Assessment of Suitable Habitats and Identification of Key Protection Areas for Polyplectron katsumatae in Jianfengling, Hainan Province, China
Abstract
:1. Introduction
2. Materials and Methods
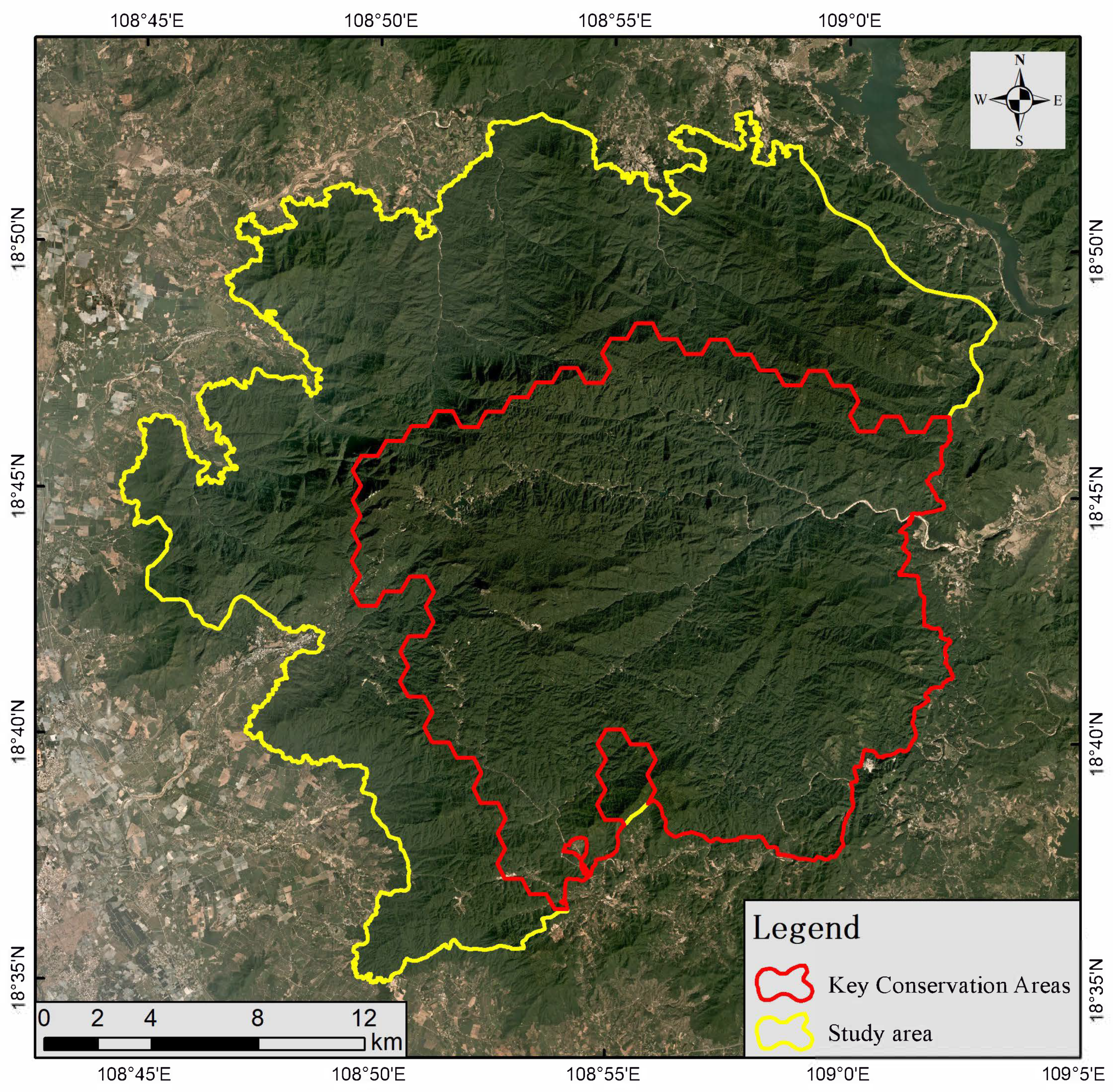
2.1. Study Area
2.2. Species Distribution Data
2.3. Environmental Factor Data
2.4. Species Distribution Modeling and Accuracy Evaluation
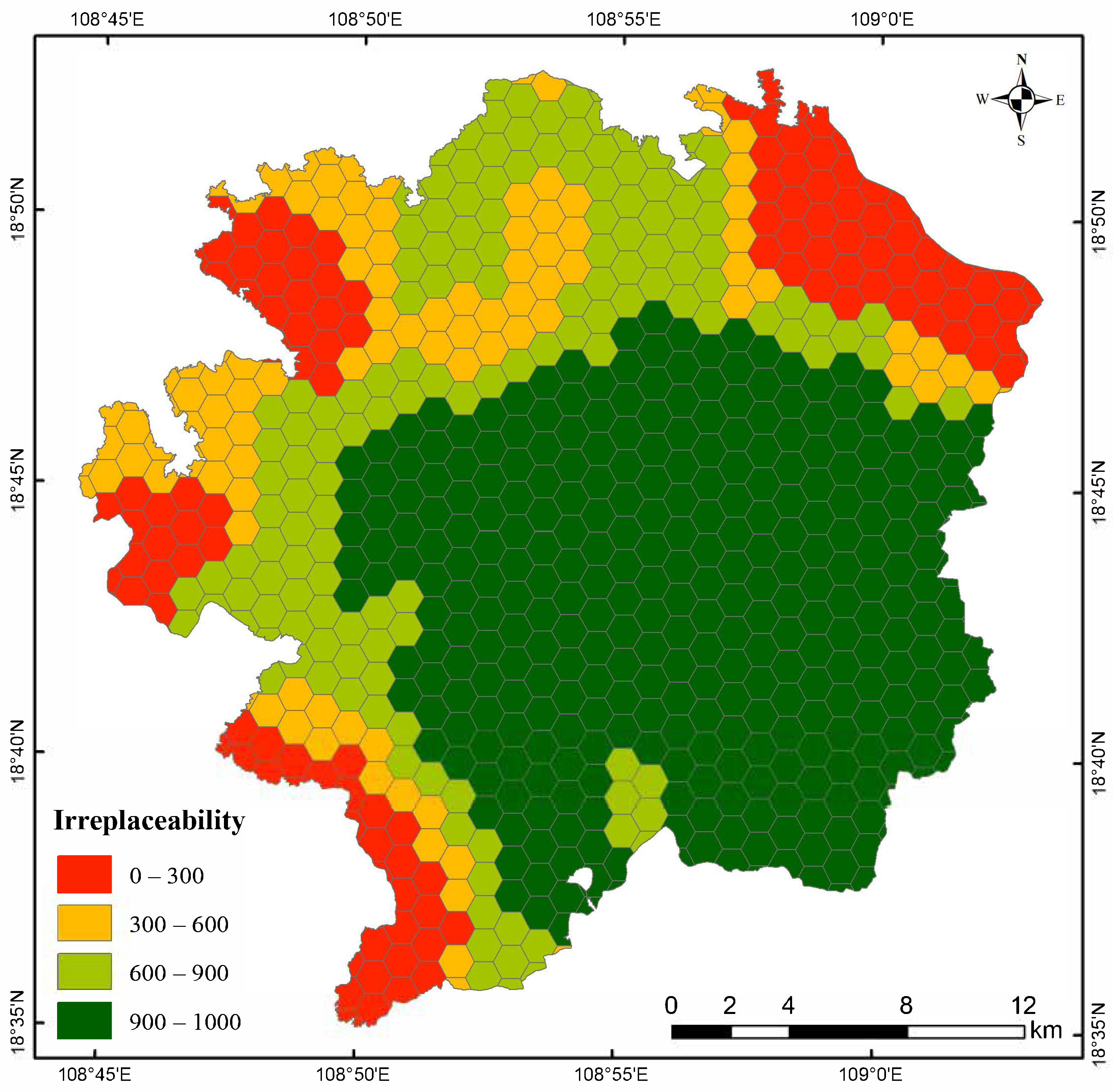
2.5. Identification of Key Protection Areas
3. Results
3.1. Evaluating Predictive Accuracy and Environmental Factors Impacts in ESDM
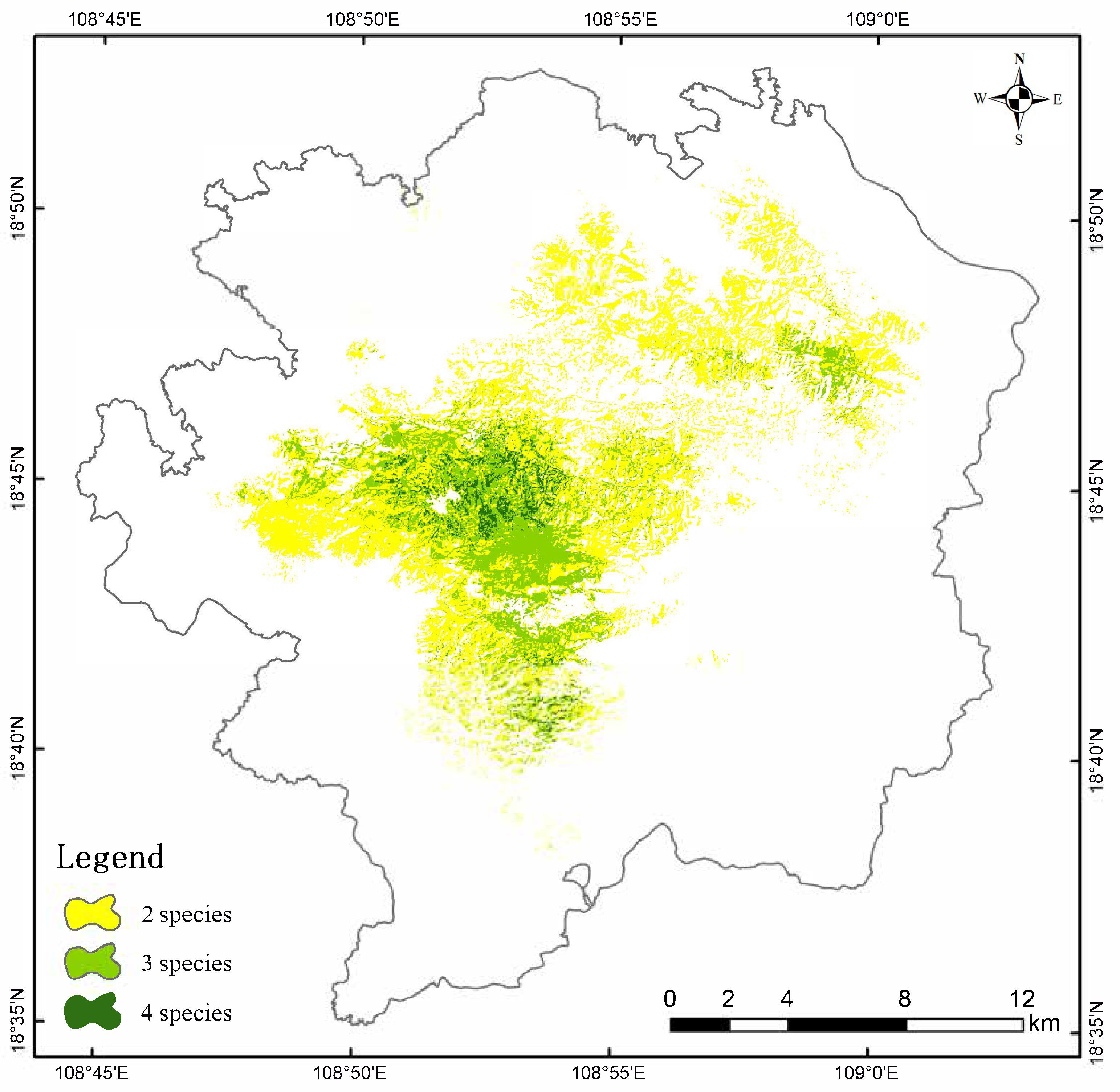
3.2. Habitat Suitability Spatial Distribution and Geographic Overlap Analysis
3.3. Key Protection Areas of P. katsumatae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konoshima, M.; Yoshimoto, A. Balancing timber production and habitat conservation of Okinawa Rails (Gallirallus okinawae): Application of a harvest scheduling optimization model in subtropical forest in Okinawa, Japan. J. Mt. Sci. 2019, 16, 2770–2782. [Google Scholar] [CrossRef]
- Apriyani, V.; Holle, M.J.M.; Mumbunan, S. A systematic map of evidence on the relationship between agricultural production and biodiversity in tropical rainforest areas. Environ. Evid. 2024, 13, 17. [Google Scholar] [CrossRef]
- Zook, D. Tropical rainforests as dynamic symbiospheres of life. Symbiosis 2010, 51, 27–36. [Google Scholar] [CrossRef]
- Delarue, E.M.P.; Kerr, S.E.; Rymer, T.L. Habitat complexity, environmental change and personality: A tropical perspective. Behav. Process. 2015, 120, 101–110. [Google Scholar] [CrossRef]
- Struebig, M.J.; Kingston, T.; Petit, E.J.; Le Comber, S.C.; Zubaid, A.; Mohd-Adnan, A.; Rossiter, S.J. Parallel declines in species and genetic diversity in tropical forest fragments. Ecol. Lett. 2011, 14, 582–590. [Google Scholar] [CrossRef]
- Lan, C.W.; Lo, M.H.; Chou, C.; Kumar, S. Terrestrial water flux responses to global warming in tropical rainforest areas. Earth’s Future 2016, 4, 210–224. [Google Scholar] [CrossRef]
- Pillay, R.; Watson, J.E.M.; Hansen, A.J.; Burns, P.; Virnig, A.L.S.; Supples, C.; Armenteras, D.; González-del-Pliegoh, P.; Aragon-Osejo, J.; Jantz, P.A.; et al. Global rarity of high- integrity tropical rainforests for threatened and declining terrestrial vertebrates. Proc. Natl. Acad. Sci. USA 2024, 121, e2413325121. [Google Scholar] [CrossRef]
- Mo, J.; Ji, Y.; Xu, H.; Li, D.; Liu, F. Camera-trapping survey on mammals and birds in a forest dynamics plot in Hainan Jianfengling National Nature Reserve. Biodivers. Sci. 2021, 29, 819–824. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, j. Conservation and Development Strategies for Hylobates concolor hainanus. Trop. For. 2003, 31, 16–17. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Z. Hainan Peacock Pheasant (Polyplectron katsumatae):an endangered and rare tropical forest bird. Chin. Birds 2011, 2, 111–116. [Google Scholar] [CrossRef]
- Chang, J.; Wang, B.; Zhang, Y.Y.; Liu, Y.; Liang, W.; Wang, J.C.; Shi, H.T.; Su, W.B.; Zhang, Z.W. Molecular evidence for species status of the endangered Hainan peacock pheasant. Zool. Sci. 2008, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Leader-Williams, N.; Turvey, S.T. Exploitation Histories of Pangolins and Endemic Pheasants on Hainan Island, China: Baselines and Shifting Social Norms. Front. Ecol. Evol. 2021, 9, 608057. [Google Scholar] [CrossRef]
- Turvey, S.T.; Ma, H.D.; Zhou, T.L.; Teng, T.T.; Yu, C.Y.; Archer, L.J.; Rao, X.D.; Dowell, S.D.; Liang, W.; Liu, H. Local ecological knowledge and regional sighting histories of Hainan Peacock-pheasant Polyplectron katsumatae: Pessimism or optimism for a threatened island endemic? Bird Conserv. Int. 2022, 33, e25. [Google Scholar] [CrossRef]
- Mo, J. A Research on Characteristic and Influence Facotrs of Terrestrial Birds and Mamammls Diversities in Hainan Jianfengling National Nature Reserve. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2021. [Google Scholar]
- McCallum, J. Changing use of camera traps in mammalian field research: Habitats, taxa and study types. Mammal Rev. 2013, 43, 196–206. [Google Scholar] [CrossRef]
- Cabello, J.; Mairota, P.; Alcaraz-Segura, D.; Arenas-Castro, S.; Escribano, P.; Leitao, P.J.; Martinez-Lopez, J.; Regos, A.; Requena-Mullor, J.M. IEEE Satellite Remote Sensing of Ecosystem Functions: Opportunities and Challenges for Reporting Obligations of the EU Habitat Directive. In Proceedings of the IGARSS 2018-2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 6604–6607. [Google Scholar]
- McDermid, G.J.; Hall, R.J.; Sanchez-Azofeifa, G.A.; Franklin, S.E.; Stenhouse, G.B.; Kobliuk, T.; LeDrew, E.F. Remote sensing and forest inventory for wildlife habitat assessment. For. Ecol. Manag. 2009, 257, 2262–2269. [Google Scholar] [CrossRef]
- Zannetos, S.P.; Theodorou, K.; Zevgolis, Y.G.; Galinou, E.; Akriotis, T. Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach. Life 2024, 14, 1231. [Google Scholar] [CrossRef]
- Moradi, E.; Tavili, A.; Darabi, H.; Muchová, Z. Assessing wildfire impact on Trigonella elliptica habitat using random forest modeling. J. Environ. Manag. 2024, 353, 120209. [Google Scholar] [CrossRef]
- Sedighkia, M.; Datta, B. Linking SVM based habitat model and evolutionary optimisation for managing environmental impacts of hydropower plants. River Res. Appl. 2023, 39, 897–910. [Google Scholar] [CrossRef]
- Rahmanian, S.; Pourghasemi, H.R.; Pouyan, S.; Karami, S. Habitat potential modelling and mapping of Teucrium polium using machine learning techniques. Environ. Monit. Assess. 2021, 193, 759. [Google Scholar] [CrossRef]
- Vázquez, R.F.; Vimos-Lojano, D.; Hampel, H. Habitat Suitability Curves for Freshwater Macroinvertebrates of Tropical Andean Rivers. Water 2020, 12, 2703. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Lawer, E.A. Predicting the impact of climate change on the potential distribution of a critically endangered avian scavenger, Hooded Vulture Necrosyrtes monachus, in Ghana. Glob. Ecol. Conserv. 2024, 49, e02804. [Google Scholar] [CrossRef]
- Shivambu, T.C.; Shivambu, N.; Nelufule, T.; Moshobane, M.C.; Seoraj-Pillai, N.; Nangammbi, T.C. Returning to the Wilderness: Potential Habitat Suitability of Non-Native Pet Birds in South Africa. Biology 2024, 13, 483. [Google Scholar] [CrossRef]
- Nematollahi, S.; Fakheran, S.; Jafari, A.; Pourmanafi, S.; Kienast, F. Applying a systematic conservation planning tool and ecological risk index for spatial prioritization and optimization of protected area networks in Iran. J. Nat. Conserv. 2022, 66, 126144. [Google Scholar] [CrossRef]
- Zhang, F.G.; Zhang, S.Q.; Wu, K.F.; Zhao, R.X.; Zhao, G.H.; Wang, Y.J. Potential habitat areas and priority protected areas of Tilia amurensis Rupr in China under the context of climate change. Front. Plant Sci. 2024, 15, 1365264. [Google Scholar] [CrossRef] [PubMed]
- Dehaghi, I.M.; Salmanmahiny, A.; Karimi, S.; Shabani, A.A. Multi-criteria evaluation and simulated annealing for delimiting high priority habitats of Alectoris chukar and Phasianus colchicus in Iran. Anim. Biodivers. Conserv. 2018, 41, 185–193. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Yao, W.; Shang, E.; Zhang, S.; Chen, F.; Zeng, Y. Ecological assessment and identification strategy of key conservation areas: A case study of the south Taihang Mountains region. Biol. Conserv. 2024, 296, 110705. [Google Scholar] [CrossRef]
- Li, D. Spatial Distribution Pattern and Activity Rhythm of Mammals in Jianfengling Forest Area, Hainan. Master’s Thesis, Shanxi University of Technology, Hanzhong, China, 2022. [Google Scholar]
- Ma, Y.; Liu, L.X.; Yao, W.T.; Zeng, Z.G.; Zhang, M.J.; Shang, E.R.; Zhang, S.Y.; Yang, J. Spatial and Temporal Changes and Assessment of Multi-Species Habitat in Hainan Jianfengling Protected Area. Remote Sens. 2025, 17, 46. [Google Scholar] [CrossRef]
- Mcgowan, P.; Kirwan, G. Silver Pheasant (Lophura nycthemera). In Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Gao, Y.R.; Yu, D.Q. The Ecology and Situation of The Hainan Subspecies of Silver Pheasant. Zool. Res. 1995, 58, 239. [Google Scholar] [CrossRef]
- Zou, F.S.; Zhang, Q.; Hiuang, J.H.; Zhang, M. A new record of bird in Guangdong-Garrulax maesi. Sichuan J. Zool. 2008, 27, 660–661. [Google Scholar]
- Fick, S.; Hijmans, R. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Barron, C.; Neis, P.; Zipf, A. A Comprehensive Framework for Intrinsic OpenStreetMap Quality Analysis. Trans. GIS 2014, 18, 877–895. [Google Scholar] [CrossRef]
- Dodsworth, E.; Nicholson, A. Academic Uses of Google Earth and Google Maps in a Library Setting. Inf. Technol. Libr. 2012, 31, 102–117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Xu, H.; Liu, W.; Wang, J.; Chen, X.; Liu, L. GLC_FCS30D: The first global 30 m land-cover dynamics monitoring product with a fine classification system for the period from 1985 to 2022 generated using dense-time-series Landsat imagery and the continuous change-detection method. Earth Syst. Sci. Data 2024, 16, 1353–1381. [Google Scholar] [CrossRef]
- Haeruddin; Irawan, J.F. Identifying of the relationship between lineament density and vegetation index at Tumpangpitu Mining Area, East Java, Indonesia. AIP Conf. Proc. 2020, 2278, 020006. [Google Scholar] [CrossRef]
- Abrams, M.; Crippen, R.; Fujisada, H. ASTER Global Digital Elevation Model (GDEM) and ASTER Global Water Body Dataset (ASTWBD). Remote Sens. 2020, 12, 1156. [Google Scholar] [CrossRef]
- Bucklin, D.N.; Basille, M.; Benscoter, A.M.; Brandt, L.A.; Mazzotti, F.J.; Romañach, S.S.; Speroterra, C.; Watling, J.I. Comparing species distribution models constructed with different subsets of environmental predictors. Divers. Distrib. 2015, 21, 23–35. [Google Scholar] [CrossRef]
- Randin, C.F.; Dirnböck, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting Multicollinearity in Ecological Multiple Regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Yao, W.; Yang, J.; Ma, Y.; Liu, L.; Shang, E.; Zhang, S. Habitat Suitability Assessment of Key Wildlife in Hainan Tropical Rainforest Based on ESDM. Life 2025, 15, 323. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Possingham, H.P.; Ball, I.R.; Cousins, E.A. Mathematical methods for spatially cohesive reserve design. Environ. Model. Assess. 2002, 7, 107–114. [Google Scholar] [CrossRef]
- Wang, S.; Yao, W.; Ma, Y.; Shang, E.; Zhang, S.; Chen, F.; Zeng, Y. Optimizing natural boundary definition and functional zoning in protected areas: An integrated framework encompassing species, landscapes and ecosystems. Glob. Ecol. Conserv. 2024, 49, e02781. [Google Scholar] [CrossRef]
- Rüdisser, J.; Tasser, E.; Tappeiner, U. Distance to nature—A new biodiversity relevant environmental indicator set at the landscape level. Ecol. Indic. 2012, 15, 208–216. [Google Scholar] [CrossRef]
- Thomas, J.; Joseph, S.; Thrivikramji, K.P. Estimation of soil erosion in a rain shadow river basin in the southern Western Ghats, India using RUSLE and transport limited sediment delivery function. Int. Soil Water Conserv. Res. 2018, 6, 111–122. [Google Scholar] [CrossRef]
- Wu, J.Y.; Luo, J.A.; Zhang, H.; Qin, S.; Yu, M.J. Projections of land use change and habitat quality assessment by coupling climate change and development patterns. Sci. Total Environ. 2022, 847, 157491. [Google Scholar] [CrossRef]
- Raines, G.L. Description and comparison of geologic maps with FRAGSTATS-a spatial statistics program. Comput. Geosci. 2002, 28, 169–177. [Google Scholar] [CrossRef]
- Yang, F.L.; Wu, R.D.; Jin, T.; Long, Y.C.; Zhao, P.; Yu, Q.; Wang, L.Z.; Wang, J.J.; Zhao, H.W.; Guo, Y. Efficiency of unlocking or locking existing protected areas for identifying complementary areas for biodiversity conservation. Sci. Total Environ. 2019, 694, 133771. [Google Scholar] [CrossRef]
- Ma, B.R.; Zeng, W.H.; Xie, Y.X.; Wang, Z.Z.; Hu, G.Z.; Li, Q.; Cao, R.X.; Zhuo, Y.; Zhang, T.Z. Boundary delineation and grading functional zoning of Sanjiangyuan National Park based on biodiversity importance evaluations. Sci. Total Environ. 2022, 825, 154068. [Google Scholar] [CrossRef]
- Ma, X.L.; Zhu, X.L.; Xie, Q.Y.; Jin, J.X.; Zhou, Y.K.; Luo, Y.P.; Liu, Y.X.; Tian, J.Q.; Zhao, Y.H. Monitoring nature’s calendar from space: Emerging topics in land surface phenology and associated opportunities for science applications. Glob. CHANGE Biol. 2022, 28, 7186–7204. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Z.; Ling, Q.P.; Pei, H.Q.; Song, Y.N.; Qiu, Z.X.; Wang, C.; Liu, T.D.; Gong, W.F. Remote Sensing of Tropical Rainforest Biomass Changes in Hainan Island, China from 2003 to 2018. Remote Sens. 2021, 13, 1696. [Google Scholar] [CrossRef]
- Powell, L.L.; Cordeiro, N.J.; Stratford, J.A. Ecology and conservation of avian insectivores of the rainforest understory: A pantropical perspective. Biol. Conserv. 2015, 188, 1–10. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; Seddon, N.; MacGregor, H.E.A.; Darski, B.; Aleixo, A.; Bonsall, M.B.; Tobias, J.A. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 2015, 96, 2692–2704. [Google Scholar] [CrossRef]
- Stratford, J.A.; Stouffer, P.C. Forest fragmentation alters microhabitat availability for Neotropical terrestrial insectivorous birds. Biol. Conserv. 2015, 188, 109–115. [Google Scholar] [CrossRef]
- Luther, D.A.; Wolfe, J.D.; Johnson, E.; Stouffer, P.C.; Batchelor, J.; Tarwater, C.E. Habitat use of Amazonian birds varies by age and foraging guild along a disturbance gradient. Proc. R. Soc. B Biol. Sci. 2024, 291, 20240866. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.H.; Slik, J.W.F. Topography related habitat associations of tree species traits, composition and diversity in a Chinese tropical forest. For. Ecol. Manag. 2014, 330, 75–81. [Google Scholar] [CrossRef]
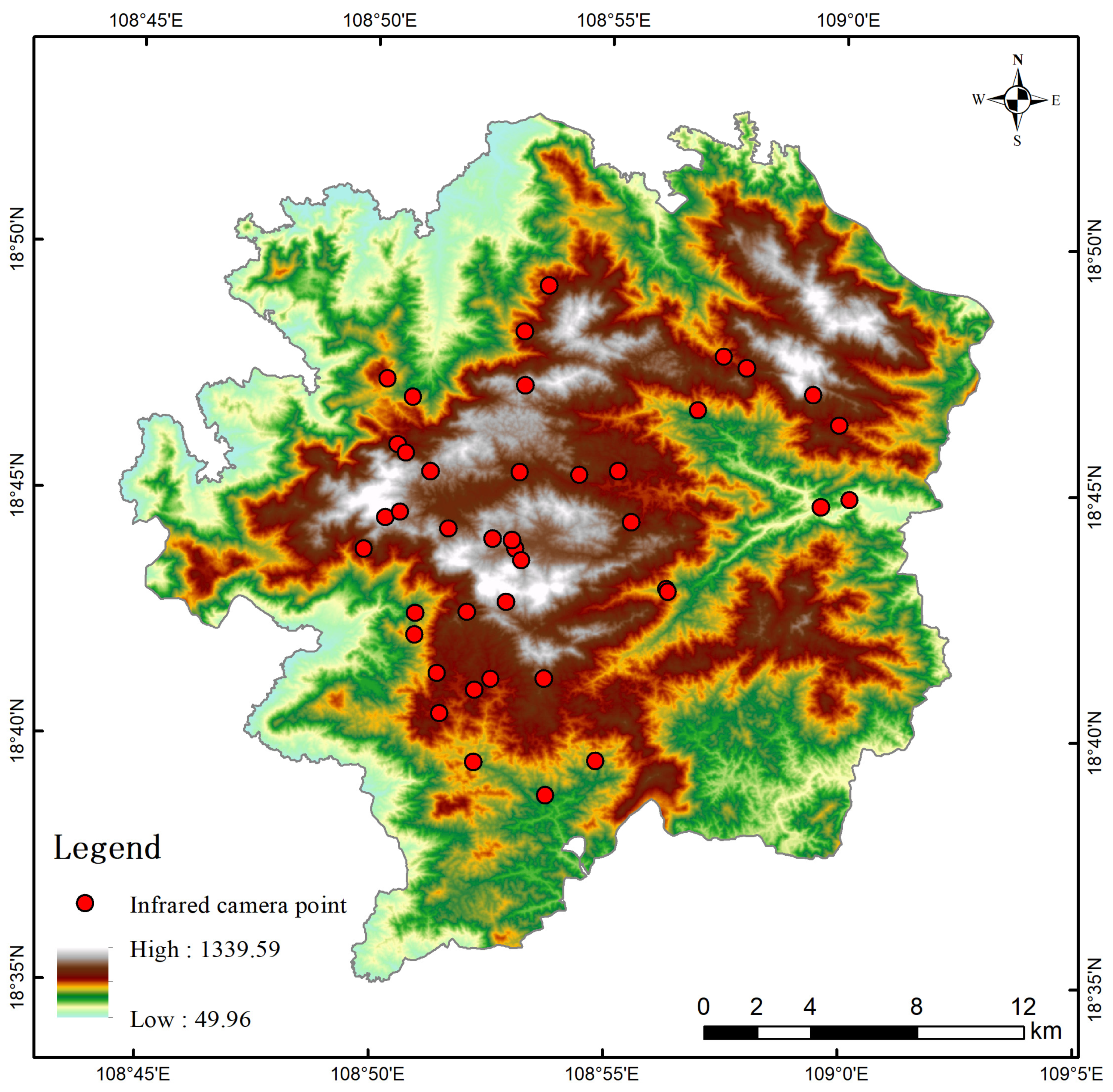
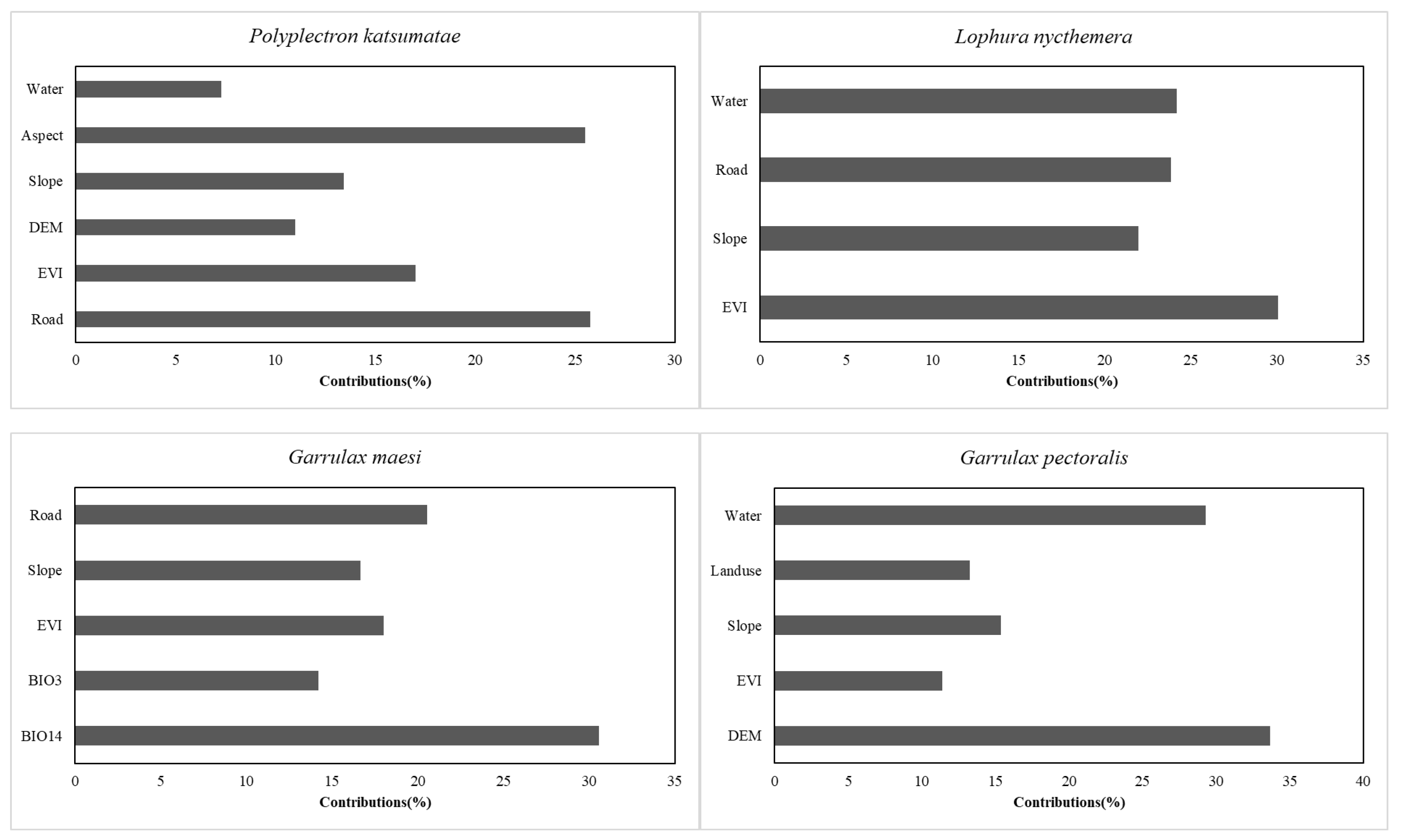
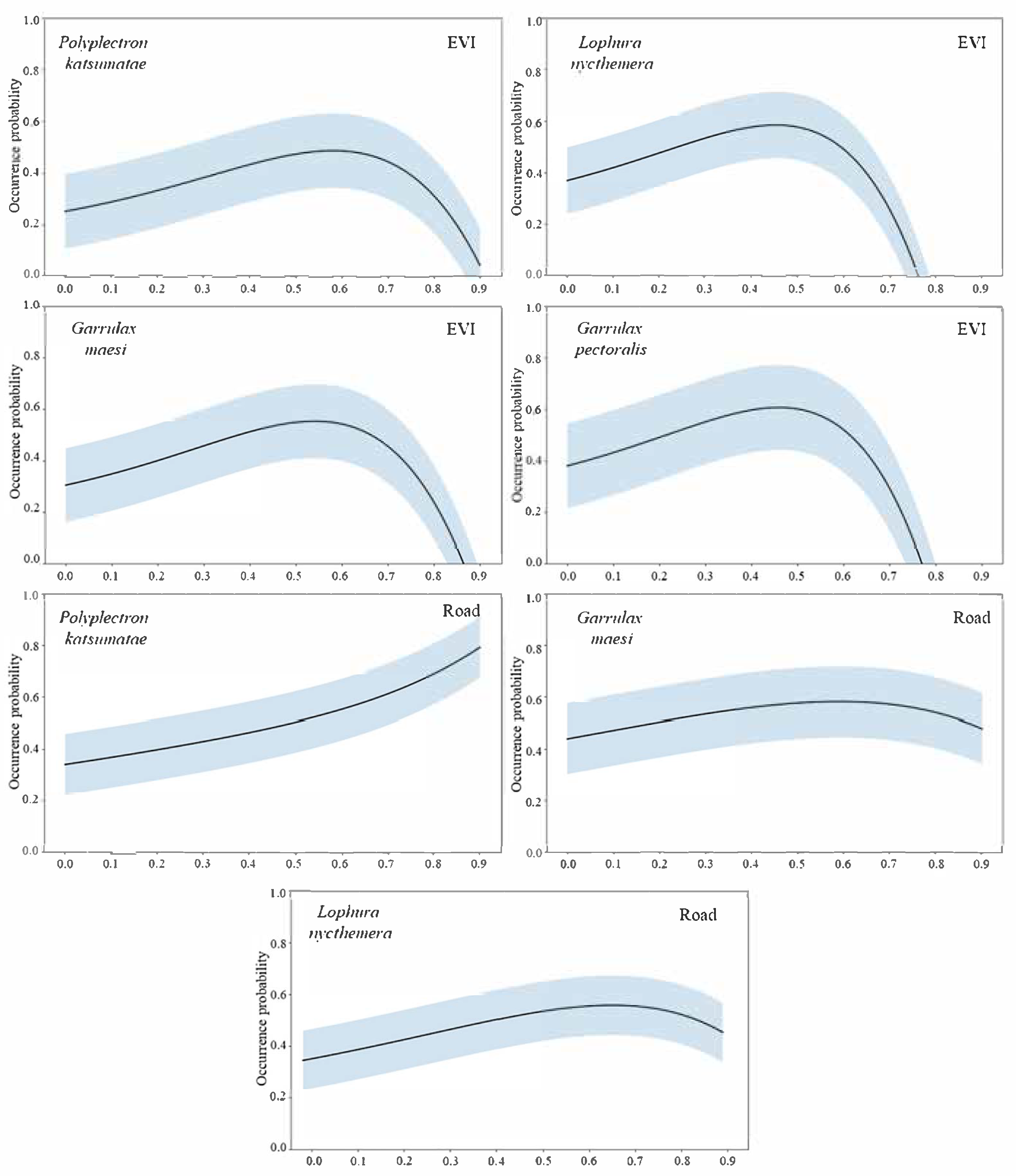

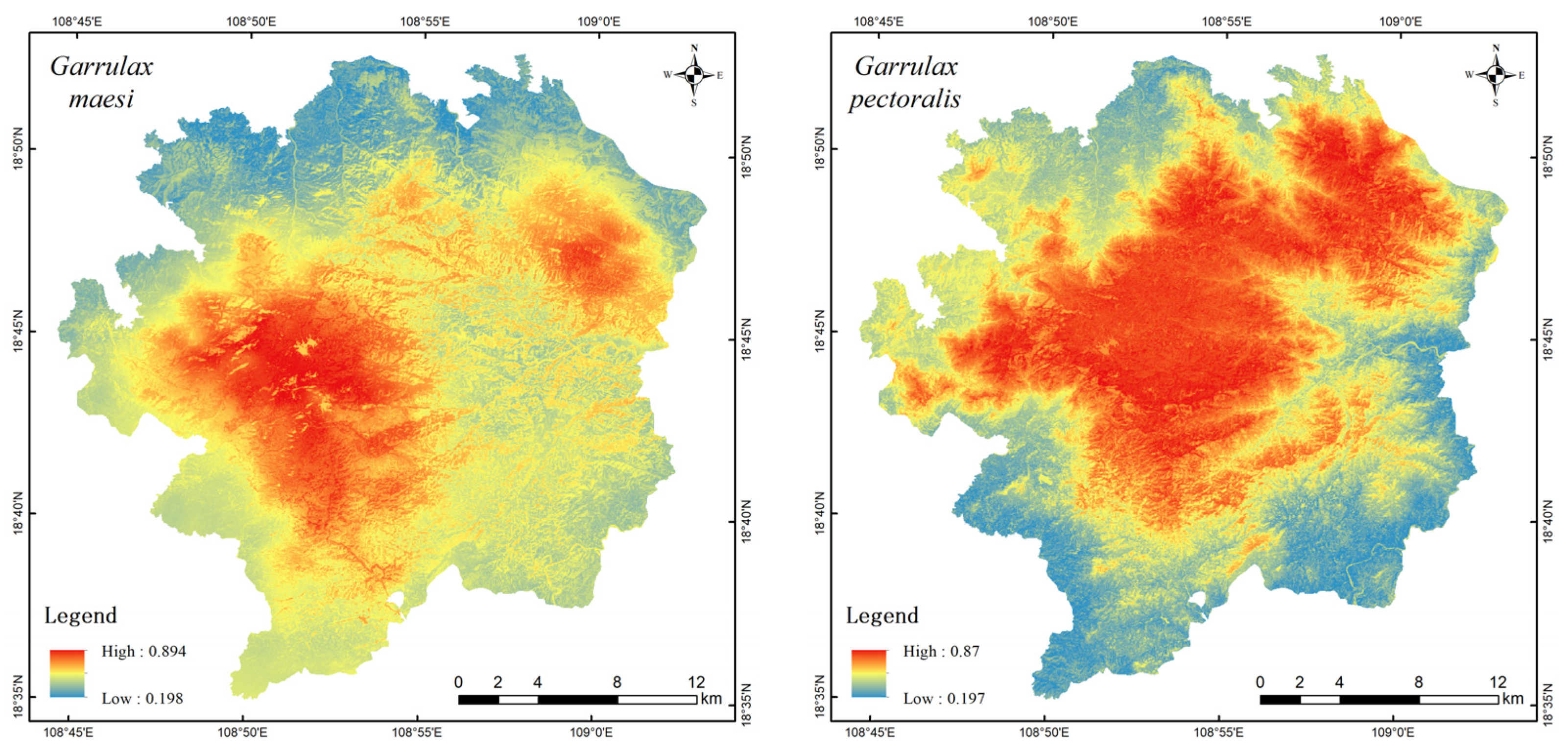
| Species | No. of Independent Photographs | RAI | National Protected Level | China’s Vertebrate Red List Status | CITES Appendix |
|---|---|---|---|---|---|
| Polyplectron katsumatae | 136 | 0.320 | I | II | EN |
| Lophura nycthemera | 723 | 1.699 | II | LC | |
| Garrulax maesi | 142 | 0.334 | LC | ||
| Garrulax pectoralis | 56 | 0.132 | LC |
| Species | Habitat | Diet | Behavior |
|---|---|---|---|
| Polyplectron katsumatae | Inhabits tropical rainforests, dense understory [10]. | Omnivorous Insects, seeds, fruits [11]. | Usually solitary or found in pairs, with a secretive nature adept at avoiding predators [10]. |
| Lophura nycthemera | Found in montane forests, bamboo groves, and secondary forests [32]. | Omnivorous Seeds, fruits, insects, small invertebrates [32]. | Social bird, often seen in pairs or small groups, with strong territorial behavior [33]. |
| Garrulax maesi | Resides in lowland evergreen forests, secondary forests, and dense understory [34]. | Omnivorous Insects, fruits. | Social bird, typical understory bird, adept at hiding and often seen in groups [34]. |
| Garrulax pectoralis | Occupies lowland forests, shrub lands, and bamboo groves. | Omnivorous Insects, fruits, seeds. | Social bird, often seen in noisy groups. |
| Data Types | Specific Data | Data Set | Spatial/Temporal Resolution | Period |
|---|---|---|---|---|
| Climate data | 19 Bioclimatic variables (BIO1~BIO19) | WorldClim Version 2.1 | 30s (~1 km), month | Average value |
| Water data | Distance from surface water bodies such as rivers and lakes (Water) | Provided by the protected area management authority (field surveys, remote sensing) | Vector | 2019 |
| Road data | Distance from road (Road) | OpenStreetMap (OSM) | Vector | 2019 |
| Land use/cover data | Landuse | National Center for Geographic Resources Science | 30 m, year | 2019 |
| Vegetation data | Enhanced Vegetation Index, (EVI) | LANDSAT/LC08/C01/T1_RT_TOA | 30 m, year | 2013–2019 |
| Topographic data | DEM/Slope/Aspect | ASETER GDEM v3 | 30 m | 2019 |
| Species | AUC | TSS | Omission. Rate | Prop. Correct | Kappa |
|---|---|---|---|---|---|
| Polyplectron katsumatae | 0.863 | 0.565 | 0.233 | 0.767 | 0.511 |
| Lophura nycthemera | 0.850 | 0.537 | 0.247 | 0.753 | 0.532 |
| Garrulax maesi | 0.881 | 0.488 | 0.279 | 0.721 | 0.446 |
| Garrulax pectoralis | 0.856 | 0.575 | 0.232 | 0.768 | 0.530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Ma, Y.; Long, A.; Liu, L.; Shang, E.; Zhang, S.; Yang, J.; Gao, T. Assessment of Suitable Habitats and Identification of Key Protection Areas for Polyplectron katsumatae in Jianfengling, Hainan Province, China. Life 2025, 15, 826. https://doi.org/10.3390/life15050826
Yao W, Ma Y, Long A, Liu L, Shang E, Zhang S, Yang J, Gao T. Assessment of Suitable Habitats and Identification of Key Protection Areas for Polyplectron katsumatae in Jianfengling, Hainan Province, China. Life. 2025; 15(5):826. https://doi.org/10.3390/life15050826
Chicago/Turabian StyleYao, Wutao, Yong Ma, An Long, Lixi Liu, Erping Shang, Shuyan Zhang, Jin Yang, and Tianxiong Gao. 2025. "Assessment of Suitable Habitats and Identification of Key Protection Areas for Polyplectron katsumatae in Jianfengling, Hainan Province, China" Life 15, no. 5: 826. https://doi.org/10.3390/life15050826
APA StyleYao, W., Ma, Y., Long, A., Liu, L., Shang, E., Zhang, S., Yang, J., & Gao, T. (2025). Assessment of Suitable Habitats and Identification of Key Protection Areas for Polyplectron katsumatae in Jianfengling, Hainan Province, China. Life, 15(5), 826. https://doi.org/10.3390/life15050826







