Electrocauterization versus Ligation of Lymphatic Vessels to Prevent Lymphocele Development after Kidney Transplantation—A Meta-Analysis
Abstract
:1. Introduction
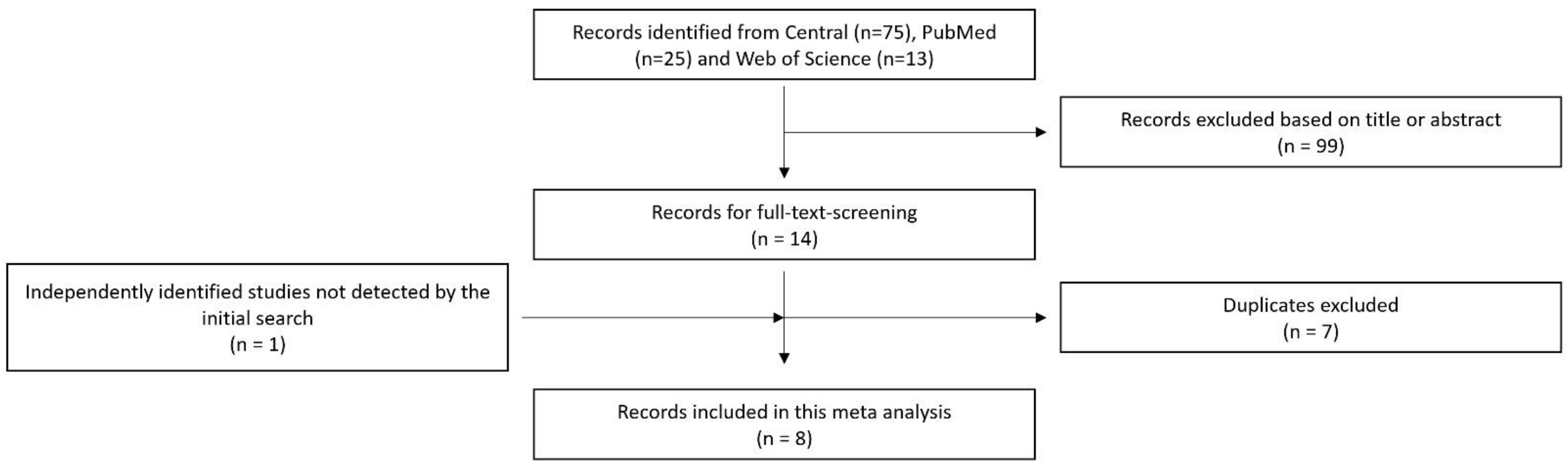
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
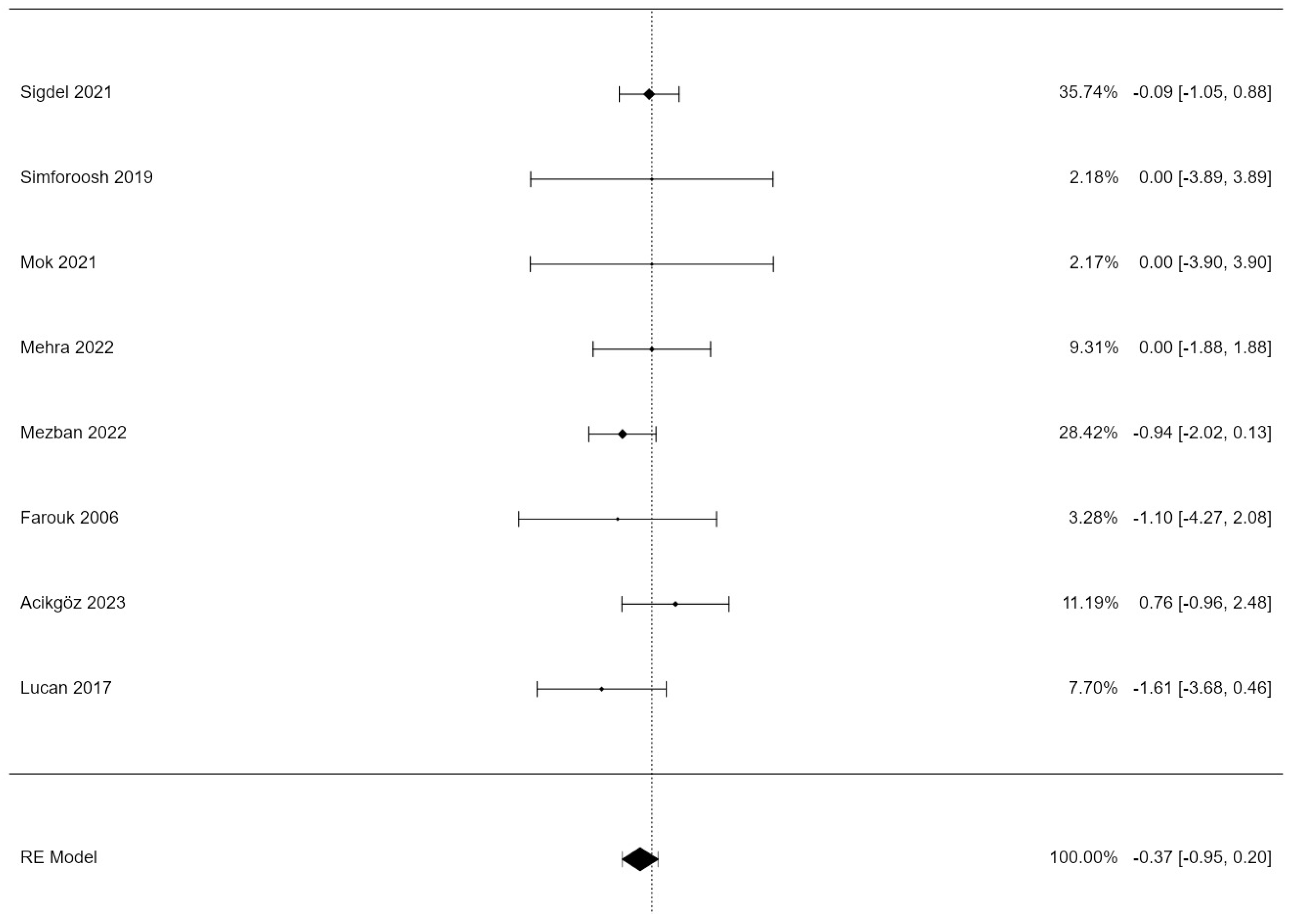
3.1. Lymphocele Incidence
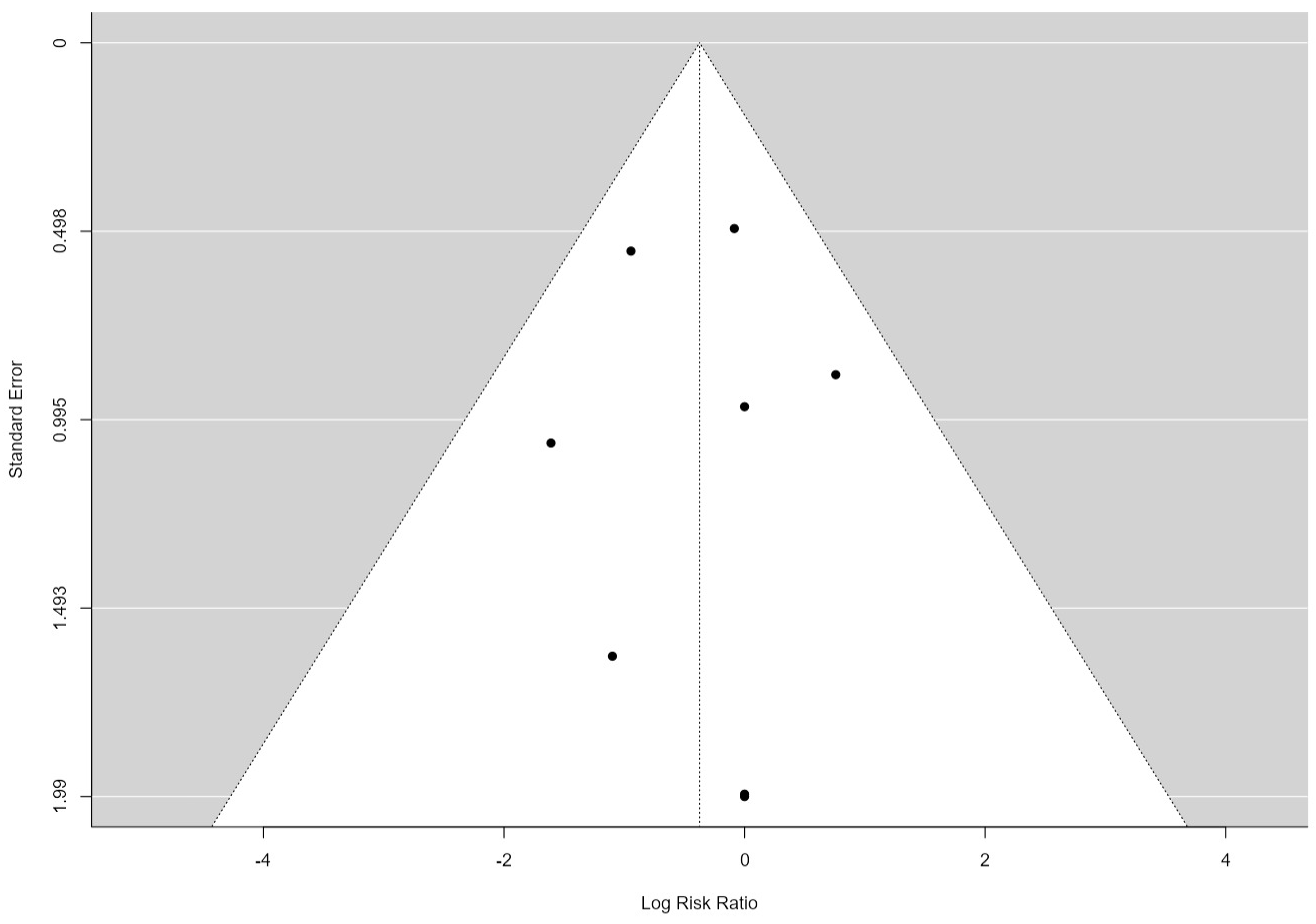
3.2. Publication Bias Assessment
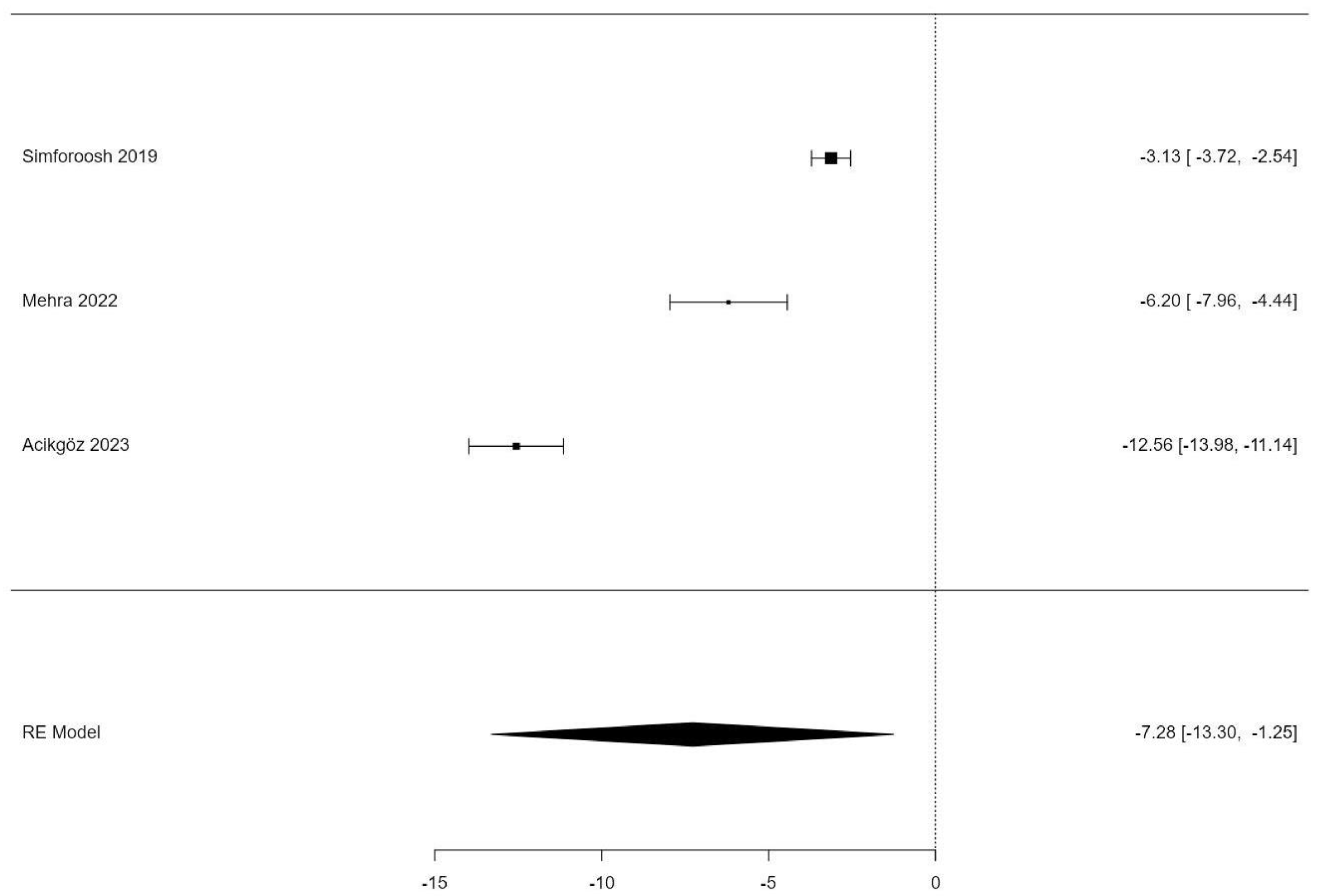
3.3. Lymphatic Sealing Time
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhry, D.; Chaudhry, A.; Peracha, J.; Sharif, A. Survival for Waitlisted Kidney Failure Patients Receiving Transplantation versus Remaining on Waiting List: Systematic Review and Meta-Analysis. BMJ 2022, 376, e068769. [Google Scholar] [CrossRef]
- Samhan, M.; Al-Mousawi, M. Lymphocele Following Renal Transplantation. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2006, 17, 34–37. [Google Scholar]
- Khauli, R.B.; Stoff, J.S.; Lovewell, T.; Ghavamian, R.; Baker, S. Post-Transplant Lymphoceles: A Critical Look into the Risk Factors, Pathophysiology and Management. J. Urol. 1993, 150, 22–26. [Google Scholar] [CrossRef]
- Joosten, M.; d’Ancona, F.C.; van der Meijden, W.A.; Poyck, P.P. Predictors of Symptomatic Lymphocele after Kidney Transplantation. Int. Urol. Nephrol. 2019, 51, 2161–2167. [Google Scholar] [CrossRef]
- Lucewicz, A.; Wong, G.; Lam, V.W.T.; Hawthorne, W.J.; Allen, R.; Craig, J.C.; Pleass, H.C.C. Management of Primary Symptomatic Lymphocele after Kidney Transplantation: A Systematic Review. Transplantation 2011, 92, 663. [Google Scholar] [CrossRef]
- Lehner, L.J.; Hohberger, A.; Marschke, L.; Lachmann, N.; Peters, R.; Friedersdorff, F.; Khadzhynov, D.; Halleck, F.; Budde, K.; Staeck, O.; et al. Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles. J. Clin. Med. 2020, 9, 2841. [Google Scholar] [CrossRef]
- Serirodom, M.; Taweemonkongsap, T.; Chotikawanich, E.; Jitpraphai, S.; Woranisarakul, V.; Shrestha, S.; Hansomwong, T. Lymphocele in Kidney Transplantation: A Comparison of Ligation and Non-Ligation Technique of Iliac Lymphatic Dissection. Transplant. Proc. 2022, 54, 2197–2204. [Google Scholar] [CrossRef]
- Hwang, K. Tracing the Use of Cautery in the Modern Surgery. J. Craniofac. Surg. 2018, 29, 12–13. [Google Scholar] [CrossRef]
- Prakash, O.; Chandrashekar, S.; Mattews, J.; George, R.; Suprej, K.; Mattew, J.; Francis, F.L.; Shajan, F.R.; Alex, J.; Kamath, G.D. Bipolar Cautery versus Conventional Suture Ligation of Vascular Pedicles in Thyroidectomy: A Comparative Clinical Study. Int. Surg. J. 2021, 8, 1181–1184. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 August 2023).
- Farsad-Naeimi, A.; Asjodi, F.; Omidian, M.; Askari, M.; Nouri, M.; Pizarro, A.B.; Daneshzad, E. Sugar Consumption, Sugar Sweetened Beverages and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2020, 53, 102512. [Google Scholar] [CrossRef]
- Forte, A.J.; Guliyeva, G.; McLeod, H.; Dabrh, A.M.A.; Salinas, M.; Avila, F.R.; Perlman, A. The Impact of Optimism on Cancer-Related and Postsurgical Cancer Pain: A Systematic Review. J. Pain Symptom Manag. 2022, 63, e203–e211. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Farouk, K.; Afridi, Z.u.D.; Bano, U.; Ahmad, N.; Khan, A.R. Electocoagulation Versus Suture-Ligation of Lymphatics in Kidney Transplant Recipient Surgery. J. Postgrad. Med. Inst. 2006, 20. [Google Scholar]
- Lucan, C.V.; Jurchis, I.; Suciu, M.; Selicean, S.E.; Buttice, S. Modern Lymphatic Dissection Techniques for Preventing Post Renal Transplant Lymphocele. Clujul Med. 1957 2017, 90, 416–419. [Google Scholar] [CrossRef]
- Simforoosh, N.; Tabibi, A.; Rad, H.M.; Gholamrezaie, H.R. Comparison Between Bipolar Lymphatic Vessels Cautery and Suture Ligature in Prevention of Postrenal Transplant Lymphocele Formation: A Randomized Controlled Trial. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2019, 17, 26–30. [Google Scholar] [CrossRef]
- Mok, S.; Park, Y.J.; Park, S.C.; Yun, S.S. Efficacy of Lymphatic Sealing Using the LigaSure in Kidney Transplantation: A Pilot Study. Transplant. Proc. 2021, 53, 2278–2284. [Google Scholar] [CrossRef]
- Sigdel, P.R.; Gnyawali, D.; Thapa, J.; Rai, B.D.K.; Dhital, P.; Parajuli, P.; Chudal, S.; Pradhan, M.; Poudyal, S.; Chapagain, S.; et al. Bipolar Vessel Sealing System versus Silk Ligation of Lymphatic Vessels in Renal Transplant Recipient Lymphatic Complications: A Randomized Controlled Trial. Int. Urol. Nephrol. 2021, 53, 2477–2483. [Google Scholar] [CrossRef]
- Mehra, K.; Kapashi, K.; Khemchandani, S.; Modi, P.R.; Rizvi, S.J. Prospective Comparison of Suture Ligation and Electrothermal Sealing for the Control of Perivascular Lymphatics in Kidney Transplant Recipients. Korean J. Transplant. 2022, 36, 245–252. [Google Scholar] [CrossRef]
- Mezban, S.; Almayyahi, A.; Mahdi, R.; Al-Badran, M.; Al-Khammas, H.; Aledan, H. Comparative Study to Evaluate the Effect of Using an Electro-Thermal Bipolar Sealing Device and Conventional Ligation on the Incidence of Post-Renal Transplant Lymphoceles in Recipients: The Effect of Using Electro-Thermal Bipolar Sealing on Post Renal Transplant Lymphocele in Recipients. Iraqi Natl. J. Med. 2022, 4, 61–70. [Google Scholar] [CrossRef]
- Acikgoz, O.; Akinci, S. Comparison of Bipolar Electrocautery-Based Vascular Sealers with Conventional Ligation in Iliac Vessel Preparation of Renal Transplant Recipients. Transplant. Proc. 2023, 55, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Madura, J.A.; Dunbar, J.D.; Cerilli, G.J. Perirenal Lymphocele as a Complication of Renal Homotransplantation. Surgery 1970, 68, 310–313. [Google Scholar]
- Ooms, L.S.; Verhelst, J.; Jeekel, J.; Ijzermans, J.N.; Lange, J.F.; Terkivatan, T. Incidence, Risk Factors, and Treatment of Incisional Hernia after Kidney Transplantation: An Analysis of 1564 Consecutive Patients. Surgery 2016, 159, 1407–1411. [Google Scholar] [CrossRef]
- Menezes, F.G.; Wey, S.B.; Peres, C.A.; Medina-Pestana, J.O.; Camargo, L.F.A. Risk Factors for Surgical Site Infection in Kidney Transplant Recipients. Infect. Control Hosp. Epidemiol. 2008, 29, 771–773. [Google Scholar] [CrossRef]
- Childers, C.P.; Maggard-Gibbons, M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018, 153, e176233. [Google Scholar] [CrossRef]
- Smith, T.; Evans, J.; Moriel, K.; Tihista, M.; Bacak, C.; Dunn, J.; Rajani, R.; Childs, B. The Cost of OR Time Is $46.04 per Minute. J. Orthop. Bus. 2022, 2, 10–13. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Kontzoglou, K.; Dimitroulis, D.; Vlachos, D.-E.G.; Routsolias, P.; Vlachos, G.D. Electrosurgical Bipolar Vessel Sealing during Axillary Lymphadenectomy: A Systematic Review and Meta-Analysis. Breast Dis. 2015, 35, 5–11. [Google Scholar] [CrossRef]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-Term Kidney Transplant Graft Survival-Making Progress When Most Needed. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 2824–2832. [Google Scholar] [CrossRef]
- Bender, R.; Friede, T.; Koch, A.; Kuss, O.; Schlattmann, P.; Schwarzer, G.; Skipka, G. Methods for Evidence Synthesis in the Case of Very Few Studies. Res. Synth. Methods 2018, 9, 382–392. [Google Scholar] [CrossRef]
| Name | Study Type | Newcastle–Ottawa Scale | Exclusion Criteria | Lymphocele Definition | Follow-Up Regime | Study Size | Average Age in Years (SD) | Duration (in Minutes) Comparison (SD) |
|---|---|---|---|---|---|---|---|---|
| Farouk, 2006 [14] | Prospective RCT | - | None | Not specified | Repeated ultrasound exams in the first six months | 90 | 38 (NA) | OT: 95.98 (10.51) vs. 109.78 (12.24) |
| Lucan, 2017 [15] | Prospective RCT | - | None | Not specified | Not specified | 48 | 40.96 (12.81) | Not provided |
| Simforoosh, 2019 [16] | Prospective RCT | - | None | Not specified | Once at 5 months | 60 | 41.25 (15.98) | LST: 3.57 (1.09) vs. 6.7 (1.22) |
| Mok, 2021 [17] | Retrospective cohort study | 7 * | None | Not specified | Ultrasound after 1 month, check for symptoms for 2 more months | 100 | 47.44 (12.4) | OT:173.38(29.55) vs. 183(37.14) |
| Sigdel, 2021 [18] | Prospective RCT | - | Urinary leakage, urinoma formation, retroperitoneal hemorrhage, re-exploration or death within 3 months | >50 mL | Ultrasound after 6 weeks and after 3 months | 58 | 33.6 (10.65) | Not provided |
| Mehra, 2022 [19] | Prospective RCT | - | Not specified | Not specified | Not specified | 52 | 35.9 (13.75) | LST: 8.3 (1.9) vs. 14.5 (4) |
| Mezban, 2022 [20] | Retrospective cohort study | 6 * | Uncontrolled diabetes, high doses of immunosuppressive drugs, diuretics treatments and anti-coagulants and BMI > 24 or age >60 years | Not specified | Weekly ultrasound for 6 weeks | 130 | 34.22 (10.31) | OT: 140.33 (17.07) vs. 155.57 (17.9) |
| Acikgöz, 2023 [21] | Retrospective cohort study | 5 * | Deceased donor re-transplantation | >50 mL | Not specified | 63 | 38.29 (12.47) | LST: 7.85 (1.97) vs. 20.41 (3.62) |
| Estimate | se | Z | p | CI Lower Bound | CI Upper Bound | |
|---|---|---|---|---|---|---|
| Intercept | −0.3474 | 0.293 | −1.28 | 0.202 | −0.949 | 0.201 |
| Estimate | se | Z | p | CI Lower Bound | CI Upper Bound | |
|---|---|---|---|---|---|---|
| Intercept | −7.28 | 3.07 | −2.37 | 0.018 | −13.304 | −1.254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matrisch, L.; Lapshyn, H.; Nitschke, M.; Rau, Y. Electrocauterization versus Ligation of Lymphatic Vessels to Prevent Lymphocele Development after Kidney Transplantation—A Meta-Analysis. J. Pers. Med. 2024, 14, 256. https://doi.org/10.3390/jpm14030256
Matrisch L, Lapshyn H, Nitschke M, Rau Y. Electrocauterization versus Ligation of Lymphatic Vessels to Prevent Lymphocele Development after Kidney Transplantation—A Meta-Analysis. Journal of Personalized Medicine. 2024; 14(3):256. https://doi.org/10.3390/jpm14030256
Chicago/Turabian StyleMatrisch, Ludwig, Hryhoriy Lapshyn, Martin Nitschke, and Yannick Rau. 2024. "Electrocauterization versus Ligation of Lymphatic Vessels to Prevent Lymphocele Development after Kidney Transplantation—A Meta-Analysis" Journal of Personalized Medicine 14, no. 3: 256. https://doi.org/10.3390/jpm14030256





