A Pilot Study on Qualitative Metabolomics to Characterize Lewis Lung Carcinoma in Mice
Abstract
1. Introduction
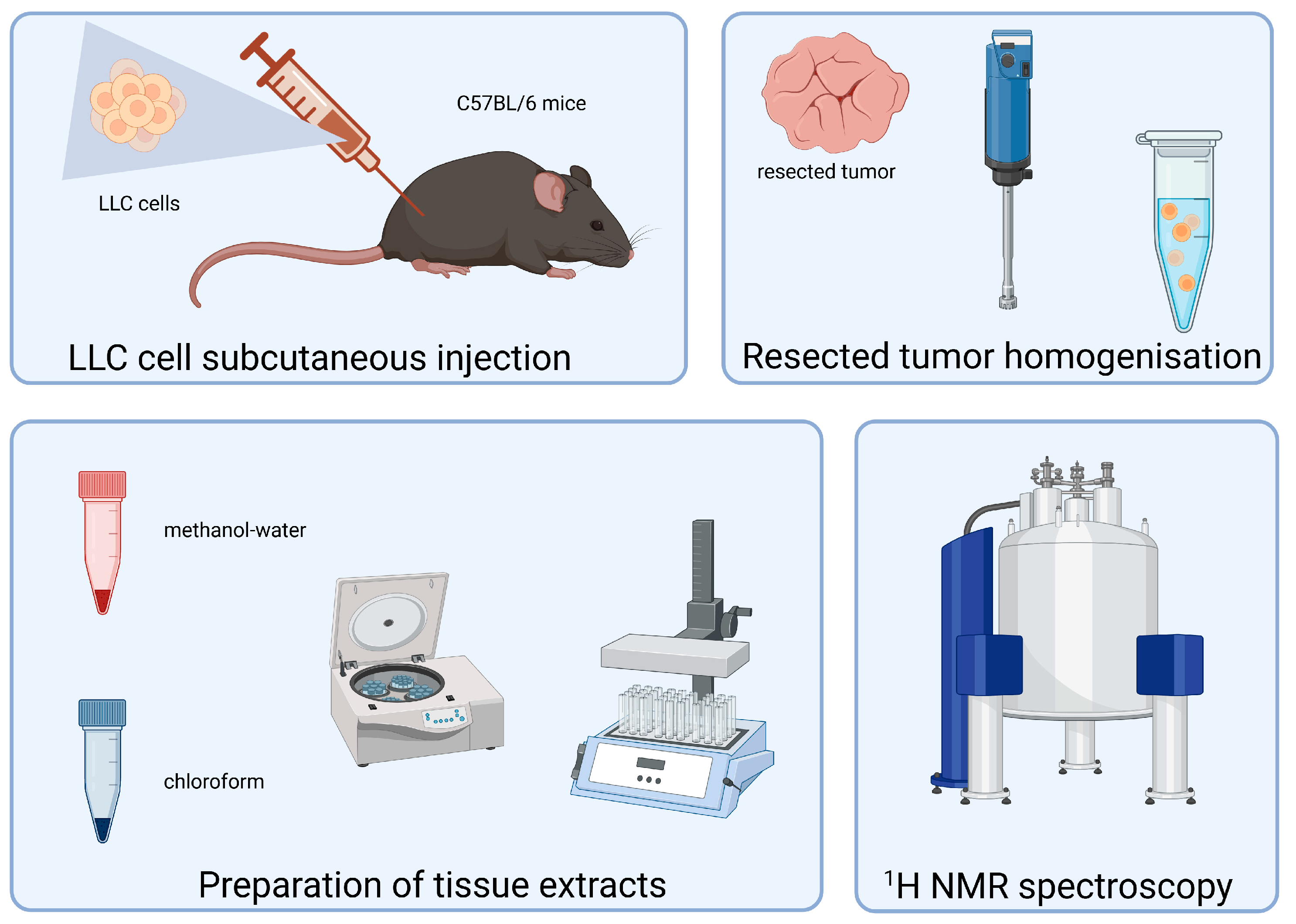
2. Materials and Methods
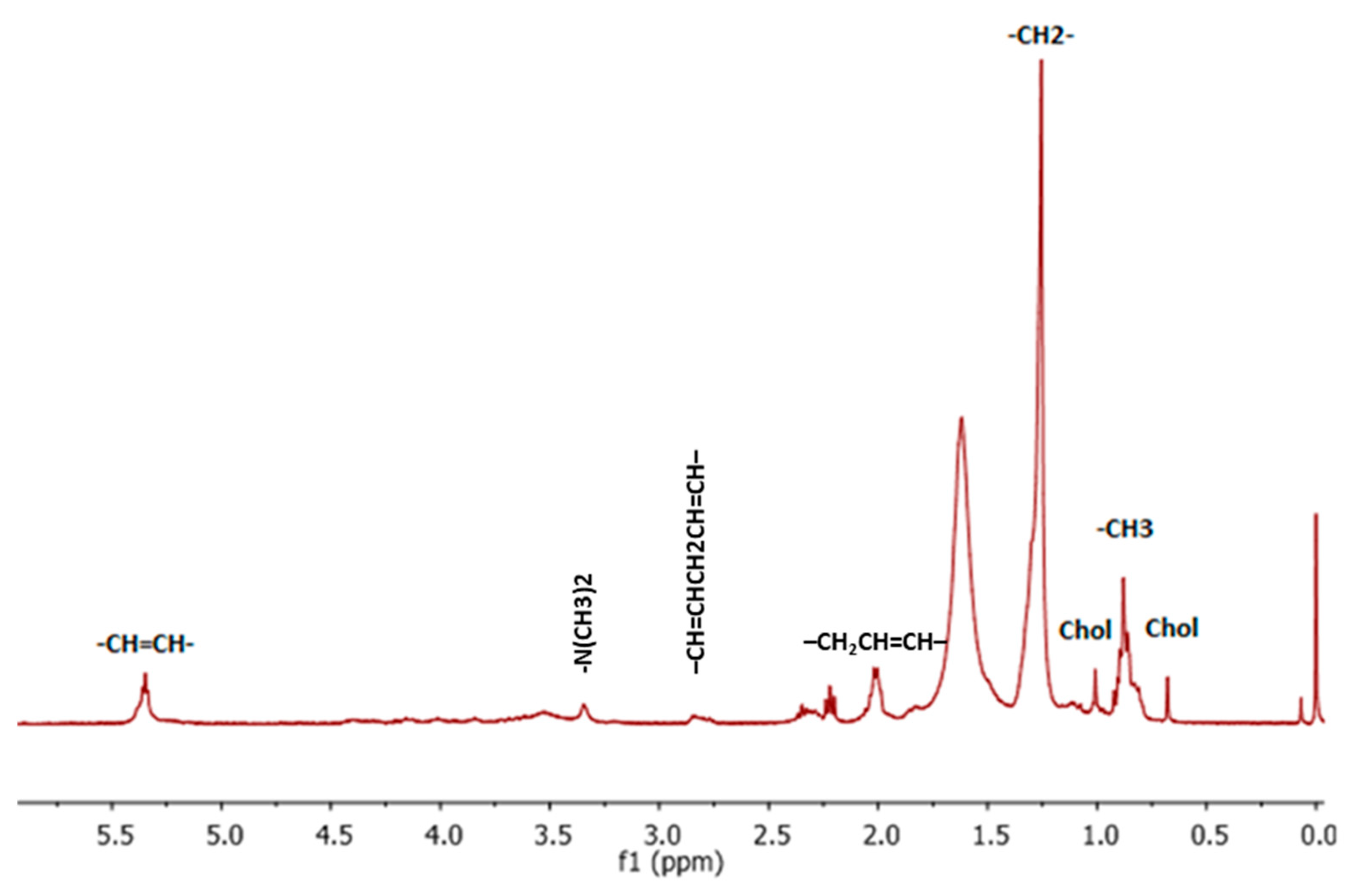
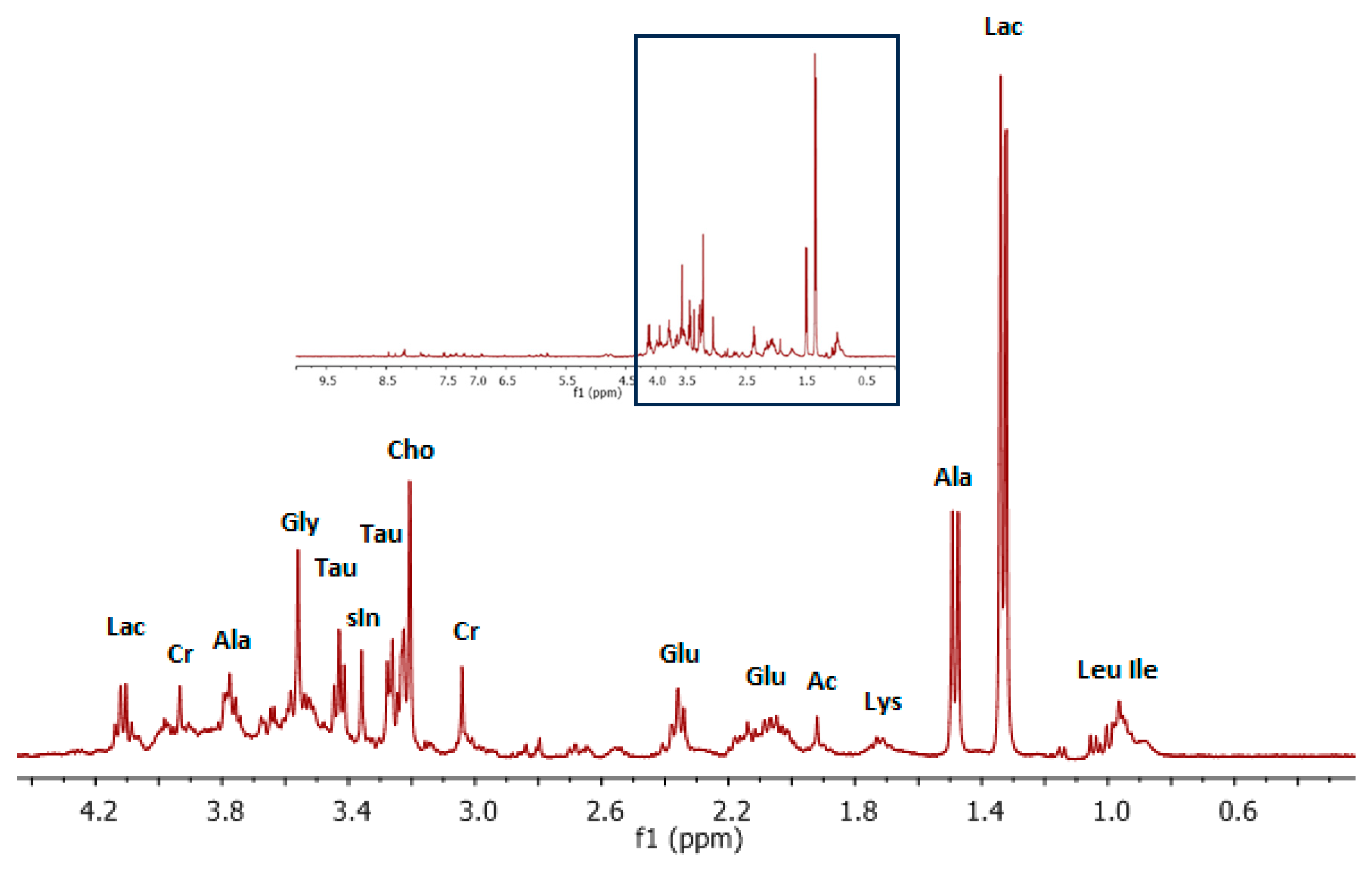
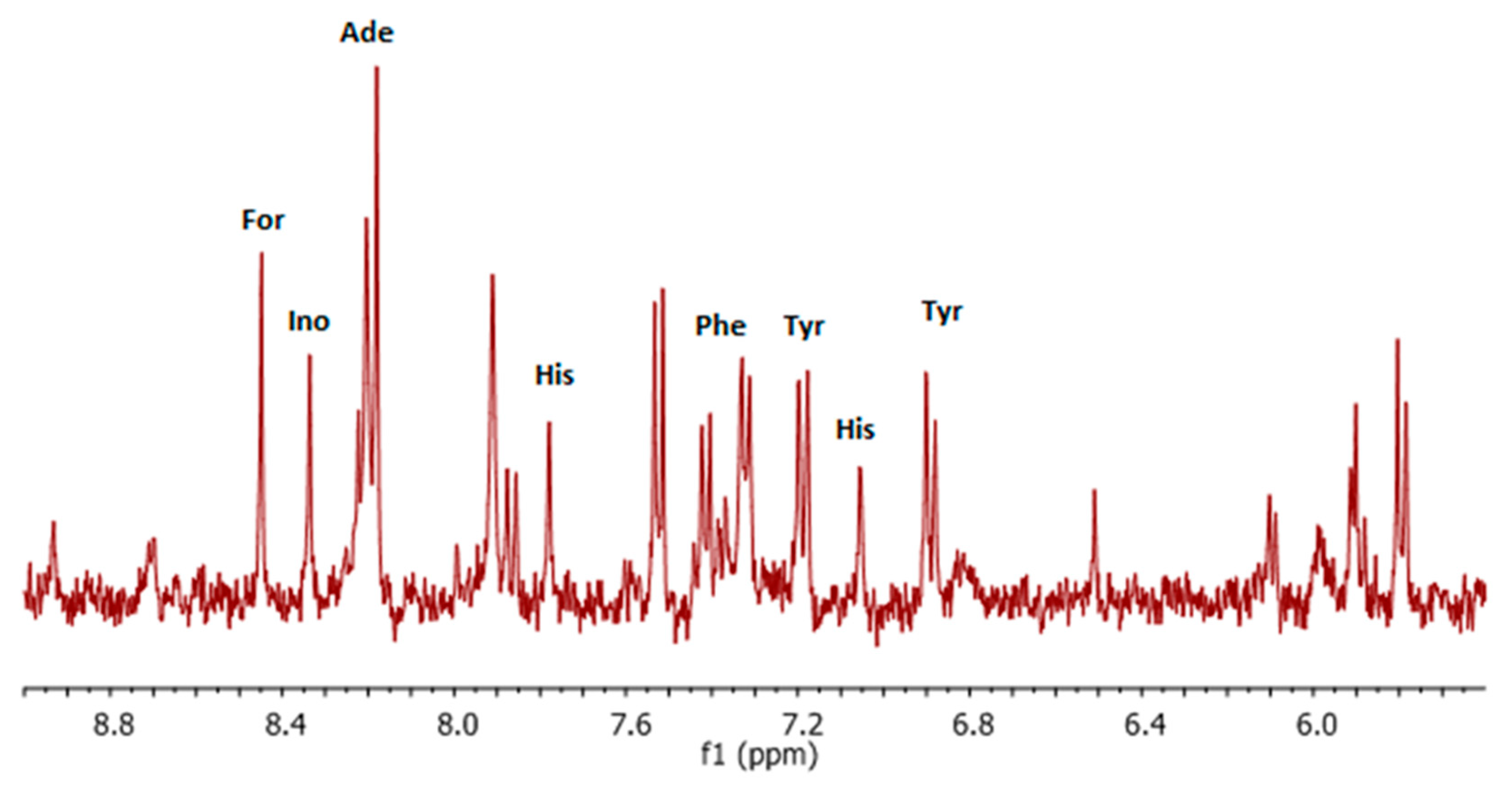
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Keshamouni, V.; Arenberg, D.; Kalemkerian, G. Lung Cancer Metastasis: Novel Biological Mechanisms and Impact on Clinical Practice; Springer: New York, NY, USA, 2009. [Google Scholar]
- Roth, J.A.; Hong, W.K.; Komaki, R.U. Lung Cancer; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Van de Luecht, M.R.; Reed, W.M. The cognitive and perceptual processes that affect observer performance in lung cancer detection: A scoping review. J. Med. Radiat. Sci. 2021, 68, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. In Lung Cancer: Treatment and Research; Reckamp, K.L., Ed.; Cancer Treatment and Research; Springer: New York, NY, USA, 2016; Volume 170, pp. 25–46. [Google Scholar]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non–Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Yuan, P.; Cao, J.L.; Rustam, A.; Zhang, C.; Yuan, X.S.; Bao, F.C.; Lv, W.; Hu, J. Time-to-Progression of NSCLC from Early to Advanced Stages: An Analysis of data from SEER Registry and a Single Institute. Sci. Rep. 2016, 6, 28477. [Google Scholar] [CrossRef]
- Wang, J.; Mahasittiwat, P.; Wong, K.K.; Quint, L.E.; Kong, F.-M. Natural growth and disease progression of non-small cell lung cancer evaluated with (18)F-fluorodeoxyglucose PET/CT. Lung Cancer 2012, 78, 51–56. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Verlicchi, A.; Rosell, R. Cancer stem cells in small cell lung cancer. Transl. Lung Cancer Res. 2016, 5, 16–25. [Google Scholar] [CrossRef]
- Meyer, M.L.; Fitzgerald, B.G.; Paz-Ares, L.; Cappuzzo, F.; Jänne, P.A.; Peters, S.; Hirsch, F.R. New promises and challenges in the treatment of advanced non-small-cell lung cancer. Lancet 2024, 404, 803–822. [Google Scholar] [CrossRef]
- Patel, S.A.; Weiss, J. Advances in the Treatment of Non-Small Cell Lung Cancer: Immunotherapy. Clin. Chest Med. 2020, 41, 237–247. [Google Scholar] [CrossRef]
- Pozza, D.H.; Andrade de Mello, R.B. Treatment Sequencing Strategies in Lung Cancer. Chin. J. Lung Cancer 2022, 25, 323–336. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Guan, J.; Xie, C.; Zhang, H.; Yang, J.; Luo, Y.; Chen, L.; Zhao, M.; Huo, B.; et al. Metabolomic differentiation of benign vs. malignant pulmonary nodules with high specificity via high-resolution mass spectrometry analysis of patient sera. Nat. Commun. 2023, 14, 2339. [Google Scholar] [CrossRef]
- Nie, M.; Chen, N.; Pang, H.; Jiang, T.; Jiang, W.; Tian, P.; Yao, L.; Chen, Y.; DeBerardinis, R.J.; Li, W.; et al. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR-mutant lung cancer and impedes tumor relapse. J. Clin. Investig. 2022, 132, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bafiti, V.; Thanou, E.; Ouzounis, S.; Kotsakis, A.; Georgoulias, V.; Lianidou, E.; Katsila, T.; Markou, A. Profiling Plasma Extracellular Vesicle Metabotypes and miRNAs: An Unobserved Clue for Predicting Relapse in Patients with Early-Stage NSCLC. Cancers 2024, 16, 3729. [Google Scholar] [CrossRef] [PubMed]
- Siddique, F.; Shehata, M.; Ghazal, M.; Contractor, S.; El-Baz, A. Lung Cancer Subtyping: A Short Review. Cancers 2024, 16, 2643. [Google Scholar] [CrossRef]
- Yan, L.; Sundaram, S.; Rust, B.M.; Picklo, M.J.; Bukowski, M.R. Metabolomes of Lewis lung carcinoma metastases and normal lung tissue from mice fed different diets. J. Nutr. Biochem. 2022, 107, 109051. [Google Scholar] [CrossRef]
- Stinkens, K.; Vanhove, K.; Thomeer, M. Metabolomics a novel biomarker in lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, e46. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Lewis, K.E.; Beckmann, M.; Allison, G.G.; Ghosal, R.; Lewis, P.D.; Mur, L.A.J. The metabolomic detection of lung cancer biomarkers in sputum. Lung Cancer 2016, 94, 88–95. [Google Scholar] [CrossRef]
- Yu, S.; Li, J.; Gao, W.; Wu, Y.; Qin, X.; Li, Z. Uncovering the anticancer mechanism of petroleum extracts of Farfarae Flos against Lewis lung cancer by metabolomics and network pharmacology analysis. Biomed. Chromatogr. 2020, 34, e4878. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Lazar, L.; Fang, Z.; Tang, C.; Zhao, J. Metabolomics workflow for lung cancer: Discovery of biomarkers. Clin. Chim. Acta 2019, 495, 436–445. [Google Scholar] [CrossRef]
- Hori, S.; Nishiumi, S.; Kobayashi, K.; Shinohara, M.; Hatakeyama, Y.; Kotani, Y.; Hatano, N.; Maniwa, Y.; Nishio, W.; Bamba, T.; et al. A metabolomic approach to lung cancer. Lung Cancer 2011, 74, 284–292. [Google Scholar] [CrossRef]
- Griffin, J.L.; Kauppinen, R.A. Tumour metabolomics in animal models of human cancer. J. Proteome Res. 2007, 6, 498–505. [Google Scholar] [CrossRef]
- Noreldeen, H.A.A.; Liu, X.; Xu, G. Metabolomics of lung cancer: Analytical platforms and their applications. J. Sep. Sci. 2020, 43, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Fuss, T.L.; Cheng, L.L. Evaluation of Cancer Metabolomics Using ex vivo High Resolution Magic Angle Spinning (HRMAS) Magnetic Resonance Spectroscopy (MRS). Metabolites 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Singh, R.K.; Fidler, I.J.; Raz, A. Murine Models to Evaluate Novel and Conventional Therapeutic Strategies for Cancer. Am. J. Pathol. 2007, 170, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. BioMed Res. Int. 2015, 2015, 17. [Google Scholar] [CrossRef]
- Bertram, J.S.; Janik, P. Establishment of a cloned line of lewis lung-carcinoma cells adapted to cell-culture. Cancer Lett. 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Lu, H.; Lan, W.X.; Bo, L.; Niu, C.; Zhou, J.J.; Zhu, H.L. Metabolic response of LLC xenografted mice to oxythiamine, as measured by H-1 NMR spectroscopy. Genet. Mol. Res. 2015, 14, 11043–11051. [Google Scholar] [CrossRef]
- Weljie, A.M.; Jirik, F.R. Hypoxia-induced metabolic shifts in cancer cells: Moving beyond the Warburg effect. Int. J. Biochem. Cell Biol. 2011, 43, 981–989. [Google Scholar] [CrossRef]
- Li, Y.; Huang, P.; Peng, H.J.; Yue, H.C.; Wu, M.; Liu, S.S.; Qin, R.S.; Fan, J.; Han, Y.W. Antitumor effects of Endostar(rh-endostatin) combined with gemcitabine in different administration sequences to treat Lewis lung carcinoma. Cancer Manag. Res. 2019, 11, 3469–3479. [Google Scholar] [CrossRef]
- Ratai, E.M.; Pilkenton, S.; Lentz, M.R.; Greco, J.B.; Fuller, R.A.; Kim, J.P.; He, J.; Cheng, L.L.; Gonzalez, R.G. Comparisons of brain metabolites observed by HRMAS 1H NMR of intact tissue and solution 1H NMR of tissue extracts in SIV-infected macaques. NMR Biomed. 2005, 18, 242–251. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Pradhan, S.; Gowda, G.A.; Kumar, R. In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: One possible diagnostic view. NMR Biomed. 2010, 23, 113–122. [Google Scholar] [CrossRef]
- Gottschalk, M.; Ivanova, G.; Collins, D.M.; Eustace, A.; O’Connor, R.; Brougham, D.F. Metabolomic studies of human lung carcinoma cell lines using in vitro (1)H NMR of whole cells and cellular extracts. NMR Biomed. 2008, 21, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. The role of short-chain fatty acids in cancer prevention and cancer treatment. Arch. Biochem. Biophys. 2024, 761, 110172. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef]
- Hoxha, M.; Zappacosta, B. A review on the role of fatty acids in colorectal cancer progression. Front. Pharmacol. 2022, 13, 1032806. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Olivares-Rubio, H.F.; Espinosa-Aguirre, J.J. Role of epoxyeicosatrienoic acids in the lung. Prostaglandins Other Lipid Mediat. 2020, 149, 106451. [Google Scholar] [CrossRef]
- Merino Salvador, M.; Gómez de Cedrón, M.; Moreno Rubio, J.; Falagán Martínez, S.; Sánchez Martínez, R.; Casado, E.; Ramírez de Molina, A.; Sereno, M. Lipid metabolism and lung cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Pham, D.V.; Park, P.H. Adiponectin triggers breast cancer cell death via fatty acid metabolic reprogramming. J. Exp. Clin. Cancer Res. 2022, 41, 9. [Google Scholar] [CrossRef]
- Akbar, S.; Rahman, A.; Ahmad, N.; Imran, M.; Hafeez, Z. Understanding the Role of Polyunsaturated Fatty Acids in the Development and Prevention of Cancer. Cancer Treat. Res. 2024, 191, 57–93. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Wang, H.; Yung, M.M.; Chen, F.; Chan, W.S.; Chan, Y.S.; Tsui, S.K.; Ngan, H.Y.; Chan, K.K.; Chan, D.W. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics 2022, 12, 3534–3552. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.M.; Jones, C.L.; Pollyea, D.A.; Culp-Hill, R.; D’Alessandro, A.; Winters, A.; Krug, A.; Abbott, D.; Goosman, M.; Pei, S.; et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat. Cancer 2020, 1, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, H.; Xie, J.; Xu, Y.; Xu, C. Circulating fatty acids and risk of hepatocellular carcinoma and chronic liver disease mortality in the UK Biobank. Nat. Commun. 2024, 15, 3707. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Chandel, N.S. Targeting fatty acid metabolism in glioblastoma. J. Clin. Investig. 2023, 133, 1–11. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Poliakova, M.; Aebersold, D.M.; Zimmer, Y.; Medova, M. The relevance of tyrosine kinase inhibitors for global metabolic pathways in cancer. Mol. Cancer 2018, 17, 27. [Google Scholar] [CrossRef]
- Sjobakk, T.E.; Vettukattil, R.; Gulati, M.; Gulati, S.; Lundgren, S.; Gribbestad, I.S.; Torp, S.H.; Bathen, T.F. Metabolic Profiles of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 2104–2118. [Google Scholar] [CrossRef]
- Griffin, J.L.; Shockcor, J.P. Metabolic profiles of cancer cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Feng, J.; Liu, M.L.; Hu, J.Z. H-1 NMR metabolomics study of metastatic melanoma in C57BL/6J mouse spleen. Metabolomics 2014, 10, 1129–1144. [Google Scholar] [CrossRef]
- Vorland, M.; Thorsen, V.A.T.; Holmsen, H. Phospholipase D in platelets and other cells. Platelets 2008, 19, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.A.; Xu, L.Z. Phospholipase D in cell proliferation and cancer. Mol. Cancer Res. 2003, 1, 789–800. [Google Scholar] [PubMed]
- Puchades-Carrasco, L.; Jantus-Lewintre, E.; Perez-Rambla, C.; Garcia-Garcia, F.; Lucas, R.; Calabuig, S.; Blasco, A.; Dopazo, J.; Camps, C.; Pineda-Lucena, A. Serum metabolomic profiling facilitates the non-invasive identification of metabolic biomarkers associated with the onset and progression of non-small cell lung cancer. Oncotarget 2016, 7, 12904–12916. [Google Scholar] [CrossRef]
- Guo, Y.M.; Wang, X.M.; Qiu, L.; Qin, X.Z.; Liu, H.; Wang, Y.Y.; Li, F.; Wang, X.D.; Chen, G.Q.; Song, G.G.; et al. Probing gender-specific lipid metabolites and diagnostic biomarkers for lung cancer using Fourier transform ion cyclotron resonance mass spectrometry. Clin. Chim. Acta 2012, 414, 135–141. [Google Scholar] [CrossRef]
- Yu, Z.T.; Chen, H.K.; Ai, J.M.; Zhu, Y.; Li, Y.; Borgia, J.A.; Yang, J.S.; Zhang, J.C.; Jiang, B.; Gu, W.; et al. Global lipidomics identified plasma lipids as novel biomarkers for early detection of lung cancer. Oncotarget 2017, 8, 107899–107906. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Grapov, D.; DeFelice, B.C.; Taylor, S.; Kim, K.; Kelly, K.; Wikoff, W.R.; Pass, H.; Rom, W.N.; Fiehn, O.; et al. Serum phosphatidylethanolamine levels distinguish benign from malignant solitary pulmonary nodules and represent a potential diagnostic biomarker for lung cancer. Cancer Biomark. 2016, 16, 609–617. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef]
- Paige, M.; Saprito, M.S.; Bunyan, D.A.; Shim, Y.M. HPLC quantification of 5-hydroxyeicosatetraenoic acid in human lung cancer tissues. Biomed. Chromatogr. 2009, 23, 817–821. [Google Scholar] [CrossRef]
- Chen, Y.R.; Ma, Z.H.; Zhong, J.; Li, L.Q.; Min, L.S.; Xu, L.M.; Li, H.W.; Zhang, J.B.; Wu, W.; Dai, L.C. Simultaneous quantification of serum monounsaturated and polyunsaturated phosphatidylcholines as potential biomarkers for diagnosing nonsmall cell lung cancer. Sci. Rep. 2018, 8, 7137. [Google Scholar] [CrossRef]
- Al-Okaili, R.N.; Krejza, J.; Wang, S.; Woo, J.H.; Melhem, E.R. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics 2006, 26, S173–S189. [Google Scholar] [CrossRef]
- Han, Z.G.; Ke, M.X.; Liu, X.; Wang, J.; Guan, Z.Q.; Qiao, L.N.; Wu, Z.X.; Sun, Y.Y.; Sun, X.L. Molecular Imaging, How Close to Clinical Precision Medicine in Lung, Brain, Prostate and Breast Cancers. Mol. Imaging Biol. 2022, 24, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Asselin, M.C.; Reymen, B.; Jackson, A.; Lambin, P.; West, C.M.L.; O’Connor, J.P.B.; Faivre-Finn, C. Targeting Hypoxia to Improve Non-Small Cell Lung Cancer Outcome. J. Natl. Cancer Inst. 2018, 110, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Ancel, J.; Perotin, J.M.; Dewolf, M.; Launois, C.; Mulette, P.; Nawrocki-Raby, B.; Dalstein, V.; Gilles, C.; Deslee, G.; Polette, M.; et al. Hypoxia in Lung Cancer Management: A Translational Approach. Cancers 2021, 13, 3421. [Google Scholar] [CrossRef]
- Buil-Bruna, N.; Sahota, T.; López-Picazo, J.M.; Moreno-Jiménez, M.; Martín-Algarra, S.; Ribba, B.; Trocóniz, I.F. Early Prediction of Disease Progression in Small Cell Lung Cancer: Toward Model-Based Personalized Medicine in Oncology. Cancer Res. 2015, 75, 2416–2425. [Google Scholar] [CrossRef]
- Hsieh, A.H.C.; Tahkar, H.; Koczwara, B.; Kichenadasse, G.; Beckmann, K.; Karapetis, C.; Sukumaran, S. Pre-treatment serum lactate dehydrogenase as a biomarker in small cell lung cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, E64–E70. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, J.Z.; Hou, Q.; Cao, X.; Yao, N.N.; Sun, B.C.; Feng, P.X.; Zhang, W.J.; Niu, J.L. Prognostic Value of Lactate Dehydrogenase-to-Lymphocyte Ratio in Limited-Stage Small Cell Lung Cancer. J. Biol. Regul. Homeost. Agents 2023, 37, 5961–5969. [Google Scholar] [CrossRef]
- Tjokrowidjaja, A.; Lord, S.J.; John, T.; Lewis, C.R.; Kok, P.S.; Marschner, I.C.; Lee, C.K. Pre- and on-treatment lactate dehydrogenase as a prognostic and predictive biomarker in advanced non-small cell lung cancer. Cancer 2022, 128, 1574–1583. [Google Scholar] [CrossRef]
- Li, J.Q.; Eu, J.Q.; Kong, L.R.; Wang, L.Z.; Lim, Y.C.; Goh, B.C.; Wong, A.L.A. Targeting Metabolism in Cancer Cells and the Tumour Microenvironment for Cancer Therapy. Molecules 2020, 25, 4831. [Google Scholar] [CrossRef]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef]
- Yang, Y. Metabolic reprogramming of human lung cancer cells and ex vivo tissues revealed by UHR-FTMS analysis of small amino and carboxyl metabolites. Toxicol. Cancer Biol. 2017, 16, 1–124. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Liu, Y.; Li, B.; Li, J.; Zheng, L.; Wang, L. Metabonomic analysis of metastatic lung tissue in breast cancer mice by an integrated NMR-based metabonomics approach. RSC Adv. 2017, 7, 28001–28008. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhan, X.; Wang, B.; Wu, J.; Zhou, A. A UPLC-Q-TOF/MS-based plasma metabolomics approach reveals the mechanism of Compound Kushen Injection-based intervention against non-small cell lung cancer in Lewis tumor-bearing mice. Phytomedicine 2020, 76, 153259. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, X.W.; Yin, B.J.; Xia, G.H.; Shen, Z.J.; Gu, W.Z.; Wu, M.H. Role of the stromal cell derived factor-1/CXC chemokine receptor 4 axis in the invasion and metastasis of lung cancer and mechanism. J. Thorac. Dis. 2017, 9, 4947–4959. [Google Scholar] [CrossRef]
- Williams, M.D.; Xian, L.L.; Huso, T.; Park, J.J.; Huso, D.; Cope, L.M.; Gang, D.R.; Siems, W.F.; Resar, L.; Reeves, R.; et al. Fecal Metabolome in Hmga1 Transgenic Mice with Polyposis: Evidence for Potential Screen for Early Detection of Precursor Lesions in Colorectal Cancer. J. Proteome Res. 2016, 15, 4176–4187. [Google Scholar] [CrossRef]
- Yu, J.; Kim, A.K. Effect of Taurine on Antioxidant Enzyme System in B16F10 Melanoma Cells. In Taurine 7; Azuma, J., Schaffer, S.W., Ito, T., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2009; Volume 643, pp. 491–499. [Google Scholar]
- Yi, H.W.; Talmon, G.; Wang, J. Glutamate in cancers: From metabolism to signaling. J. Biomed. Res. 2020, 34, 260–270. [Google Scholar] [CrossRef]
- Sun, R.L.; Gu, J.F.; Chang, X.W.; Liu, F.Y.; Liang, Y.; Yang, X.Y.; Liang, L.; Tang, D.C. Metabonomics study on orthotopic transplantion mice model of colon cancer treated with Astragalus membranaceus-Curcuma wenyujin in different proportions via UPLC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2021, 193, 113708. [Google Scholar] [CrossRef]
- Yan, L.; Rust, B.M.; Picklo, M.J. Plasma Metabolomic Changes in Mice with Time-restricted Feeding-attenuated Spontaneous Metastasis of Lewis Lung Carcinoma. Anticancer. Res. 2020, 40, 1833–1841. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Li, T.; Deng, P.C. Nuclear Magnetic Resonance technique in tumor metabolism. Genes Dis. 2017, 4, 28–36. [Google Scholar] [CrossRef]
- Merz, A.L.; Serkova, N.J. Use of nuclear magnetic resonance-based metabolomics in detecting drug resistance in cancer. Biomark. Med. 2009, 3, 289–306. [Google Scholar] [CrossRef]
- Hu, C.; Iwasaki, M.; Liu, Z.G.; Wang, B.C.; Li, X.M.; Lin, H.; Li, J.; Li, J.V.; Lian, Q.Q.; Ma, D.Q. Lung but not brain cancer cell malignancy inhibited by commonly used anesthetic propofol during surgery: Implication of reducing cancer recurrence risk. J. Adv. Res. 2021, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qiao, T.Y. Unraveling tumor microenvironment of small-cell lung cancer: Implications for immunotherapy. Semin. Cancer Biol. 2022, 86, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Castro, M.D.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef]
- He, Q.; Sun, C.; Pan, Y. Whole-exome sequencing reveals Lewis lung carcinoma is a hypermutated Kras/Nras–mutant cancer with extensive regional mutation clusters in its genome. Sci. Rep. 2024, 14, 100. [Google Scholar] [CrossRef]
- Wu, W.S.; Wu, H.Y.; Wang, P.H.; Chen, T.Y.; Chen, K.R.; Chang, C.W.; Lee, D.E.; Lin, B.H.; Chang, W.C.W.; Liao, P.C. LCMD: Lung Cancer Metabolome Database. Comput. Struct. Biotechnol. J. 2022, 20, 65–78. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, B.B. Challenges and Opportunities in Cancer Metabolomics. Proteomics 2019, 19, 1900042. [Google Scholar] [CrossRef]
- Bensussan, A.V.; Lin, J.; Guo, C.X.; Katz, R.; Krishnamurthy, S.; Cressman, E.; Eberlin, L.S. Distinguishing Non-Small Cell Lung Cancer Subtypes in Fine Needle Aspiration Biopsies by Desorption Electrospray Ionization Mass Spectrometry Imaging. Clin. Chem. 2020, 66, 1424–1433. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Kharybin, O.N.; Archakov, A.I. Diagnosis of lung cancer based on direct-infusion electrospray mass spectrometry of blood plasma metabolites. Int. J. Mass Spectrom. 2012, 309, 200–205. [Google Scholar] [CrossRef]
- Zeng, X.M.; Hood, B.L.; Zhao, T.; Conrads, T.P.; Sun, M.; Gopalakrishnan, V.; Grover, H.; Day, R.S.; Weissfeld, J.L.; Wilson, D.O.; et al. Lung Cancer Serum Biomarker Discovery Using Label-Free Liquid Chromatography-Tandem Mass Spectrometry. J. Thorac. Oncol. 2011, 6, 725–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarska, A.; Bamburowicz-Klimkowska, M.; Pisklak, D.M.; Gawlak, M.; Grudzinski, I.P. A Pilot Study on Qualitative Metabolomics to Characterize Lewis Lung Carcinoma in Mice. Life 2025, 15, 202. https://doi.org/10.3390/life15020202
Stawarska A, Bamburowicz-Klimkowska M, Pisklak DM, Gawlak M, Grudzinski IP. A Pilot Study on Qualitative Metabolomics to Characterize Lewis Lung Carcinoma in Mice. Life. 2025; 15(2):202. https://doi.org/10.3390/life15020202
Chicago/Turabian StyleStawarska, Agnieszka, Magdalena Bamburowicz-Klimkowska, Dariusz Maciej Pisklak, Maciej Gawlak, and Ireneusz P. Grudzinski. 2025. "A Pilot Study on Qualitative Metabolomics to Characterize Lewis Lung Carcinoma in Mice" Life 15, no. 2: 202. https://doi.org/10.3390/life15020202
APA StyleStawarska, A., Bamburowicz-Klimkowska, M., Pisklak, D. M., Gawlak, M., & Grudzinski, I. P. (2025). A Pilot Study on Qualitative Metabolomics to Characterize Lewis Lung Carcinoma in Mice. Life, 15(2), 202. https://doi.org/10.3390/life15020202







