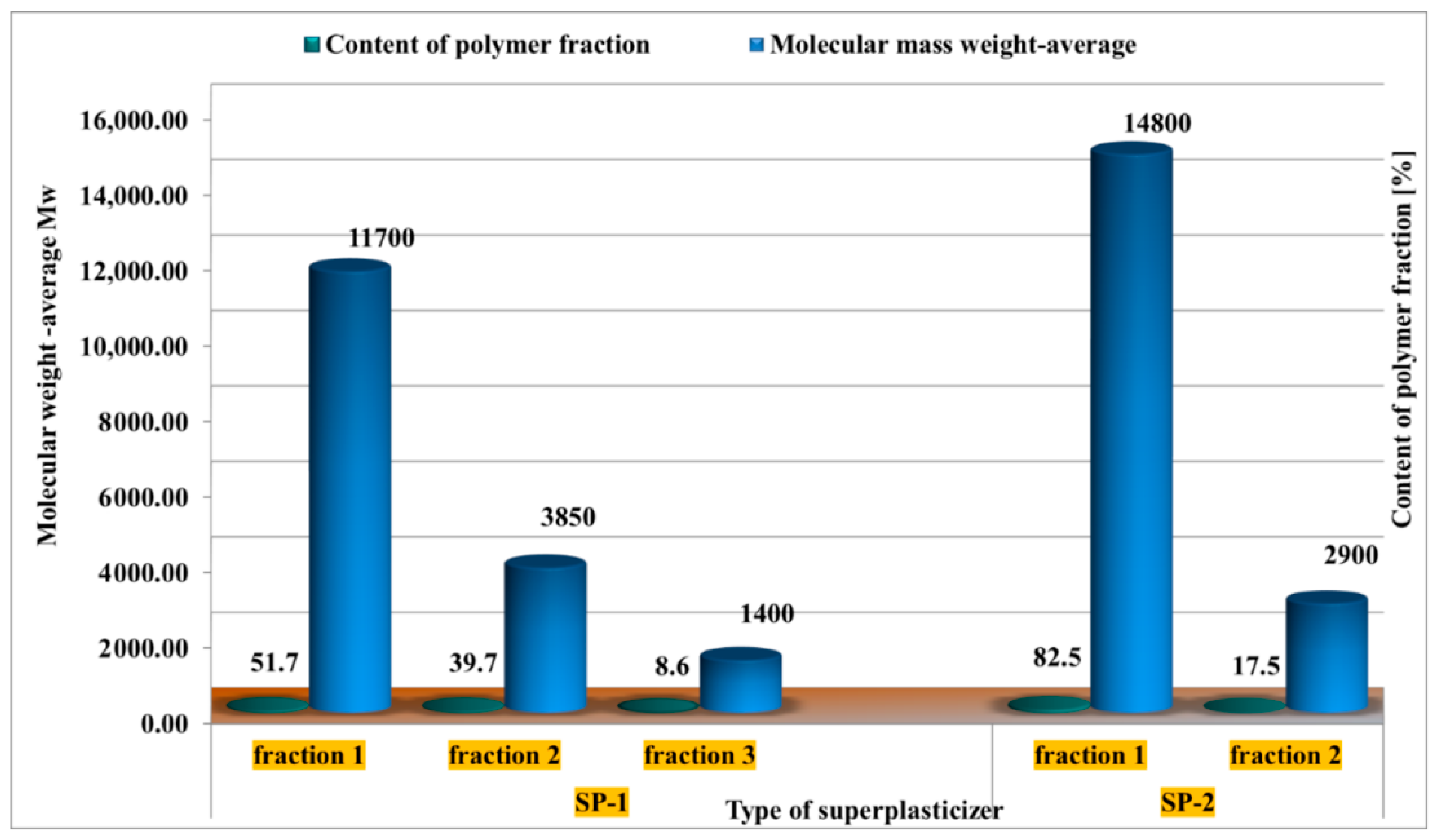
3.1. Gel Permeation Chromatography (GPC)
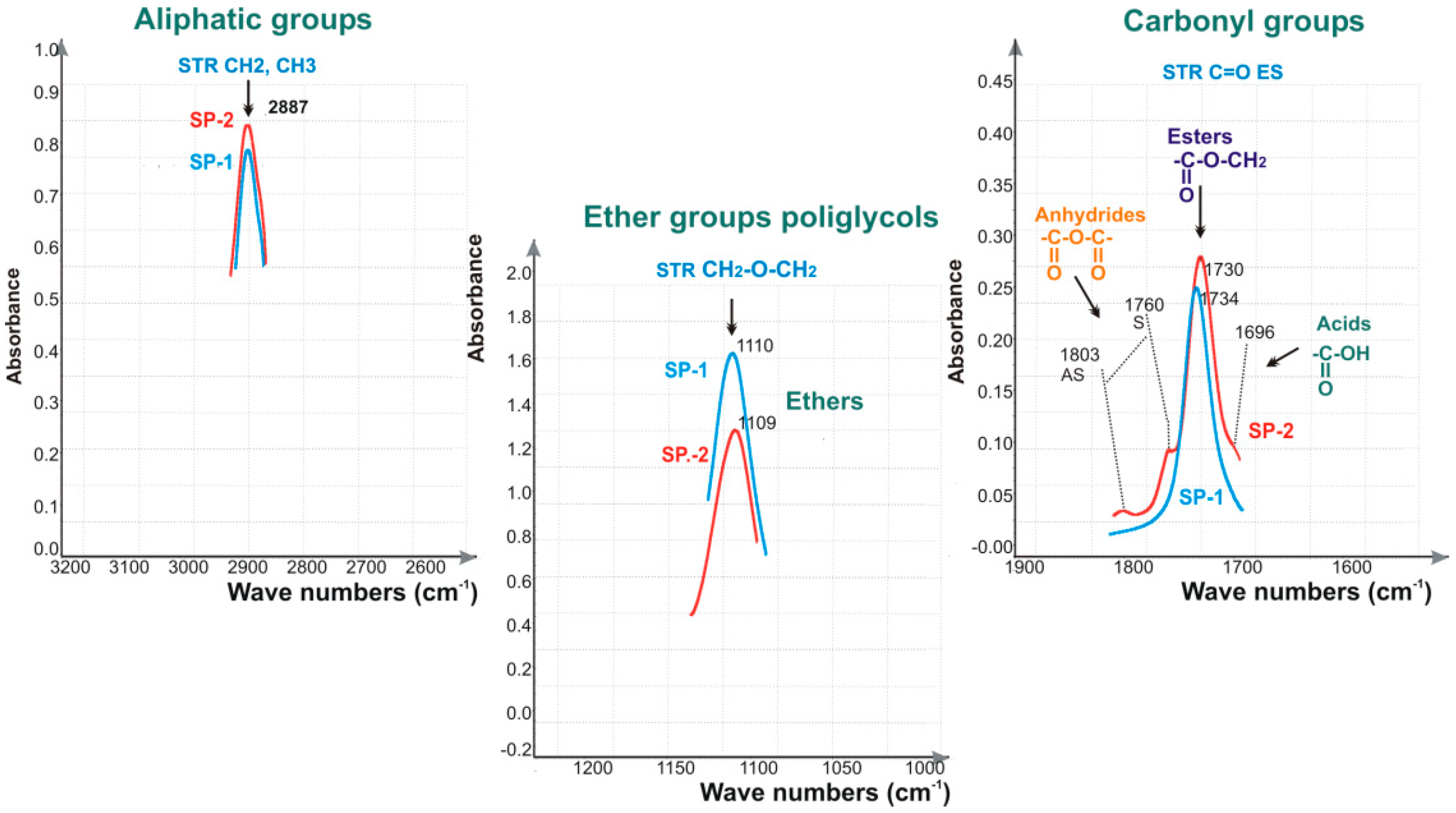
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Rheological Testing
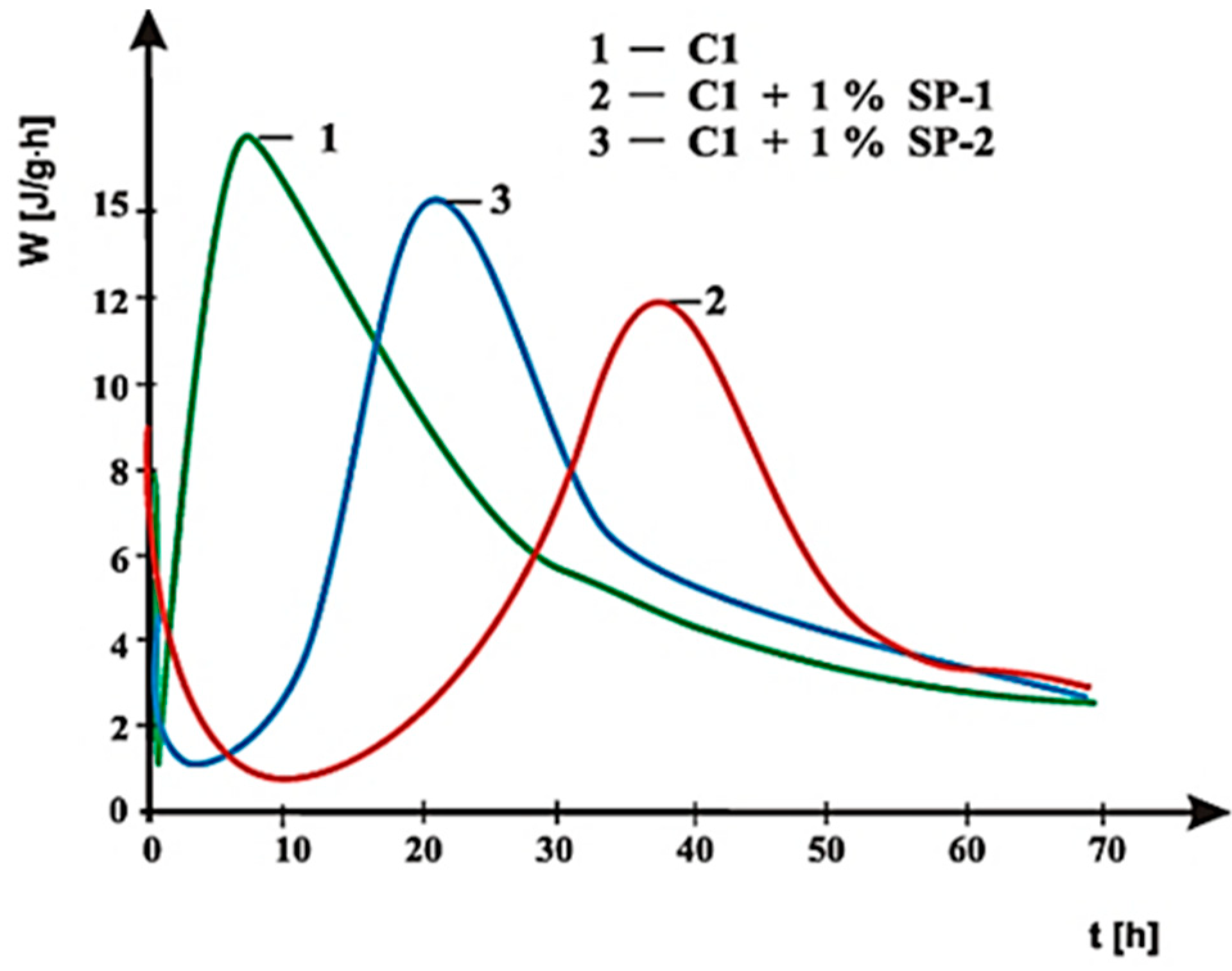
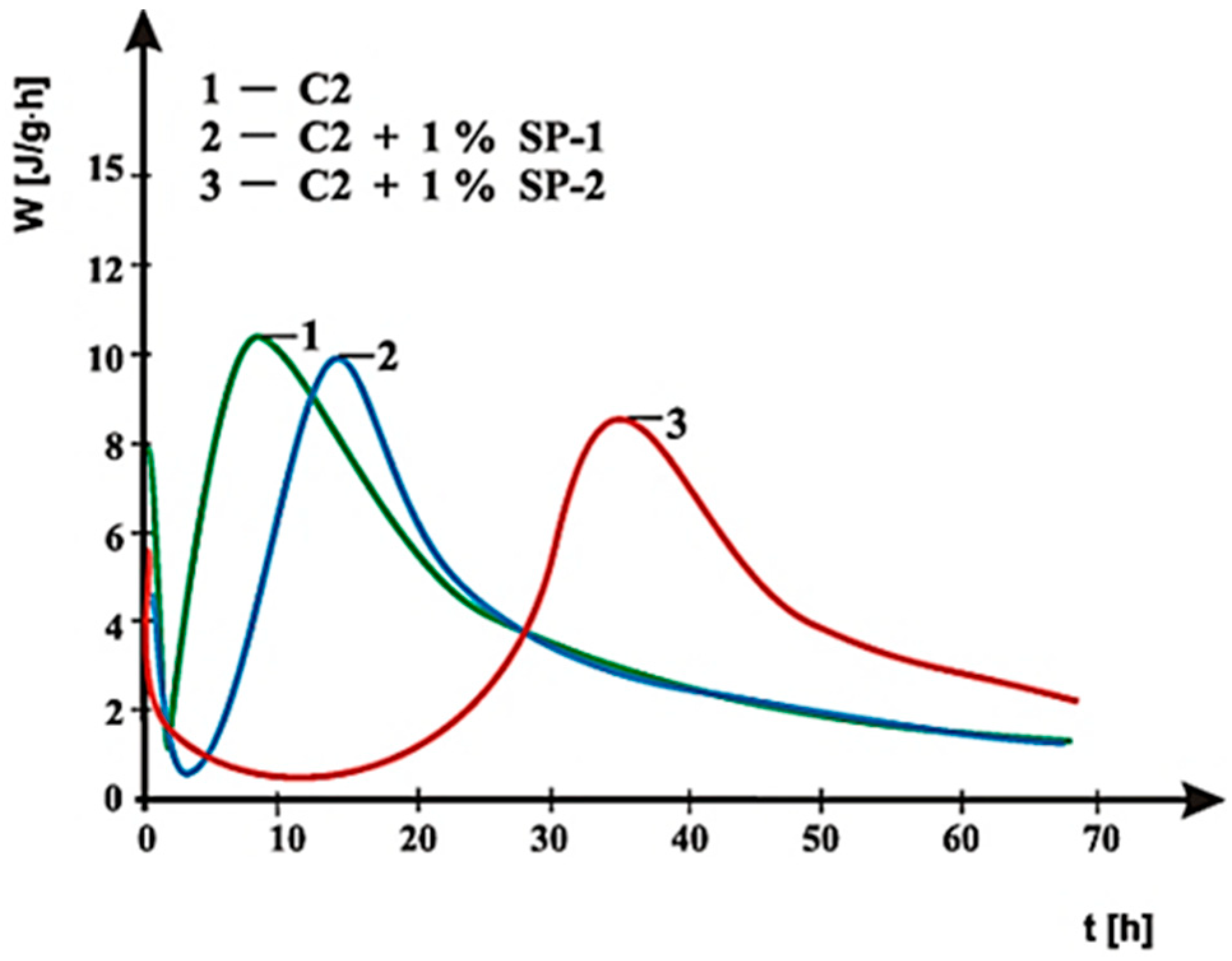
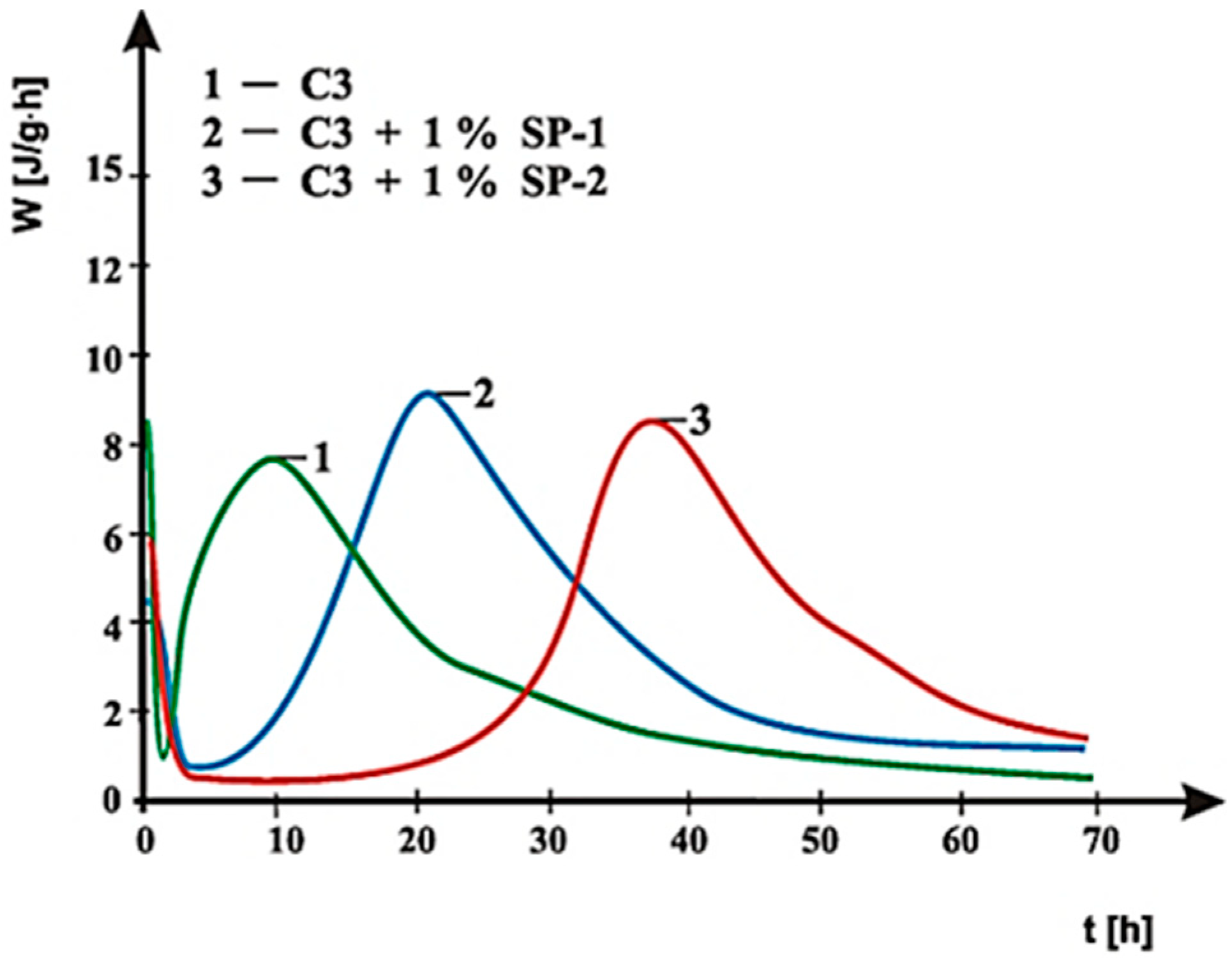
3.4. Hydration Heat Testing
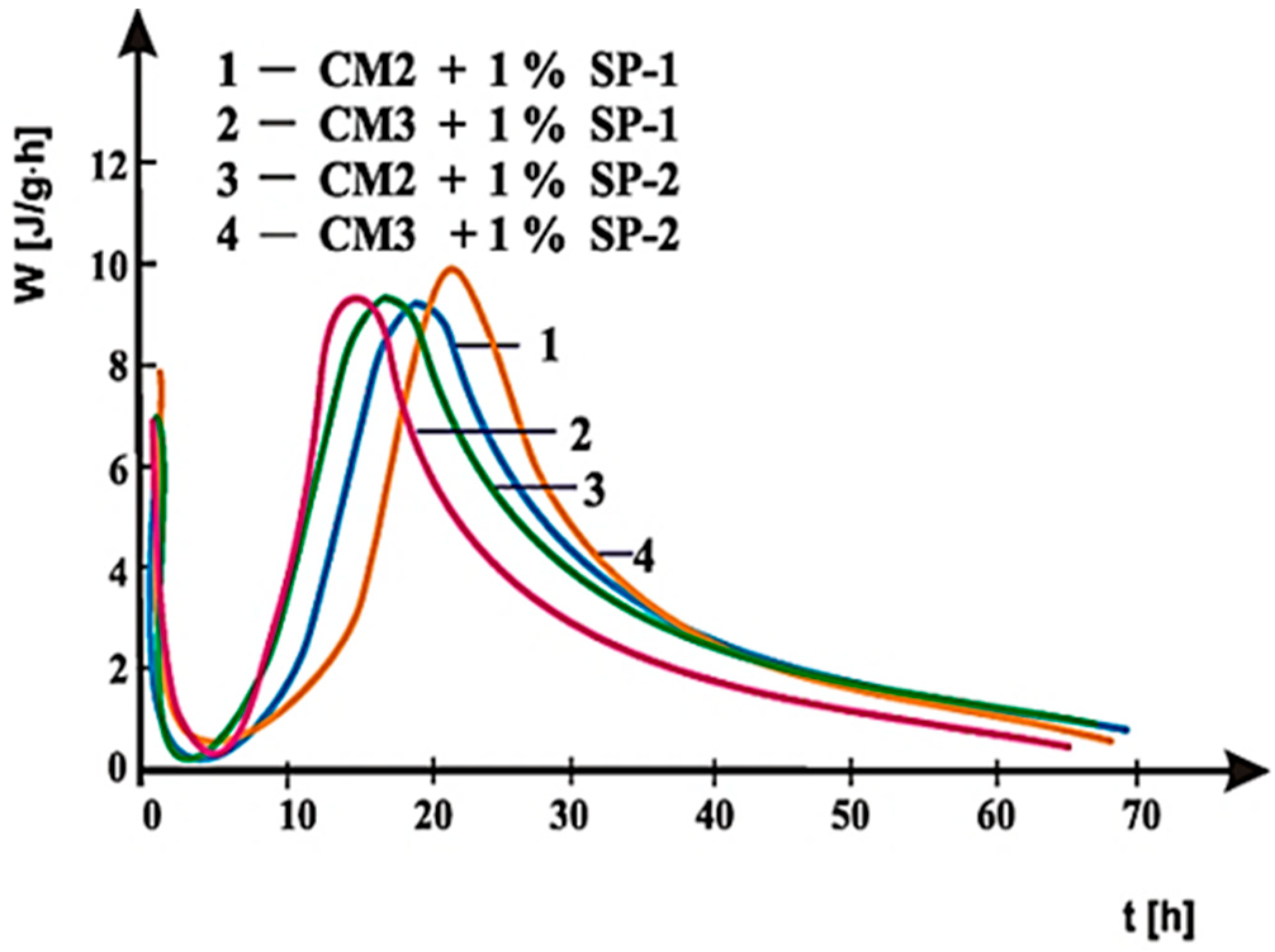
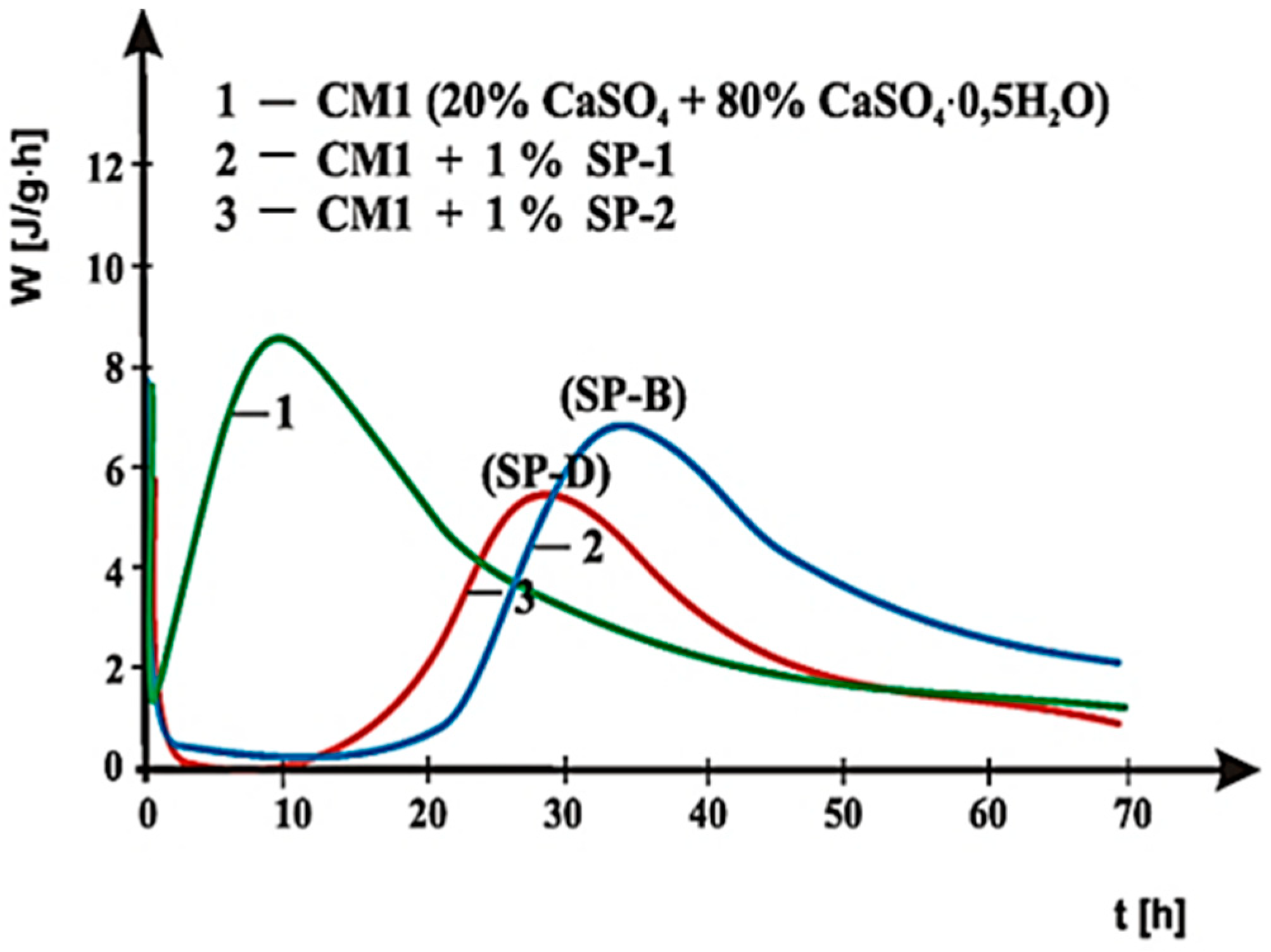
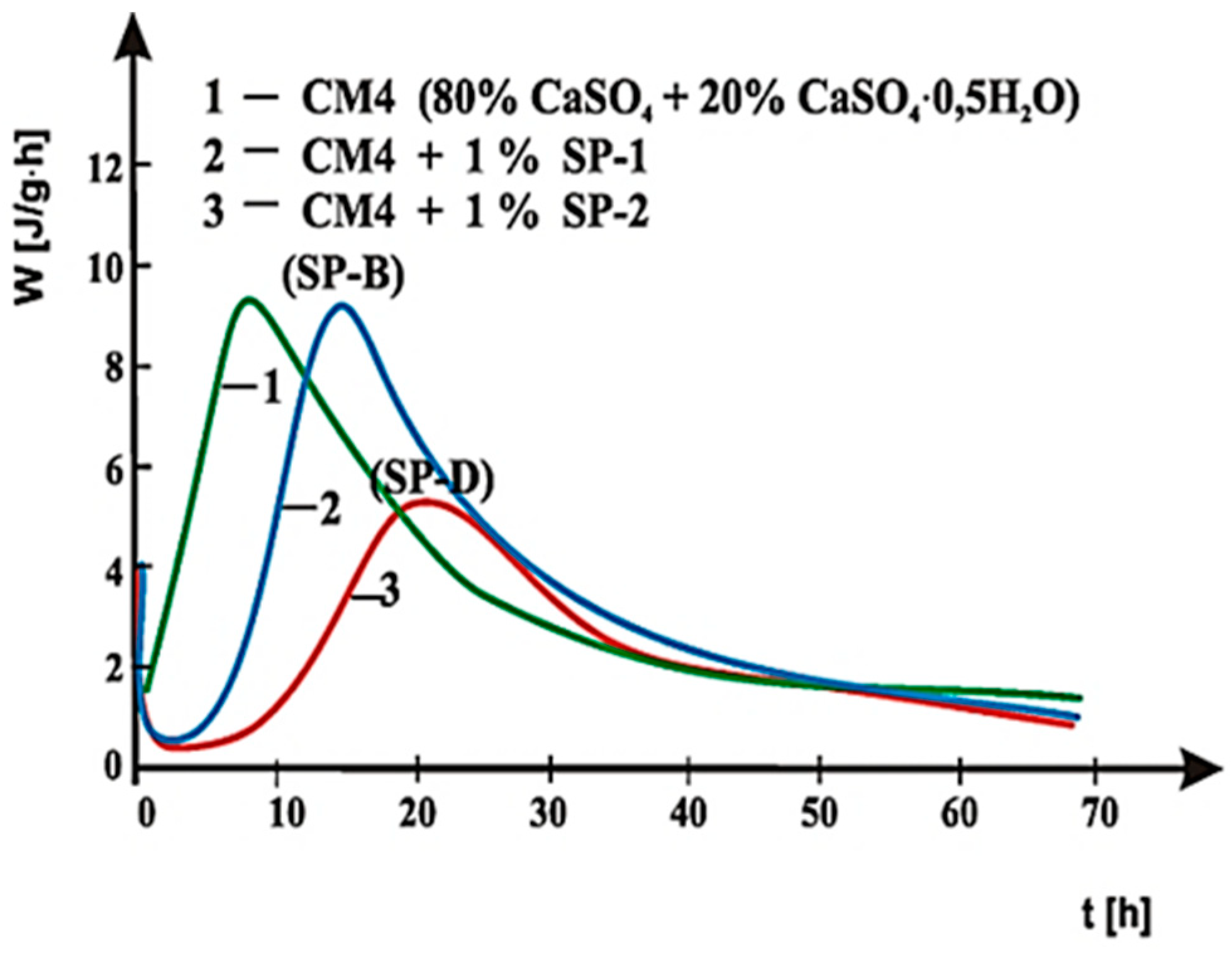
4. Results of Testing and their Interpretation
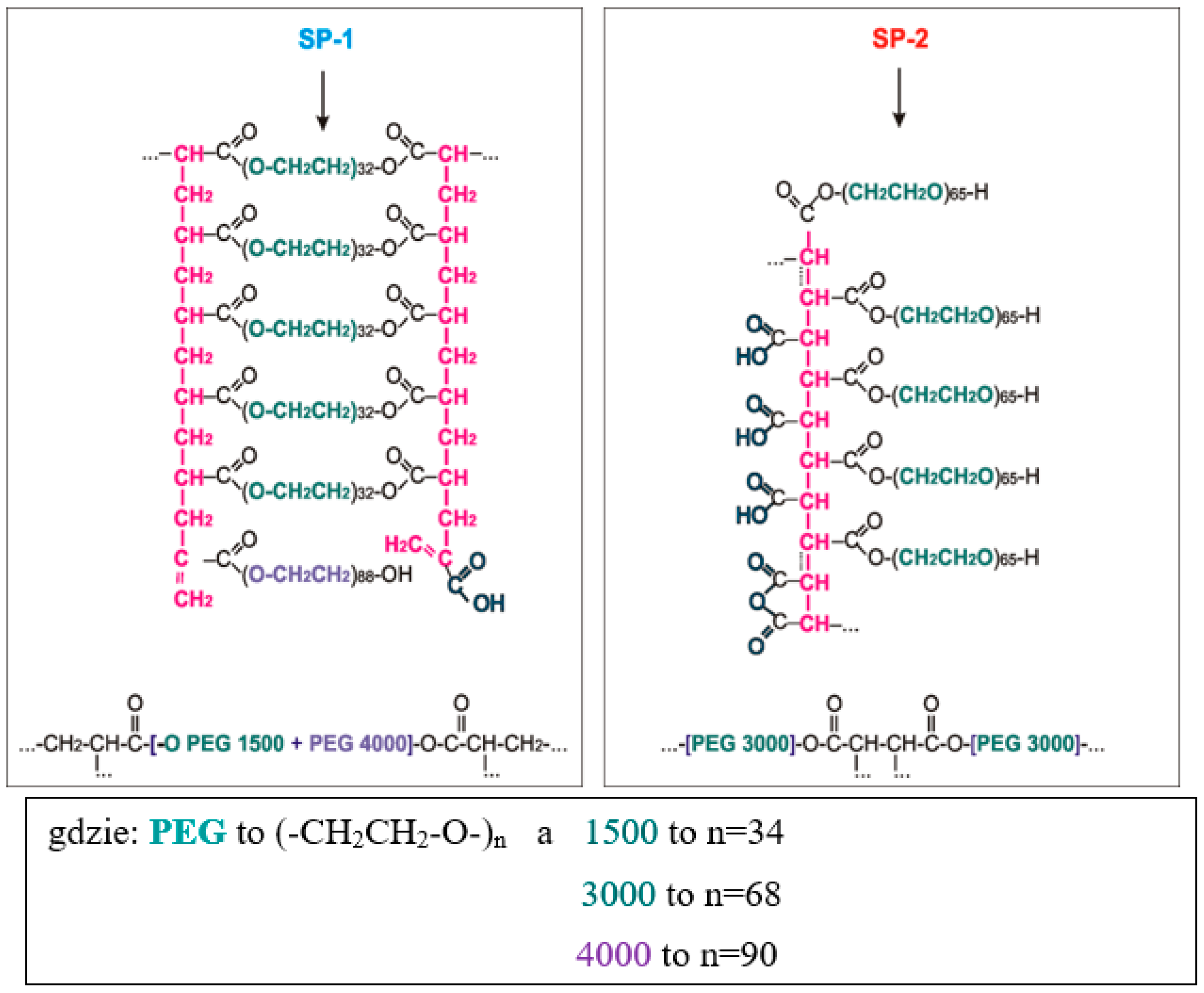
4.1. Test Results of Superplasticizers Structure
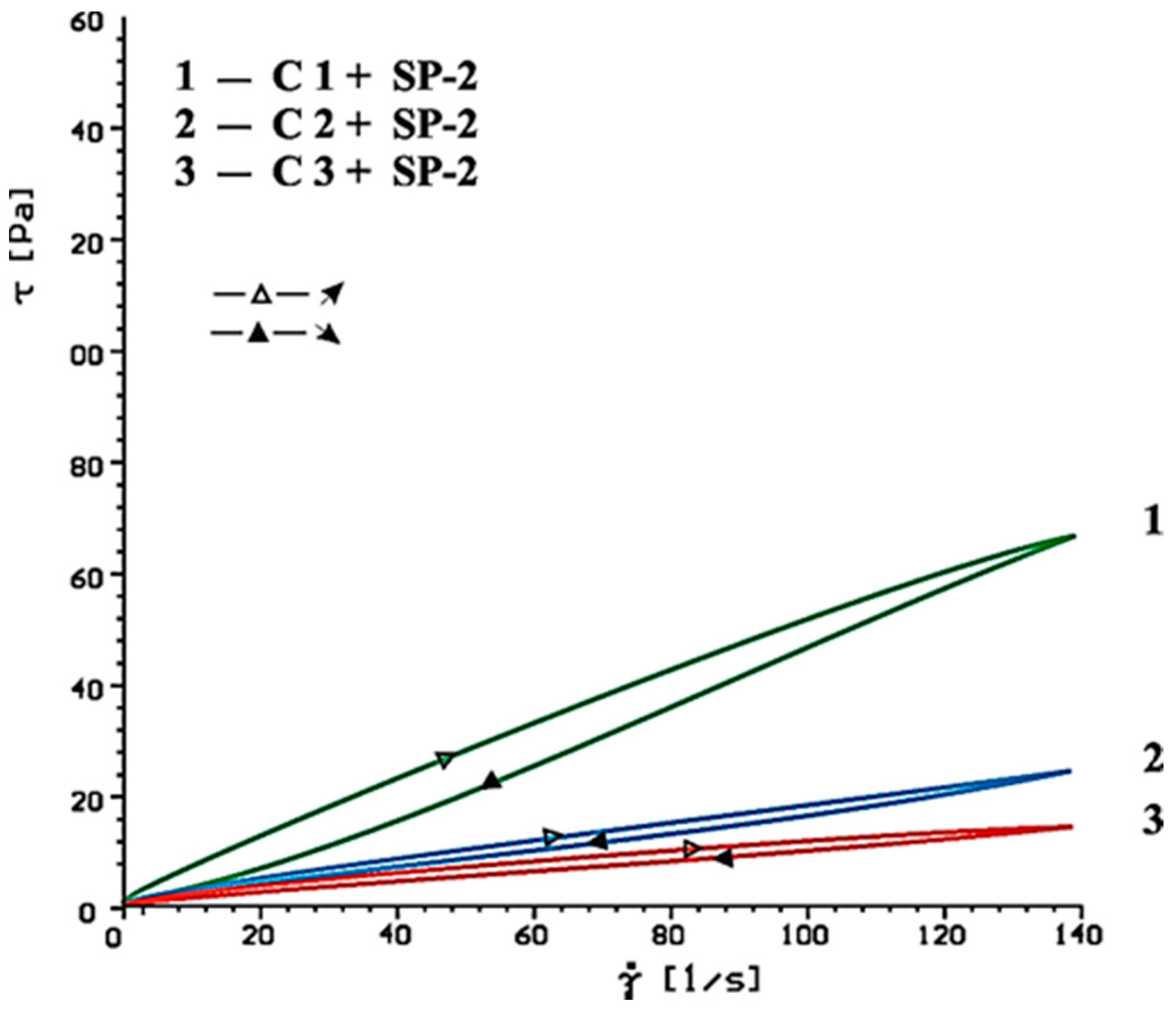
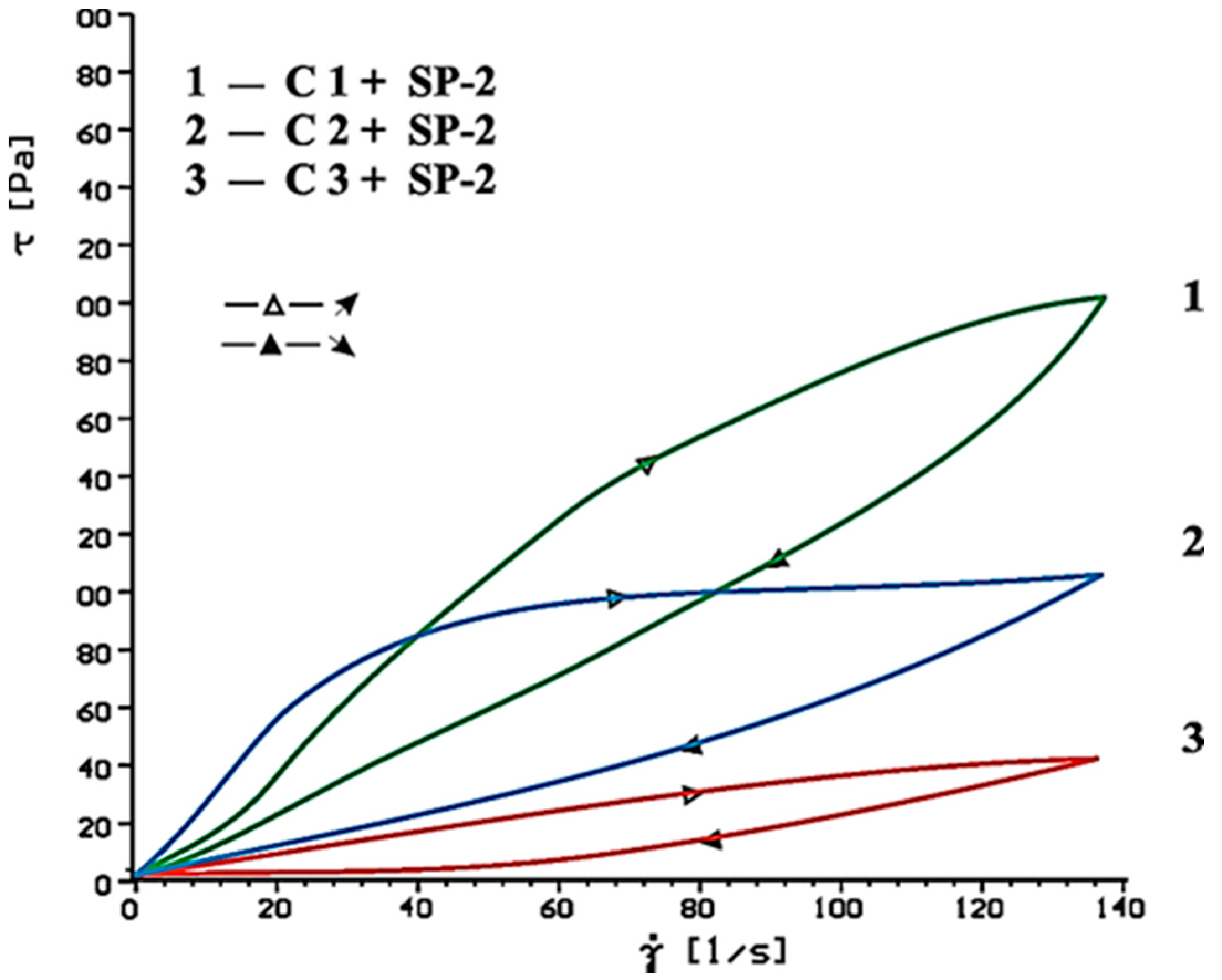
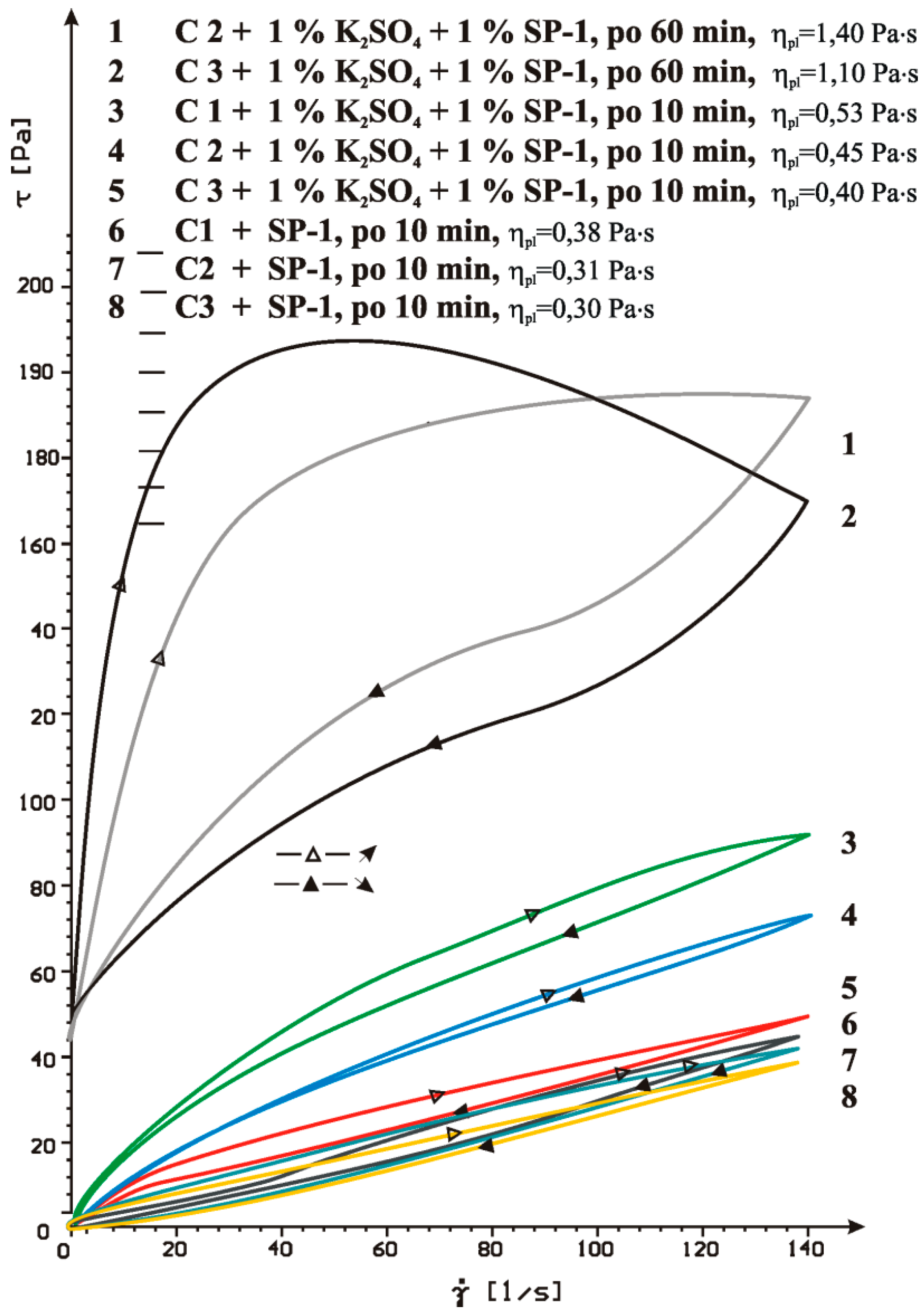
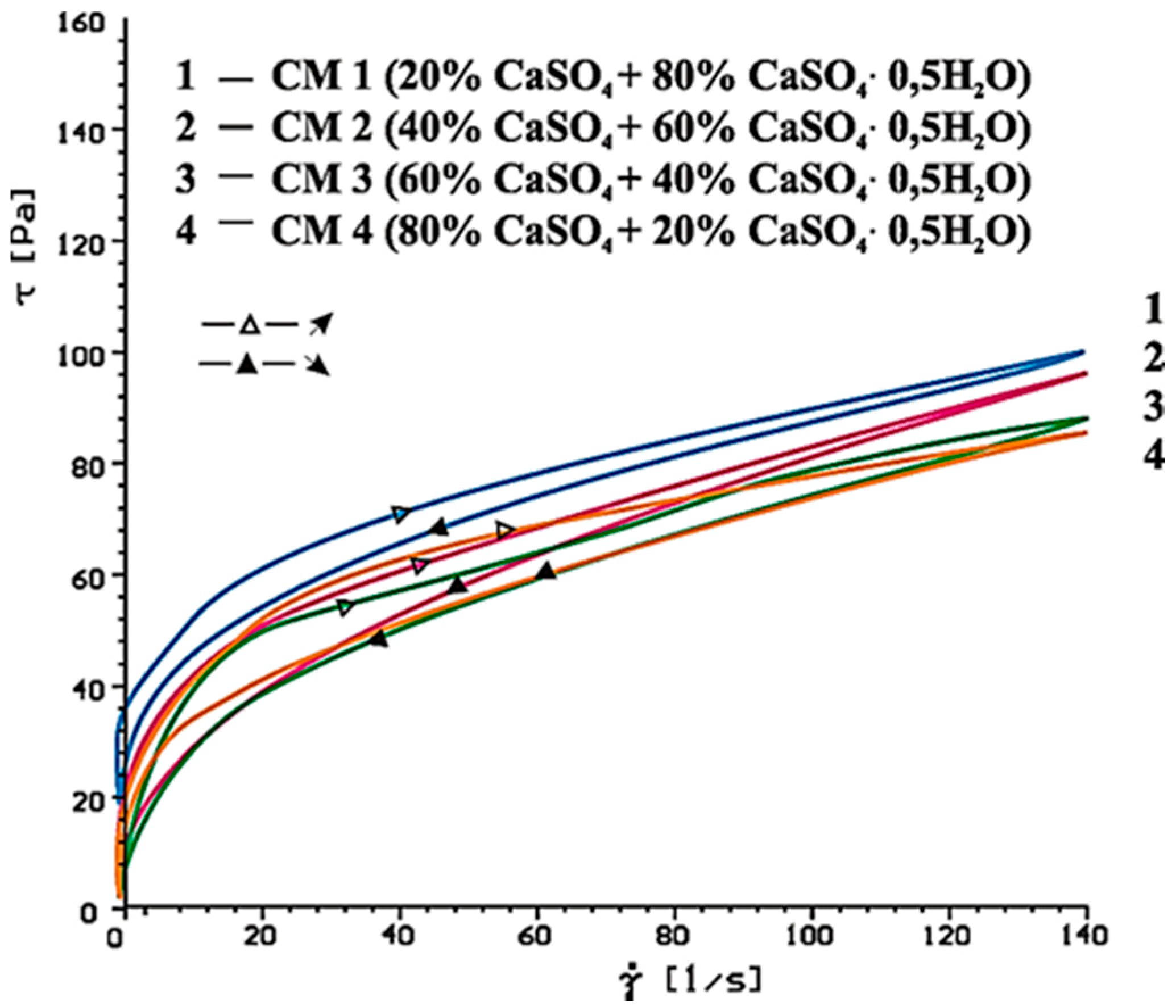
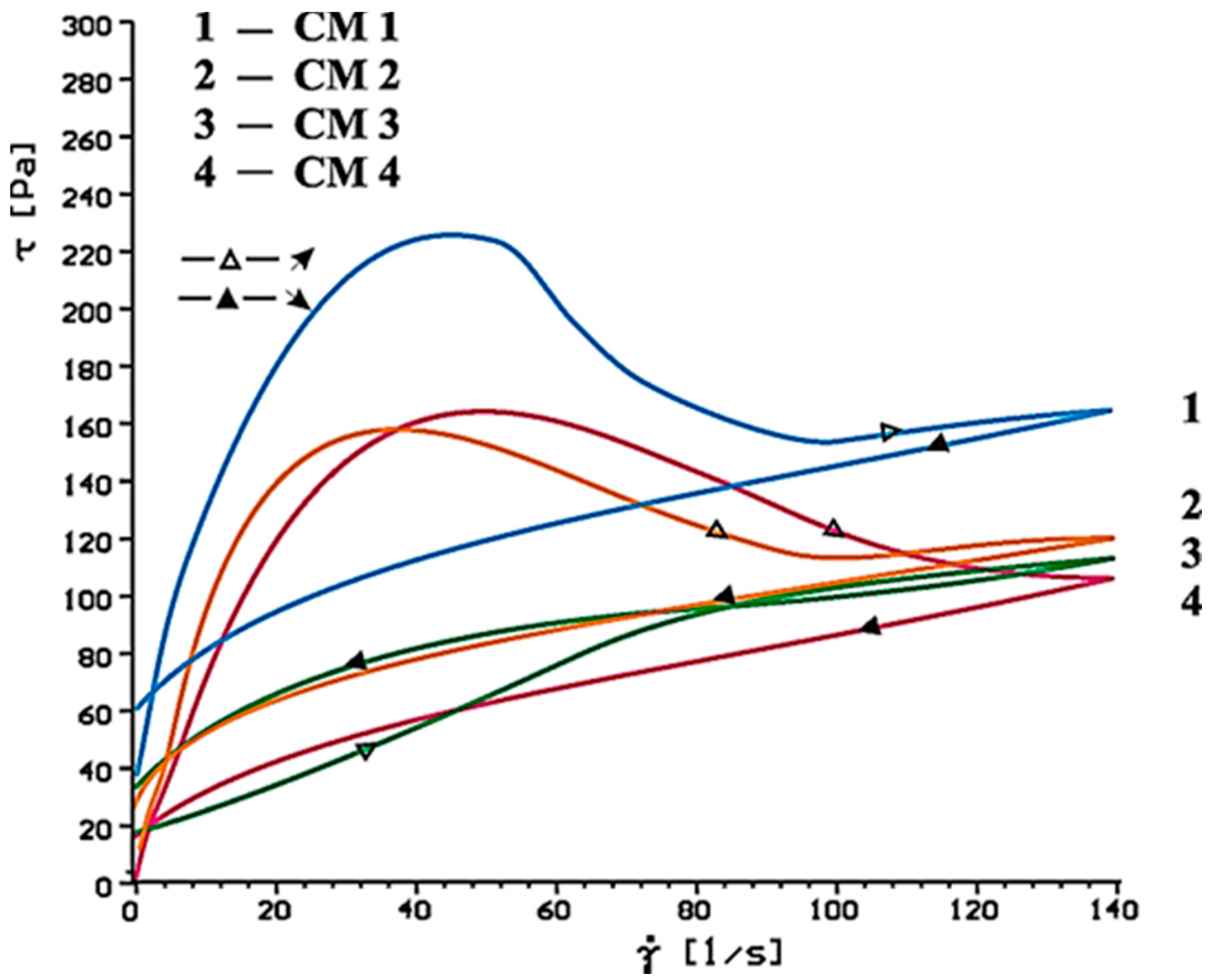
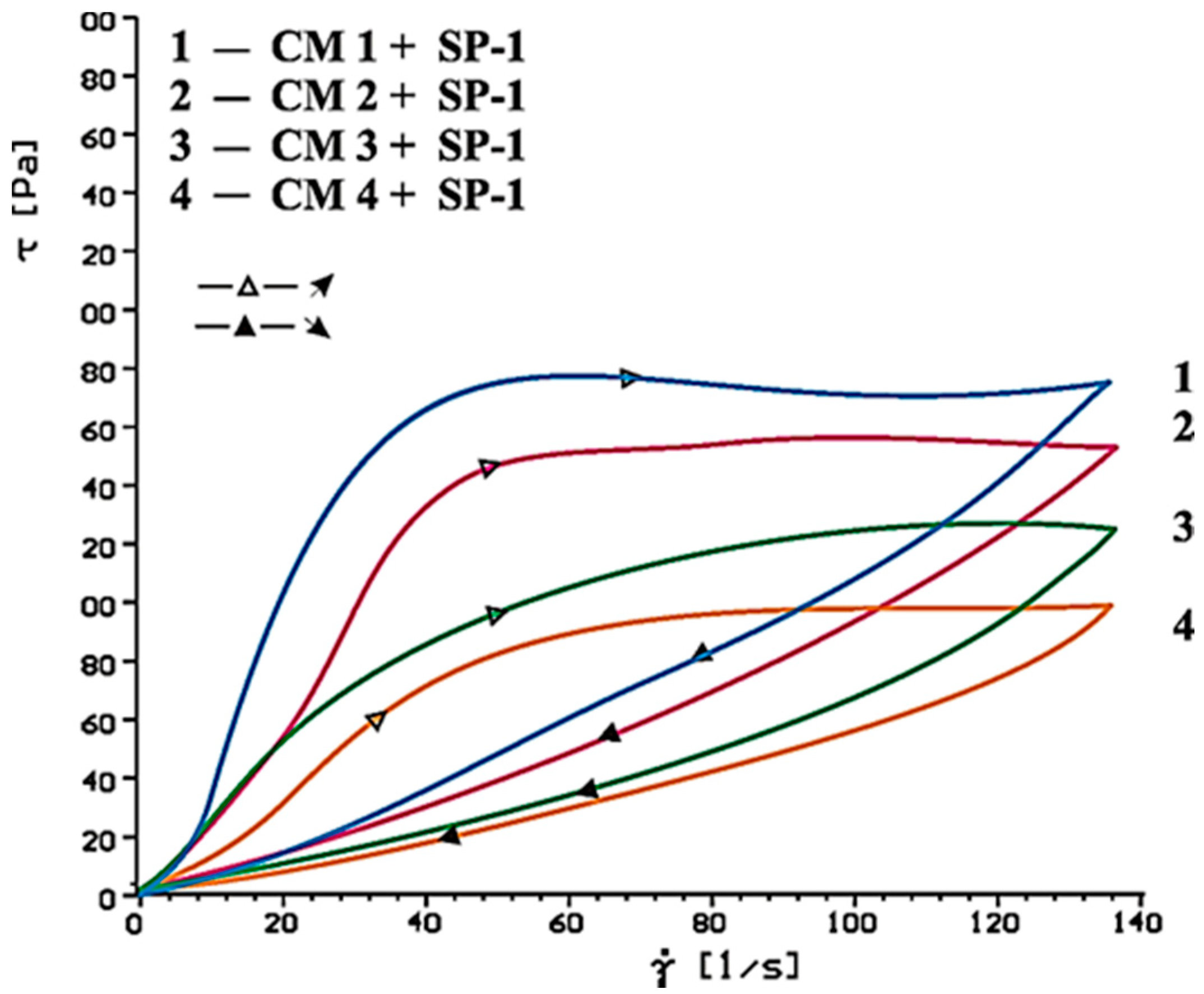
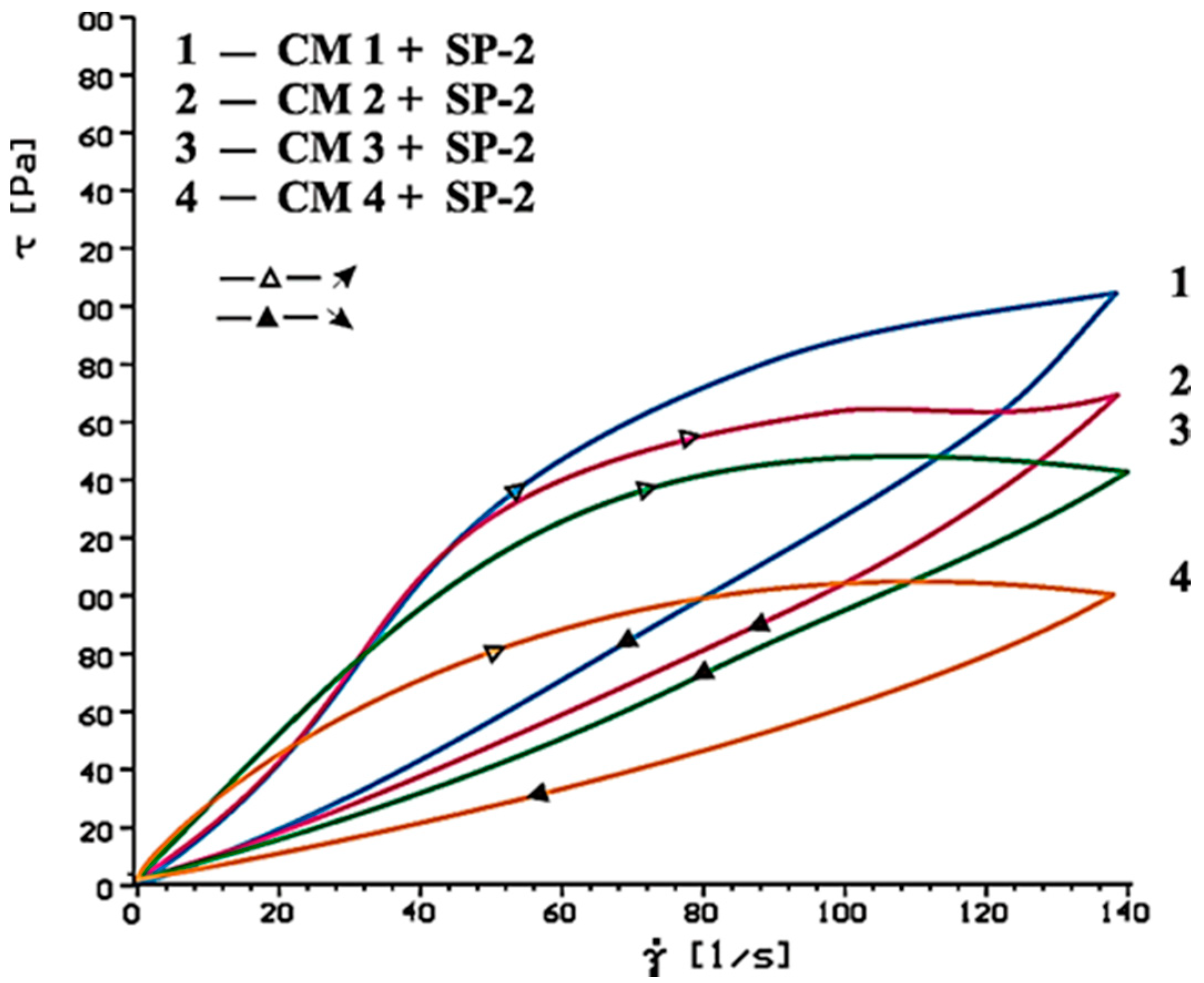
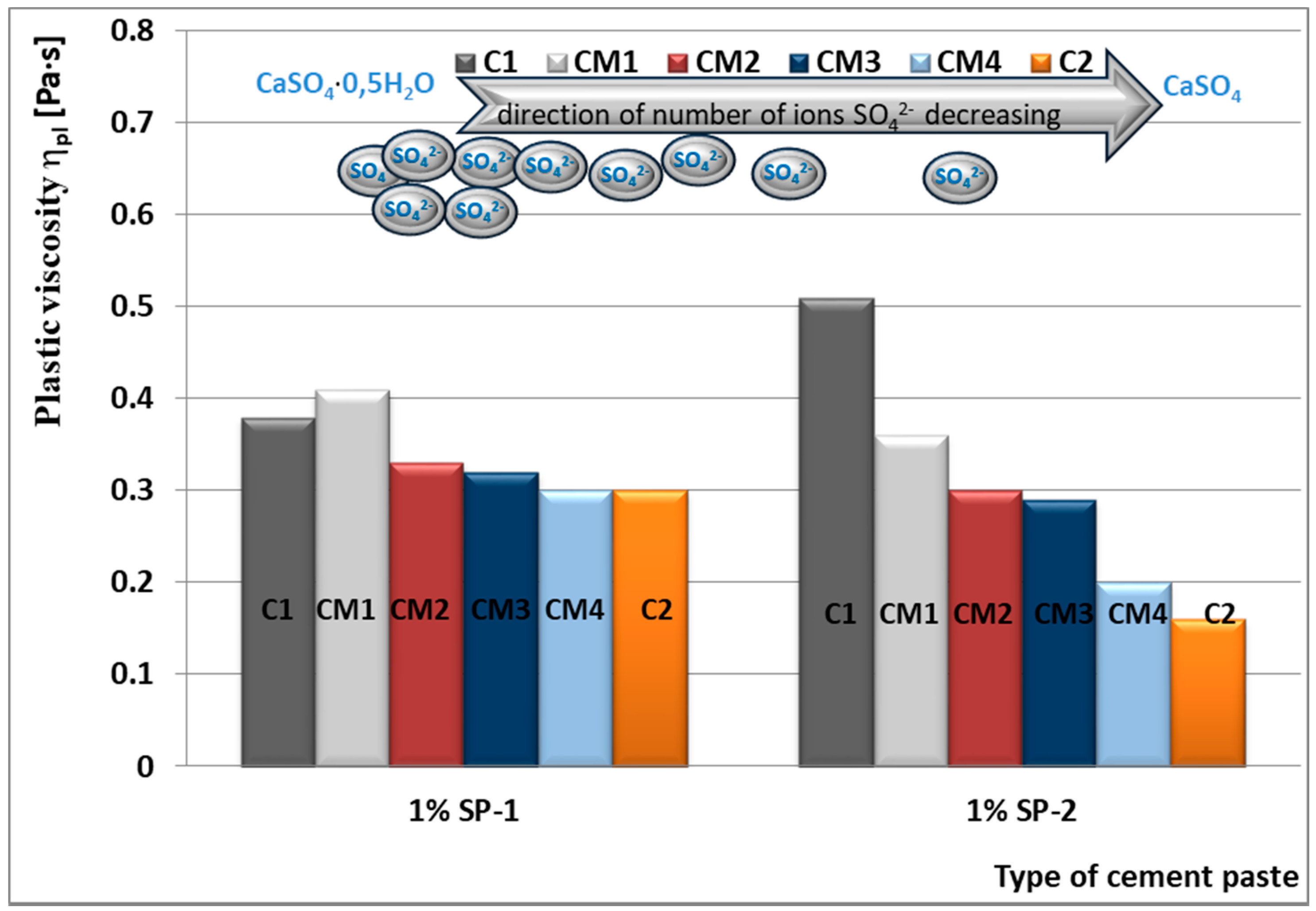
4.2. Results of Rheological Testing
5. Discussion
6. Conclusions
- A superplasticizer efficiency depends on its molecular structure. The most significant features are: Hydrophilicity, pure polymer content, weight-averaged molecular mass (Mw) of the superplasticizer polymer, presence of free carboxylic acids and anhydrides in solid samples, content of free poly(ethylene glycols) non-reacted with acid and anhydride.
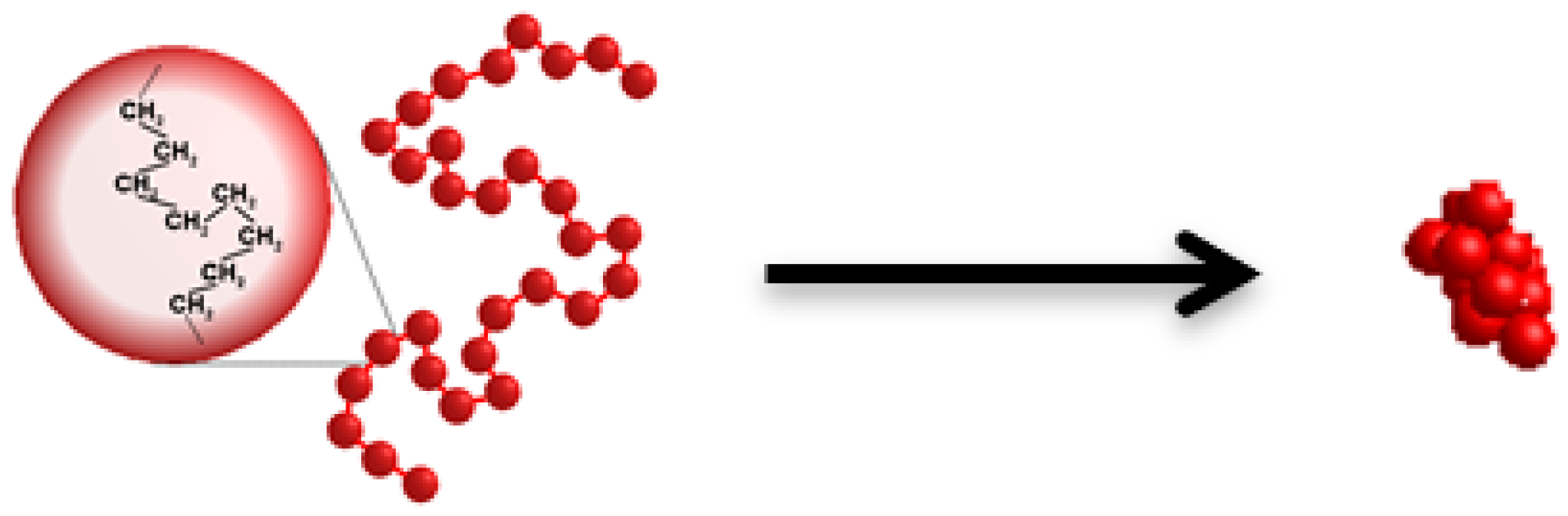
- SP-2 superplasticizer that contains the shorter backbone chain, long side chains and greater number of carboxylate groups (COO−) shows higher hydrophilicity than SP-1 superplasticizer that is built of the longer backbone chain with shorter side chains and contains fewer COO− groups.
- It was found that the superplasticizer (SP-2), with the higher hydrophilicity showed the higher efficiency in pastes containing calcium sulphates (gypsum dihydrate and anhydrite) used as the setting time regulator, than the superplasticizer with lower hydrophilicity (SP-1), despite belonging to the same polycarboxylate group.
- SP-1 superplasticizer with lower hydrophilicity and shorter side chains with the long backbone chain that contains small number of COO− group, is more resistant to the impact of sulphates. The increase of SO42− ions content in the paste by the introduction of CaSO4·0.5H2O or increasing amounts of CaSO4·0.5H2O in the mixture with CaSO4 generally does not change the viscosity of pastes, but it does promote greater delay of silicates hydration.
- In case of SP-2 superplasticizer that shows higher hydrophilicity and has the short backbone chain and more COO− groups (which suggests its better efficiency) the increase of SO42− ions content in the paste by introduction of CaSO4·0.5H2O or increasing amount of CaSO4·0.5H2O in the mixture with CaSO4, causes deterioration of rheological properties (reduction of fluidity degree) and affects the acceleration of silicates hydration.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, B.; Peng, Y.; Tan, H.; Lv, Z.; Deng, X. Effect of Polyacrylic Acid on Rheology of Cement Paste Plasticized by Polycarboxylate. Materials 2018, 11, 1081. [Google Scholar] [CrossRef] [Green Version]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Saout, G.L.; Müller, C.J.; Famy, C. Influence of the Calcium Sulphate Source on the Hydration Mechanism of Portland Cement–Calcium Sulphoaluminate Clinker–Calcium Sulphate Binders. Cem. Concr. Compos. 2011, 33, 551–561. [Google Scholar] [CrossRef]
- Locher, R.W.; Richartz, W.; Sprung, S. Erstarren von Zement. Teil II: Einfluß des Calciumsulfatzusatzes. Zem. Kalk Gips 1980, 33, 271–277. (In German) [Google Scholar]
- Borsoi, A.; Collepardi, S.; Copolla, L.; Troli, R.; Collepardi, E.M. Advances in Superplasticizers for Concrete Mixtures. Il Cemento 1999, 69, 234–244. [Google Scholar]
- Hu, K.; Sun, Z.; Yang, H. Effects of Polycarboxylate Superplasticizers with Different Functional Units on the Early Hydration Behavior of Cement Paste. J. Mater. Civ. Eng. 2019. [Google Scholar] [CrossRef]
- Kong, F.R.; Pan, L.S.; Wang, C.M.; Xu, N. Effects of Polycarboxylate Superplasticizers with Different Molecular Structure on the Hydration Behavior of Cement Paste. Constr. Build. Mater. 2016, 105, 545–553. [Google Scholar] [CrossRef]
- Ohta, A.; Sugiyama, T.; Tanaka, Y. Fluidizing Mechanism and Application of Polycarboxylate-Based Superplasticizers. In Proceedings of the 5th CANMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete (ACI. SP173), Rome, Italy, 7–10 October 1997; Malhotra, V.M., Ed.; American Concrete Institute: Farmington Hills, MI, USA, 1997; pp. 359–378. [Google Scholar]
- Wu, H.; Guo, H.; Lei, J.; Zhang, R.; Liu, Y. Research on Synthesis and Action Mechanism of Polycarboxylate Superplasticizer. Front. Chem. China 2007, 2, 322–325. [Google Scholar] [CrossRef]
- Uchikawa, H.; Hanechara, S.; Sawaki, D. The Role of Steric Repulsive Force in the Dispersion of Cement Particles with Organic Admixtures. Cem. Concr. Res. 1997, 27, 37–50. [Google Scholar] [CrossRef]
- Ran, Q.P.; Somasundara, P.; Miao, C.W.; Liu, J.P.; Shen, W.P. Effect of the Length of the Side Chains of Comb-Like Copolymer Dispersants on Dispersion and Rheological Properties of Concentrated Cement Suspensions. J. Colloid Interface Sci. 2009, 336, 624–633. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Wang, Z.; Zhu, J.; Cui, S.; Lan, M.; Li, H. Performances and Working Mechanism of a Novel Polycarboxylate Superplasticizer Synthesized through Changing Molecular Topological Structure. J. Colloid Interface Sci. 2017, 504, 12–24. [Google Scholar] [CrossRef]
- Holly, R.; Peemoeller, H.; Zhang, M.; Reardon, E.; Hansson, C.M. Magnetic Resonance in Situ Study of Tricalcium Aluminate Hydration in the Presence of Gypsum. J. Am. Ceram. Soc. 2006, 89, 1022–1027. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of Zeta Potential of Early Cement Hydration Phases on Superplasticizer Adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Plank, J.; Pöllmann, K.; Zouaoui, N.; Andres, P.R.; Schaefer, C. Synthesis and Performance of Methacrylic Ester Based Polycarboxylate Superplasticizers Possessing Hydroxy Terminated Poly(Ethylene Glycol) Side Chains. Cem. Concr. Res. 2008, 38, 1210–1216. [Google Scholar] [CrossRef]
- Yoshioka, K.; Tazawa, E.I.; Kawai, K.; Enohata, T. Adsorption Characteristics of Superplasticizers on Cement Component Minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B.; de Reese, J. Experimental Determination of the Thermodynamic Parameters Affecting the Adsorption Behaviour and Dispersion Effectiveness of PCE Superplasticizers. Cem. Concr. Res. 2001, 40, 699–709. [Google Scholar] [CrossRef]
- Yamada, K.; Hanehara, S.; Honma, K. Effects of the Chemical Structure on the Properties of Polycarboxylate-Type Superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Ohta, A.; Sugiyama, T.; Uomoto, T. Study of Dispersing Effects of Polycarboxylate-Based Dispersant on Fine Particles. In Proceedings of the 6th CANMET/ACI International Conference Superplasticizers and other Chemical Admixtures in Concrete (SP-195-14), Nice, France, 10–13 October 2000; Malhotra, V.M., Ed.; American Concrete Institute: Farmington Hills, MI, USA, 2000; pp. 211–228. [Google Scholar]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Gotz, T. Effects of the Molecular Architecture of Comb-Shaped Superplasticizers on Their Performance in Cementitious Systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Kissa, E. Dispersions: Characterization. Testing and Measurement (Surfactant Science. No. 84); CRC Press/M. Dekker: New York, NY, USA, 15 June 1999. [Google Scholar]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of Polycarboxylate-Based Superplasticizers with Cements Containing Different C3A Amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
- Bjömström, J.; Chandra, S. Effect of Superplasticizers on the Rheological Properties of Cements. Mater. Struct. Matriaux Constr. 2003, 36, 685–692. [Google Scholar] [CrossRef]
- Flatt, R.J.; Houst, J.F. A Simplified View on Chemical Effects Perturbing the Action of Superplasticizers. Cem. Concr. Res. 2001, 31, 1169–1176. [Google Scholar] [CrossRef]
- Kim, B.G.; Jiang, S.; Jolicoeur, C.; Aitcin, P.-C. The Adsorption Behavior of PNS Superplasticizer and its Relation to Fluidity of Cement Paste. Cem. Concr. Res. 2000, 30, 887–893. [Google Scholar] [CrossRef]
- Pourchet, S.; Liautaud, S.; Rinaldi, D.; Pochard, I. Effect of the Repartition of the PEG Side Chains on the Adsorption and Dispersion Behaviors of PCP in Presence of Sulfate. Cem. Concr. Res. 2012, 42, 431–439. [Google Scholar] [CrossRef]
- Uchikawa, H.; Sawaki, D.; Hanehara, S. Influence of Kind and Added Timing of Organic Admixture on the Composition, Structure and Property of Fresh Cement Pastes. Cem. Concr. Res. 1995, 25, 353–364. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Simard, M.A. Chemical Admixture-Cement Interactions: Phenomenology and Physico-Chemical Concepts. Cem. Concr. Compos. 1998, 20, 87–101. [Google Scholar] [CrossRef]
- Grierson, L.H.; Knight, J.C.; Maharaj, R. The Role of Calcium Ions and Lignosulphonate Plasticiser in the Hydration of Cement. Cem. Concr. Res. 2005, 35, 631–636. [Google Scholar] [CrossRef]
- Bombled, J.P. Influence of Sulfates on the Rheological Behavior of Cement Pastes and on their Evolution. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, 1 January 1980; Volume VI, pp. 164–169. [Google Scholar]
- Griesser, A. Cement-Superplasticizer Interactions at Ambient Temperatures: Rheology. Phase Composition. Pore Water and Heat of Hydratation of Cementitious Systems. Master’s Thesis, Technische Wissenschaften ETH, Zürich, Switzerland, 2003. 14820. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic: London, UK, 1990. [Google Scholar]
- Jiang, S.; Kim, B.G.; Aitcin, P.C. Importance of Adequate Soluble Alkali Content to Ensure Cement/Superplasticizer Compatibility. Cem. Concr. Res. 1999, 29, 71–78. [Google Scholar] [CrossRef]
- Hanehara, S.; Yamada, K. Rheology and Early Age Properties of Cement Systems. Cem. Concr. Res. 2008, 21, 175–195. [Google Scholar] [CrossRef]
- Roberts, L.R. Dealing with Cement Admixture Interactions. In Proceedings of the 23rd Annual Convention of the Institute of Concrete Technology, Telford, UK, 11 April 1995. [Google Scholar]
- Jolicoeur, C.; Nkinamubanzi, P.C.; Simard, M.A.; Piotte, M. Progress in Understanding the Functional Properties of Superplasticizer in Fresh Concrete. Am. Concr. Inst. 1994, SP148, 63–88. [Google Scholar]
- Sakai, E.; Takayuki, K.; Tomomi, S.; Kiyoshi, A.; Masaki, D. Influence of Superplasticizers on the Hydration of Cement and the Pore Structure of Hardened Cement. Cem. Concr. Res. 2006, 36, 2049–2053. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yamada, K. The Effect of the Kind of Calcium Sulfate in Cements on the Dispersing Ability of Poly ß -Napthalene Sulfonate Condensate Superplasticizer. Cem. Concr. Res. 2004, 34, 839–844. [Google Scholar] [CrossRef]
- Plank, J.; Zhimin, D.; Keller, H.; Hössle, F.; Seidl, W. Fundamental Mechanisms for Polycarboxylate Intercalation into C3A Hydrate Phases and the Role of Sulfate Present in Cement. Cem. Concr. Res 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Hanna, E.; Ostiguy, M.; Khalifé, K.; Stoica, O.; Kim, B.G.; Bédard, C.; Saric-Coric, M.; Baalbaki, M.; Jiang, S.; Nkinamubanzi Aïtcin, P.C.; et al. The Importance of Superplasticizers in Modern Concrete Technology. In Proceedings of the CANMET/ACI International Conference on Superplasticizers and other Chemical Admixtures in Concrete, ACI (SP), Nice, France, 10–13 October 2000. [Google Scholar]
- Bensted, J. Cement science—is it simple? Cem. Lime Concr. 2001, 1, 6–18. [Google Scholar]
- Janowska-Renkas, E. The Effect of Superplasticizers’ Chemical Structure on Their Efficiency in Cement Pastes. Constr. Build. Mater. 2013, 38, 1204–1210. [Google Scholar] [CrossRef]
- Grzeszczyk, S.; Sudoł, M. Effect of the Chemical Structures of Superplasticizers upon the Rheological Properties of Cement Pastes. In Proceedings of the 7th Canmet/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Berlin, Germany, 20–23 October 2003; Supplementary papers. pp. 363–377. [Google Scholar]
- Scholz, E. Zum Einfluss des Calciumsulfates in Portlandzementen auf Konsistenz und Ansteifen von Normmörteln; Institut für Baustoffkunde und Materialprüfung: Hannover, Germany, 1992. [Google Scholar]
- Bundyra-Oracz, G. Influence of Calcium Sulphate on the Reheological Behawior of Cement Paste. In Proceedings of the Conference, Dni betonu: Tradycja i nowoczesność, Stowarzyszenie Producentów Cementu, Wisła, Cracow, Poland, 9–11 October 2006. (In Polish). [Google Scholar]
- Aïtcin, P.C. Admixtures: Essentional Components of Modern Concrete. Cem. Lime Concr. 2006, 5, 227–284. [Google Scholar]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of Polyelectrolytes and Its Influence on the Rheology. Zeta Potential and Microstructure of Various Cement and Hydrate Phases. J. Colloid Interface Sci. 2008, 32, 301–312. [Google Scholar] [CrossRef]
- Morrison, R.T.; Boyd, R.N. Organic Chemistry; Państwowe Wydawnictwo Naukowe (PWN): Warsaw, Poland, 1985. (In Polish) [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Państwowe Wydawnictwo Naukowe (PWN): Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Andersen, R.J.; Roy, D.M.; Gaidis, J.M. The Effect of Superplasticizers Molecular Weight on its Adsorption, and Dispersion of Cement. Cem. Concr. Res. 1988, 18, 980–986. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chiu, J.J.; Chen, S.D.; Tseng, Y.C. Effect of Addition Time of a Superplasticizer on Cement Adsorption and on Concrete Workabilyt. Cem. Concr. Compos. 1999, 21, 425–430. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, S.; Hanehara, S. Controlling of the Adsorption and Dispersing Force of Polycarboxylate-Type Superplasticizer by Sulfate Ion Concentration in Aqueous Phase. Cem. Concr. Res. 2001, 31, 375–383. [Google Scholar] [CrossRef]
- Lewandowski, K. Globular and Micellar Polymer Structures—Computer Simulations Study. Ph.D. Thesis, Department of Quantum Physics, Faculty of Physics, Adam Mickiewicz University, Poznań, Poland, 2012. [Google Scholar]
- Czarnecki, L. Domieszki do betonu–Możliwości i ograniczenia. Bud. Technol. Archit. Zesz. Spec. Domieszki Betonu 2003, 4–6. (In Polish) [Google Scholar]
- Ohama, Y. Polymer-Based Admixtures. Cem. Concr. Compos. 1998, 20, 189–212. [Google Scholar] [CrossRef]
- Rudnicki, T. Natural and Synthetic Admixtures Plasticize the Tail and Mechanisms of Their Interaction in the Concrete Mix. Autostrady Mag. 2004, 4, 22–25. [Google Scholar]



| Component | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | Cl− | CaOfree | C3S | C2S | C4AF | C3A | Blaine Surface [m2/kg] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinker K [wt%] | 21.0 | 3.1 | 5.3 | 65.9 | 0.8 | 1.4 | 0.1 | 0.9 | 0.04 | 0.3 | 66.0 | 11.0 | 10.0 | 8.7 | 314.0 |
| Cement | Type of Cement Setting Regulator | Clinker | SO3 Content [% by Mass] | ||
|---|---|---|---|---|---|
| [% by Mass] | CaSO4 | CaSO4·0.5H2O | CaSO4·2H2O | ||
| C1 | 100% CaSO4·0.5H2O | 95.8 | - | 2.33 | - |
| C2 | 100% CaSO4 | 96.0 | 2.33 | - | - |
| C3 | 100% CaSO4·2 H2O | 95.0 | - | - | 2.33 |
| CM1 | 20% CaSO4 + 80% CaSO4·0.5H2O | 95.8 | 0.47 | 1.86 | - |
| CM2 | 40% CaSO4 + 60% CaSO4·0.5H2O | 95.9 | 0.93 | 1.40 | - |
| CM3 | 60% CaSO4 + 40% CaSO4·0.5H2O | 95.9 | 1.40 | 0.93 | - |
| CM4 | 80% CaSO4 + 20% CaSO4·0.5H2O | 96.0 | 1.86 | 0.47 | - |
| Time [min] | Concentration of SO42− [mg/L] | ||
|---|---|---|---|
| CaSO4·0.5H2O | CaSO4·2H2O | CaSO4 | |
| 10 | 1,544.41 | 700.53 | 559.68 |
| 20 | 1,599.62 | 1078.20 | 574.91 |
| 30 | 1,777.82 | 1147.45 | 639.65 |
| 40 | 1,876.31 | 1221.47 | 898.46 |
| 60 | 1,966.06 | 1268.98 | 945.60 |
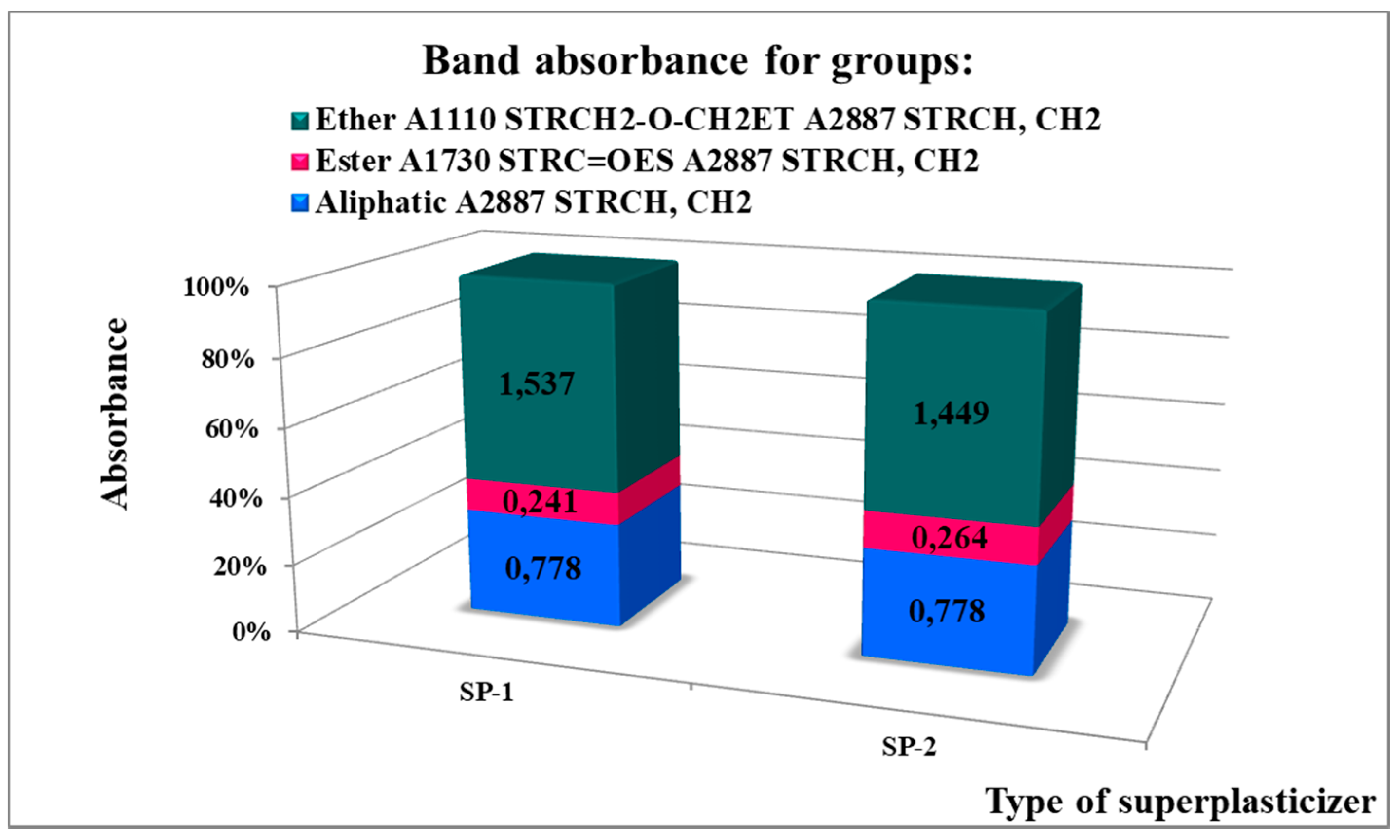
| SP Specimen | SP Hydrophilicity | Mass Fraction of Polymer | Absorbance of Ether Band | ||
|---|---|---|---|---|---|
| AET1110/AES1730 | in SP (Fraction 1) | in PEG (Fractions 2 and 3) | in SP (Fraction 1) | in PEG (Fractions 2 and 3) | |
| SP-1 | 3.30 | 0.517 | 0.483 | 0.793 | 0.742 |
| SP-2 | 4.53 | 0.825 | 0.175 | 1.195 | 0.254 |
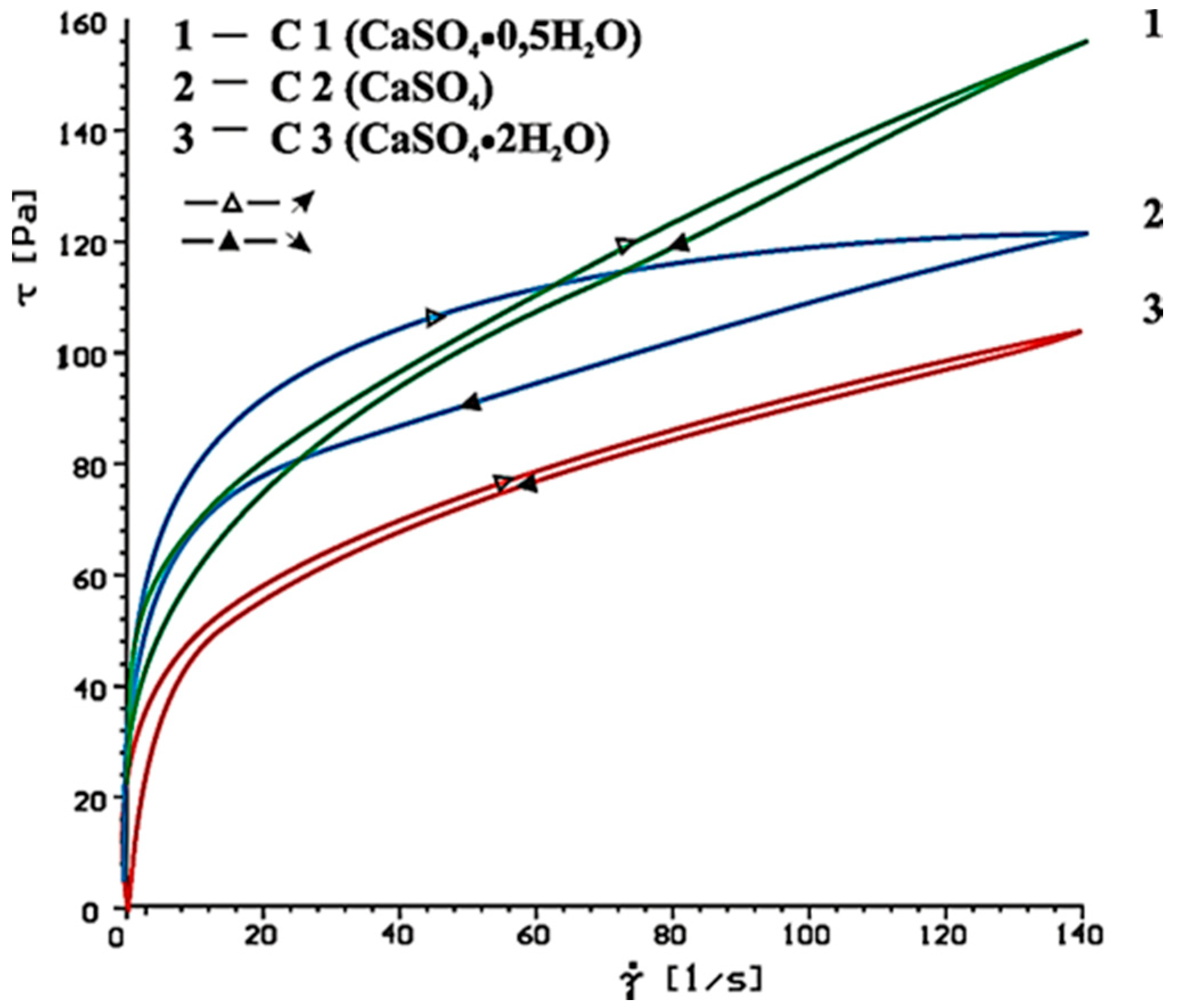
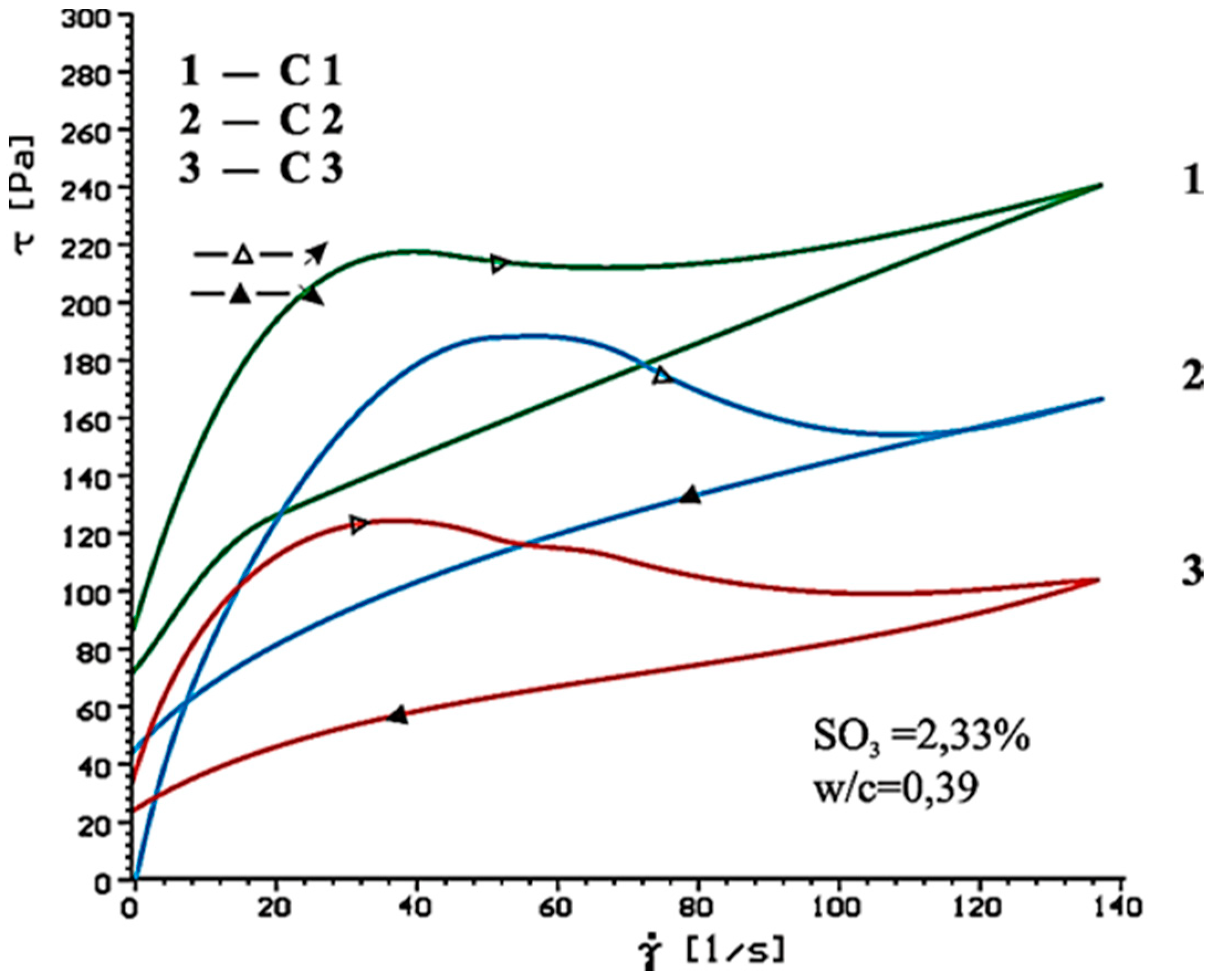
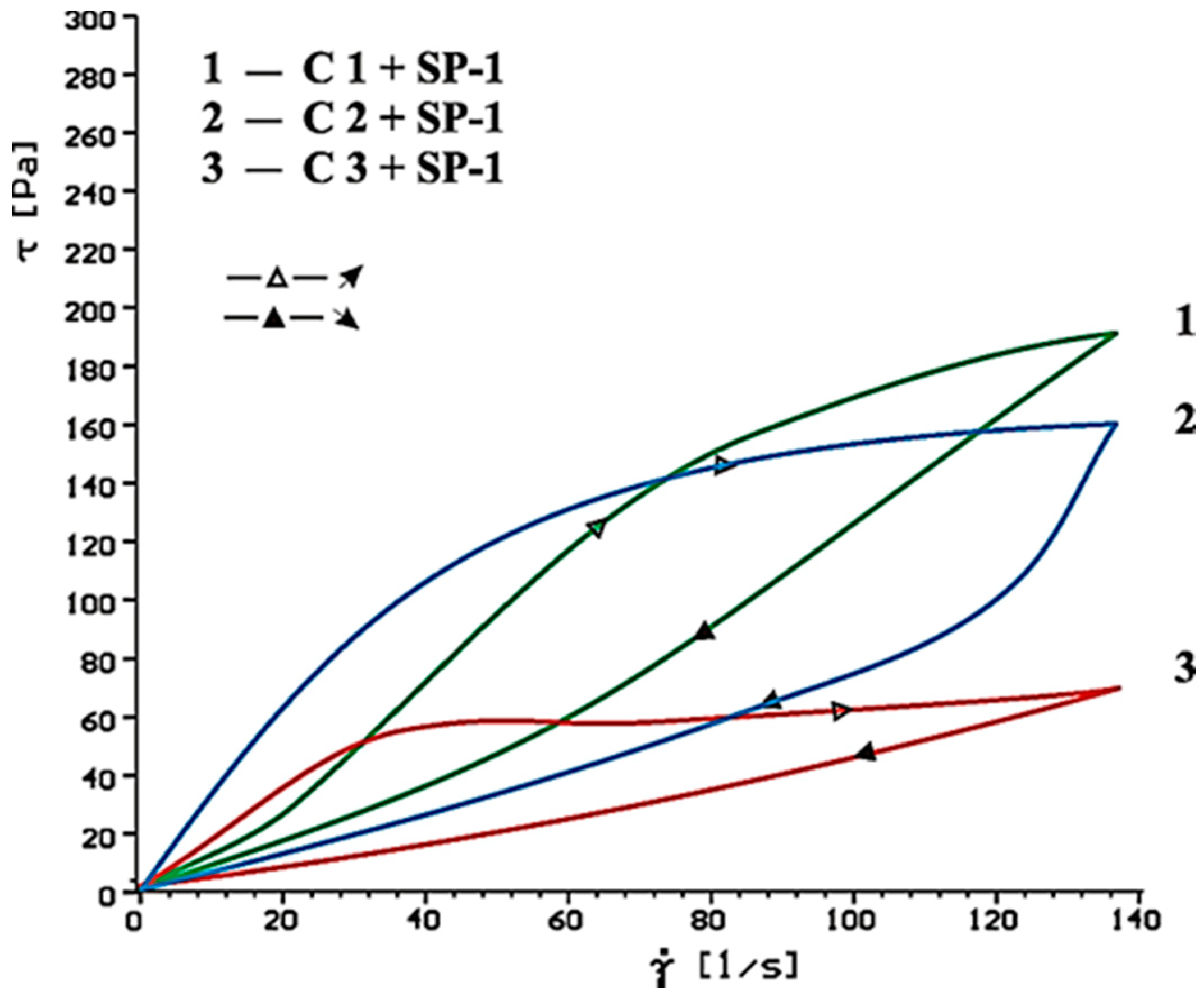
| Type of Paste | Time [min] | Without Superplasticizer | 1% SP-1 | 1% SP-2 | |
|---|---|---|---|---|---|
| τ0 [Pa] | ηpl [Paּs] | ηpl [Paּs] | ηpl [Paּs] | ||
| C1(clinker+CaSO4·0.5H2O) | 10 | 69.9 | 0.63 | 0.38 | 0.51 |
| 60 | 105.9 | 0.94 | 1.49 | 1.50 | |
| C2 (clinker + CaSO4) | 10 | 74.6 | 0.36 | 0.31 | 0.16 |
| 60 | 68.2 | 0.67 | 0.80 | 0.74 | |
| C3 (clinker + CaSO4·2H2O) | 10 | 54.9 | 0.32 | 0.30 | 0.10 |
| 60 | 52.9 | 0.43 | 0.49 | 0.33 | |
| Type of Paste | Time, t[min] | 1% SP1 | 1% SP2 |
|---|---|---|---|
| ηpl [Paּs] | ηpl [Paּs] | ||
| CM 1 (Clinker + 20% CaSO4 + 80% CaSO4·0.5 H2O) | 10 | 0.41 | 0.36 |
| 60 | 1.33 | 1.55 | |
| CM 2 (Clinker + 40% CaSO4 + 60% CaSO4·0.5 H2O) | 10 | 0.33 | 0.30 |
| 60 | 1.20 | 1.39 | |
| CM 3 (Clinker + 60% CaSO4 + 40% CaSO4·0.5 H2O) | 10 | 0.32 | 0.29 |
| 60 | 0.89 | 1.05 | |
| CM 4 (Clinker + 80% CaSO4 + 20% CaSO4·0.5 H2O) | 10 | 0.30 | 0.20 |
| 60 | 0.74 | 0.75 |
| Type of Paste | Quantity of Heat Evolved [J/g] after | ||
|---|---|---|---|
| 12 h | 24 h | 48 h | |
| C1 | 131.7 | 264.1 | 383.0 |
| C1 + SP-1 | 42.7 | 148.1 | 317.2 |
| C1 + SP-2 | 17.3 | 134.9 | 201.4 |
| C2 | 93.7 | 171.5 | 251.1 |
| C2 + SP-1 | 30.7 | 111.4 | 242.5 |
| C2 + SP-2 | 5.2 | 16.8 | 159.2 |
| C3 | 66.5 | 122.6 | 160.4 |
| C3 + SP-1 | 3.1 | 71.5 | 138.7 |
| C3 + SP-2 | 2.6 | 19.3 | 81.6 |
| CM1 | 66.1 | 133.2 | 191.5 |
| CM1 + SP-1 | 1.7 | 5.0 | 126.1 |
| CM1+ SP-2 | 4.2 | 28.6 | 125.8 |
| CM2 | 68.7 | 137.2 | 193.4 |
| CM2 + SP-1 | 10.5 | 101.4 | 184.4 |
| CM2 + SP-2 | 5.9 | 52.9 | 160.3 |
| CM3 | 69.5 | 138.9 | 198.6 |
| CM3 + SP-1 | 30.2 | 117.9 | 185.4 |
| CM3 + SP-2 | 5.5 | 84.5 | 180.8 |
| CM4 | 71.6 | 140.6 | 205.1 |
| CM4 + SP-1 | 23.2 | 112.7 | 191.3 |
| CM4 + SP-2 | 7.2 | 57.1 | 122.6 |
| Type of Cement | SP-1 | SP-2 |
|---|---|---|
| C1 | 38 | 21 |
| C2 | 13 | 35 |
| C3 | 21 | 38 |
| CM1 | 34 | 28 |
| CM2 | 16 | 18 |
| CM3 | 14 | 21 |
| CM4 | 13.5 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowska-Renkas, E. Impact of Sulphate Ions Content on Performance of Maleic and Acrylic Superplasticizers in Cement Paste. Materials 2021, 14, 2683. https://doi.org/10.3390/ma14102683
Janowska-Renkas E. Impact of Sulphate Ions Content on Performance of Maleic and Acrylic Superplasticizers in Cement Paste. Materials. 2021; 14(10):2683. https://doi.org/10.3390/ma14102683
Chicago/Turabian StyleJanowska-Renkas, Elżbieta. 2021. "Impact of Sulphate Ions Content on Performance of Maleic and Acrylic Superplasticizers in Cement Paste" Materials 14, no. 10: 2683. https://doi.org/10.3390/ma14102683
APA StyleJanowska-Renkas, E. (2021). Impact of Sulphate Ions Content on Performance of Maleic and Acrylic Superplasticizers in Cement Paste. Materials, 14(10), 2683. https://doi.org/10.3390/ma14102683






