Hypothermia: Beyond the Narrative Review—The Point of View of Emergency Physicians and Medico-Legal Considerations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Definition
- -
- Mild hypothermia: 32 °C < core body temperature < 35 °C.
- -
- Moderate hypothermia: 28 °C < core body temperature < 32 °C.
- -
- Severe hypothermia: core body temperature < 28 °C.
- -
- Primary hypothermia is due to environmental exposure, with no underlying medical condition causing the disruption of temperature regulation [21]. It therefore occurs when a person is exposed to the cold without adequate protection.
- -
- Secondary hypothermia is a complication of pathological and paraphysiological conditions that determine the hypothermia itself or cause either an alteration of the thermoregulation mechanisms, reduced heat production or increased heat dispersion.
- -
- Therapeutic hypothermia;
- -
- Trauma-induced hypothermia [22].
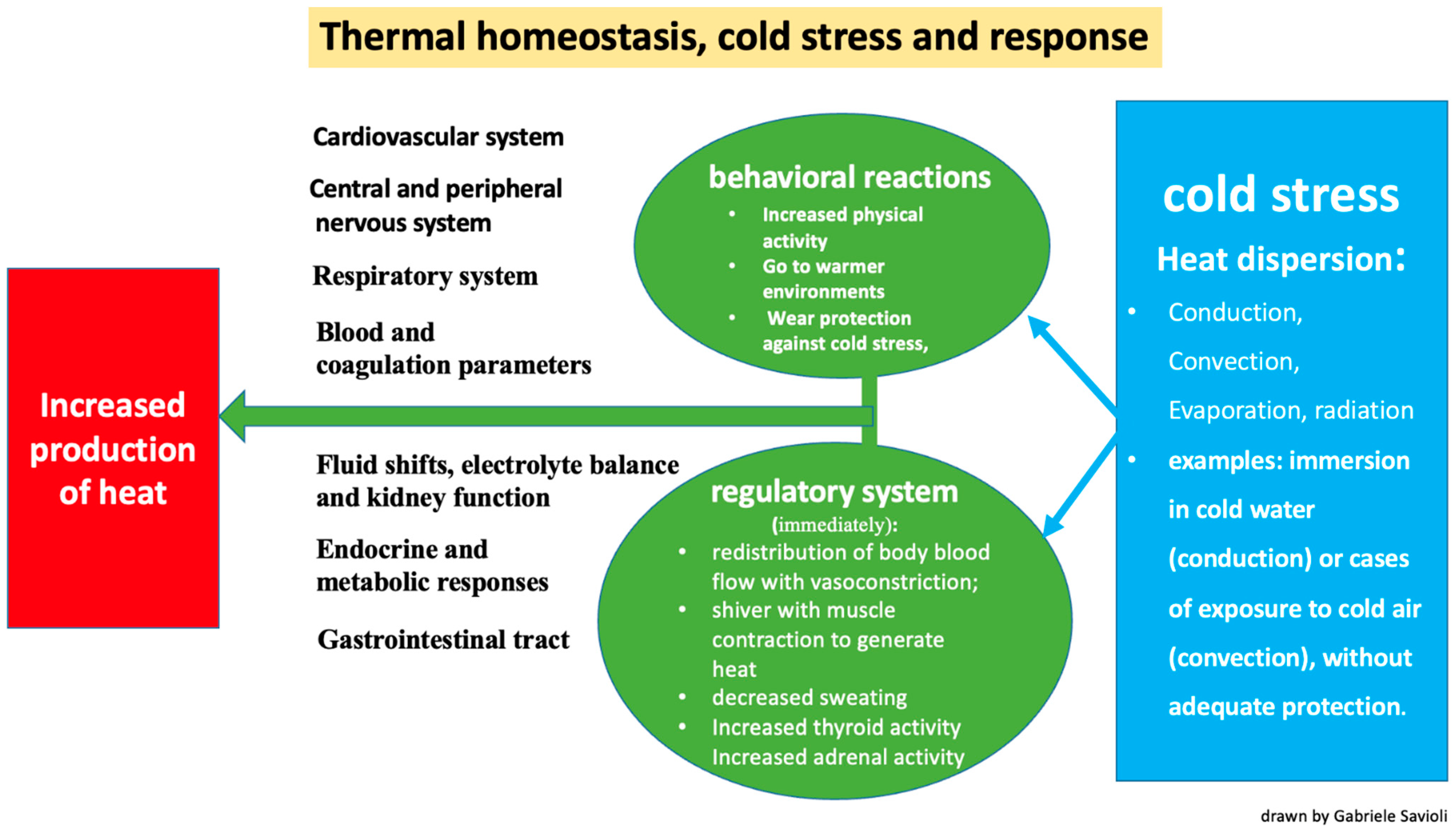
3.2. Physiopathology
- Conduction: the transfer of heat to a cooler object through direct contact (for example, when immersed in cold water).
- Convection: the transfer of heat at the body surface via air circulation (for example, when exposed to cold air or wind).
- Evaporation: cooling of the skin surfaces when sweat changes from a liquid to a vapor form.
- Radiation: occurs through the transmission of electromagnetic waves.
- -
- The redistribution of blood flow via vasoconstriction and a subsequent reduction in blood volume directed towards the skin and subcutis to reduce heat loss;
- -
- Shivering, which generates heat via muscle contraction;
- -
- Decreased sweating;
- -
- Increased thyroid activity due to hypothalamic stimulus;
- -
- Increased adrenal activity due to hypothalamic stimulus.
- -
- Increased physical activity;
- -
- A shift to warmer environments;
- -
- Wearing protection against the cold;
- -
- Taking off wet clothes and replacing them with dry clothes.
3.3. Etiology and Risk Factors
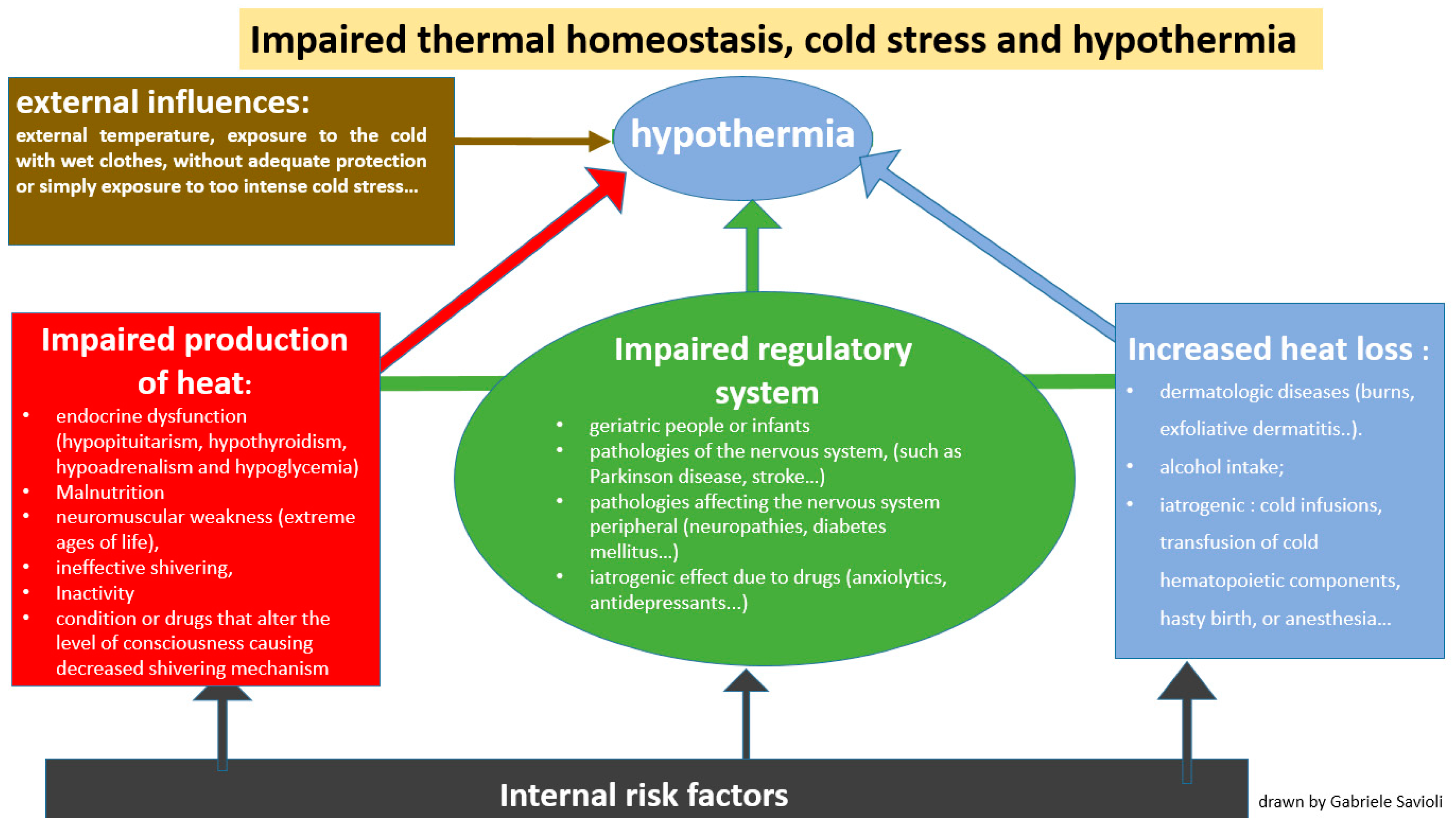
- -
- Impaired thermoregulation (Figure 2): This may be paraphysiological in the extreme ages of life (geriatric people or infants). Impaired thermoregulation may also occur following pathological conditions such as mental illnesses, or pathologies of the nervous system (such as Parkinson’s disease, stroke, multiple sclerosis, hypothalamic dysfunction, brain trauma or subarachnoid hemorrhage) or pathologies affecting the peripheral nervous system (neuropathies, diabetes mellitus, trauma to a section of the spinal cord). Impaired thermoregulation may also be due to an iatrogenic effect of drugs such as anxiolytics, antidepressants, phenothiazines, barbiturates, opioids, antipsychotics, oral antihyperglycemics, β-blockers and α-blockers [33,34].
- -
- Increased heat loss (Figure 2): This can be caused by dermatologic diseases such as burns, exfoliative dermatitis or psoriasis [35,36,37]. Heat loss can also be increased in the case of indulgent habits such as alcohol intake [38,39] but it can also have an iatrogenic origin, as in the case of cold infusions, the transfusion of cold hematopoietic components, hasty birth or anesthesia.
- -
- Decreased heat production (Figure 2): This is secondary to endocrine dysfunction (hypopituitarism, hypothyroidism, hypoadrenalism and hypoglycemia), malnutrition or either conditions or drugs that alter the level of consciousness, causing an impaired shivering mechanism.
3.4. From Pathophysiology to Clinical Manifestations
3.4.1. Cardiovascular System
3.4.2. Central and Peripheral Nervous Systems
3.4.3. Respiratory System
3.4.4. Fluid Shifts, Electrolyte Balance and Kidney Function
3.4.5. Blood and Coagulation Parameters
3.4.6. Endocrine and Metabolic Responses
3.4.7. Gastrointestinal Tract
3.5. Clinical Diagnosis
3.6. Laboratory Studies
4. Discussion and Contextualization
4.1. Point of View of the Emergency Departments: General Aspects and Management
4.1.1. Management of Hypothermic Patients in the Emergency Department (ED)
4.1.2. Inpatient Treatment of Moderate-to-Severe Hypothermia
4.1.3. CPR (Cardiopulmonary Resuscitation) and Management of Arrhythmias
4.1.4. Secondary Hypothermia in Acute Diseases
4.1.5. Recommendations for Future Research
4.2. Risk Management and the Forensic Point of View
4.2.1. Post-Mortem Changes
4.2.2. External Findings
4.2.3. Autopsy Findings
4.2.4. Microscopic Findings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hypothermia: Background, Pathophysiology, Etiology. Available online: https://emedicine.medscape.com/article/770542-overview (accessed on 15 August 2023).
- Petrone, P.; Asensio, J.A.; Marini, C.P. In brief: Hypothermia. Curr. Probl. Surg. 2014, 51, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Anna, K. Accidental shypothermia. BMJ 2006, 332, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.R.; Hedegaard, H.B. QuickStats: Death Rates* Attributed to Excessive Cold or Hypothermia† Among Persons Aged ≥15 Years, by Urban-Rural Status§ and Age Group—National Vital Statistics System, United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 258. [Google Scholar]
- van Veelen, M.J.; Brodmann Maeder, M. Hypothermia in Trauma. Int. J. Environ. Res. Public Health 2021, 18, 8719. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.K.; Maier, R.V.; Pavlin, E.G.; Anardi, D.; Copass, M.K.; Oreskovich, M.R. Incidence and effect of hypothermia in seriously injured patients. J. Trauma 1987, 27, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Forristal, C.; Van Aarsen, K.; Columbus, M.; Wei, J.; Vogt, K.; Mal, S. Predictors of Hypothermia upon Trauma Center Arrival in Severe Trauma Patients Transported to Hospital via EMS. Prehosp. Emerg. Care 2020, 24, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Meiman, J.; Anderson, H.; Tomasallo, C.; Centers for Disease Control and Prevention (CDC). Hypothermia-related deaths—Wisconsin, 2014, and United States, 2003–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 141–143. [Google Scholar] [PubMed]
- Brändström, H.; Johansson, G.; Giesbrecht, G.G.; Ängquist, K.-A.; Haney, M.F. Accidental Cold-Related Injury Leading to Hospitalization in Northern Sweden: An Eight-Year Retrospective Analysis. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 6. [Google Scholar] [CrossRef]
- Hislop, L.J.; Wyatt, J.P.; McNaughton, G.W.; Ireland, A.J.; Rainer, T.H.; Olverman, G.; Laughton, L.M. Urban hypothermia in the west of Scotland. West of Scotland Accident and Emergency Trainees Research Group. BMJ 1995, 311, 725. [Google Scholar] [CrossRef]
- Darocha, T.; Kosiński, S.; Jarosz, A.; Sobczyk, D.; Gałązkowski, R.; Sanak, T.; Hymczak, H.; Kapelak, B.; Drwiła, R. Management of hypothermia—Severe Accidental Hypothermia Centre in Krakow. Kardiol. Pol. 2015, 73, 789–794. [Google Scholar] [CrossRef]
- Petrone, P.; Asensio, J.A.; Marini, C.P. Management of accidental hypothermia and cold injury. Curr. Probl. Surg. 2014, 51, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37 (Suppl. 7), S186–S202. [Google Scholar] [CrossRef] [PubMed]
- Cappaert, T.A.; Stone, J.A.; Castellani, J.W.; Krause, B.A.; Smith, D.; Stephens, B.A. National Athletic Trainers’ Association Position Statement: Environmental Cold Injuries. J. Athl. Train. 2008, 43, 640–658. [Google Scholar] [CrossRef] [PubMed]
- Jurkovich, G.J. Environmental Cold-Induced Injury. Surg. Clin. N. Am. 2007, 87, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.S.; Rathlev, N.K. Hypothermia and localized cold injuries. Emerg. Med. Clin. N. Am. 2004, 22, 281–298. [Google Scholar] [CrossRef]
- Giesbrecht, G.G. Cold stress, near drowning and accidental hypothermia: A review. Aviat. Space Environ. Med. 2000, 71, 733–752. [Google Scholar]
- Durrer, B.; Brugger, H.; Syme, D.; Elsensohn, F.; Deslarzes, T.; Yersin, B.; Paal, P.; Gordon, L.; Strapazzon, G.; Maeder, M.B.; et al. The Medical On-site Treatment of Hypothermia: ICAR-MEDCOM Recommendation. High Alt. Med. Biol. 2003, 4, 99–103. [Google Scholar] [CrossRef]
- Long, W.B.; Edlich, R.F.; Winters, K.L.; Britt, L.D. Cold injuries. J. Long Term Eff. Med. Implant. 2005, 15, 67–78. [Google Scholar] [CrossRef]
- Søreide, K. Clinical and translational aspects of hypothermia in major trauma patients: From pathophysiology to prevention, prognosis and potential preservation. Injury 2014, 45, 647–654. [Google Scholar] [CrossRef]
- Schumacker, P.T.; Rowland, J.; Saltz, S.; Nelson, D.P.; Wood, L.D. Effects of hyperthermia and hypothermia on oxygen extraction by tissues during hypovolemia. J. Appl. Physiol. 1987, 63, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Woyke, S.; Brugger, H.; Ströhle, M.; Haller, T.; Gatterer, H.; Cappello, T.D.; Strapazzon, G. Effects of Carbon Dioxide and Temperature on the Oxygen-Hemoglobin Dissociation Curve of Human Blood: Implications for Avalanche Victims. Front. Med. 2022, 8, 808025. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; Younker, J. Correcting arterial blood gases for temperature: (When) is it clinically significant? Nurs. Crit. Care 2006, 11, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, A.; Swanepoel, C. The oxyhemoglobin dissociation curve before, during and after cardiac surgery. Scand. J. Clin. Lab. Investig. 1990, 50, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Santelli, J.; Sullivan, J.M.; Czarnik, A.; Bedolla, J. Heat illness in the emergency department: Keeping your cool. Emerg. Med. Pract. 2014, 16, 1–21. [Google Scholar] [PubMed]
- D’Angelo, J. Treating heat-related illness in the elderly. Emerg. Med. Serv. 2004, 33, 111–113. [Google Scholar]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Romanovsky, A.A. The thermoregulation system and how it works. Handb. Clin. Neurol. 2018, 156, 3–43. [Google Scholar]
- Osilla, E.V.; Marsidi, J.L.; Sharma, S. Physiology, Temperature Regulation; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507838/ (accessed on 15 August 2023).
- Atha, W.F. Heat-related illness. Emerg. Med. Clin. N. Am. 2013, 31, 1097–1108. [Google Scholar] [CrossRef]
- Severe Recurrent Hypothermia in an Elderly Patient with Refractory Mania Associated with Atypical Antipsychotic, Valproic Acid and Oxcarbazepine Therapy|BMJ Case Reports. Available online: https://casereports.bmj.com/content/2017/bcr-2017-222462 (accessed on 17 August 2023).
- Rumbus, Z.; Garami, A. Fever, hypothermia, and mortality in sepsis. Temperature 2018, 6, 101–103. [Google Scholar] [CrossRef]
- Fox, R.H.; Shuster, S.; Williams, R.; Marks, J.; Goldsmith, R.; Condon, R.E. Cardiovascular, Metabolic, and Ther Moregulatory Disturbances in Patients with Erythrodermic Skin Diseases. Br. Med. J. 1965, 1, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Cucinell, S.A. Hypothermia and Generalized Skin Disease. Arch. Dermatol. 1978, 114, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Krook, G. Hypothermia in patients with exfoliative dermatitis. Acta Derm. Venereol. 1960, 40, 142–160. [Google Scholar] [PubMed]
- Kalant, H.; Lê, A. Effects of ethanol on thermoregulation. Pharmacol. Ther. 1983, 23, 313–364. [Google Scholar] [CrossRef] [PubMed]
- Ristuccia, R.C.; Hernandez, M.; Wilmouth, C.E.; Spear, L.P. Differential Expression of Ethanol-Induced Hypothermia in Adolescent and Adult Rats Induced by Pretest Familiarization to the Handling/Injection Procedure. Alcohol. Clin. Exp. Res. 2007, 31, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.A.; Brugger, H.; Boyd, J.; Paal, P. Accidental hypothermia. N. Engl. J. Med. 2012, 367, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef] [PubMed]
- Biem, J.; Koehncke, N.; Classen, D.; Dosman, J. Out of the cold: Management of hypothermia and frostbite. CMAJ 2003, 168, 305–311. [Google Scholar]
- Tsuei, B.J.; Kearney, P.A. Hypothermia in the trauma patient. Injury 2004, 35, 7–15. [Google Scholar] [CrossRef]
- Ireland, S.; Endacott, R.; Cameron, P.; Fitzgerald, M.; Paul, E. The incidence and significance of accidental hypothermia in major trauma—A prospective observational study. Resuscitation 2011, 82, 300–306. [Google Scholar] [CrossRef]
- Danzl, D.F.; Pozos, R.S. Accidental hypothermia. N. Engl. J. Med. 1994, 331, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Deussen, A. Hyperthermia and hypothermia. Effects on the cardiovascular system. Anaesthesist 2007, 56, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.P.; Chapman, B.J.; Munday, K.A. The Effect of Hypothermia on the Cardiovascular System and the Pressor Actions of Angiotensin II. J. Therm. Biol. 1984, 9, 243–246. [Google Scholar] [CrossRef]
- Dietrichs, E.S.; McGlynn, K.; Allan, A.; Connolly, A.; Bishop, M.; Burton, F.; Kettlewell, S.; Myles, R.; Tveita, T.; Smith, G.L. Moderate but not severe hypothermia causes pro-arrhythmic changes in cardiac electrophysiology. Cardiovasc. Res. 2020, 116, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Prec, O.; Rosenman, R.; Braun, K.; Rodbard, S.; Katz, L.N. The cardiovascular effects of acutely induced hypothermia. J. Clin. Investig. 1949, 28, 293–300. [Google Scholar] [CrossRef]
- Covino, B.G.; D’Amato, H.E. Mechanism of Ventricular Fibrillation in Hypothermia. Circ. Res. 1962, 10, 148–155. [Google Scholar] [CrossRef]
- Bjørnstad, H.; Tande, P.M.; Refsum, H. Cardiac electrophysiology during hypothermia. Implications for medical treatment. Arctic Med. Res. 1991, 50 (Suppl. 6), 71–75. [Google Scholar]
- Roscher, R.; Arlock, P.; Sjöberg, T.; Steen, S. Effects of dopamine on porcine myocardial action potentials and contractions at 37 degrees C and 32 degrees C. Acta Anaesthesiol. Scand. 2001, 45, 421–426. [Google Scholar] [CrossRef]
- Ehrlich, M.P.; McCullough, J.N.; Zhang, N.; Weisz, D.J.; Juvonen, T.; Bodian, C.A.; Griepp, R.B. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann. Thorac. Surg. 2002, 73, 191–197. [Google Scholar] [CrossRef]
- McCullough, J.N.; Zhang, N.; Reich, D.L.; Juvonen, T.S.; Klein, J.J.; Spielvogel, D.; Ergin, M.; Griepp, R.B. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann. Thorac. Surg. 1999, 67, 1895–1899. [Google Scholar] [CrossRef]
- Yager, J.Y.; Asselin, J. Effect of Mild Hypothermia on Cerebral Energy Metabolism During the Evolution of Hypoxic-Ischemic Brain Damage in the Immature Rat. Stroke 1996, 27, 919–926. [Google Scholar] [CrossRef]
- Erecinska, M.; Thoresen, M.; Silver, I.A. Effects of Hypothermia on Energy Metabolism in Mammalian Central Nervous System. J. Cereb. Blood Flow Metab. 2003, 23, 513–530. [Google Scholar] [CrossRef]
- Frappell, P. Experimental Biology 1997 Symposium on Neurobiology of Thermoregulation: Role of Stress: Hypothermia and physiological control: The respiratory system. Clin. Exp. Pharmacol. Physiol. 1998, 25, 159–164. [Google Scholar] [CrossRef]
- Dill, D.B.; Forbes, W.H. Respiratory and metabolic effects of hypothermia. Am. J. Physiol. Leg. Content 1941, 132, 685–697. [Google Scholar] [CrossRef]
- Taiji, S.; Nishino, T.; Jin, H.; Shinozuka, N.; Isono, S.; Nozaki-Taguchi, N. Changes in breathing pattern during severe hypothermia and autoresuscitation from hypothermic respiratory arrest in anesthetized mice. Physiol. Rep. 2021, 9, e15139. [Google Scholar] [CrossRef]
- D’Amato, H.E.; Hegnauer, A.H. Blood Volume in the Hypothermic Dog. Am. J. Physiol. Content 1953, 173, 100–102. [Google Scholar] [CrossRef]
- Moyer, J.H. The effect of hypothermia on renal function and renal damage from ischemia. Ann. N. Y. Acad. Sci. 1959, 80, 424–434. [Google Scholar] [CrossRef]
- Rosenfeld, J.B. Acid-base and electrolyte disturbances in hypothermia. Am. J. Cardiol. 1963, 12, 678–682. [Google Scholar] [CrossRef]
- Sharma, T.; Kunkes, J.; O’Sullivan, D.; Fernandez, A.B. Elevated risk of venous thromboembolism in patients undergoing therapeutic hypothermia after cardiac arrest. Resuscitation 2021, 162, 251–256. [Google Scholar] [CrossRef]
- Polderman, K.H. Hypothermia and coagulation. Crit. Care 2012, 16 (Suppl. 2), A20. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Ma, W.; Li, Y. The effects of hypothermia in thrombosis: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 9564–9571. [Google Scholar] [CrossRef]
- Van Poucke, S.; Stevens, K.; Marcus, A.E.; Lancé, M. Hypothermia: Effects on platelet function and hemostasis. Thromb. J. 2014, 12, 31. [Google Scholar] [CrossRef]
- Paal, P.; Brugger, H.; Boyd, J. Accidental hypothermia. N. Engl. J. Med. 2013, 368, 682. [Google Scholar]
- Rohrer, M.J.; Natale, A.M. Effect of hypothermia on the coagulation cascade. Crit. Care Med. 1992, 20, 1402–1405. [Google Scholar] [CrossRef]
- Watts, D.D. Hypothermic Coagulopathy in Trauma: Effect of Varying Levels of Hypothermia on Enzyme Speed, Platelet Function and Fibrinolytic Activity. 1997. Available online: https://archive.hshsl.umaryland.edu/handle/10713/1405 (accessed on 15 August 2023).
- Martini, W.Z. Coagulopathy by Hypothermia and Acidosis: Mechanisms of Thrombin Generation and Fibrinogen Availability. J. Trauma Inj. Infect. Crit. Care 2009, 67, 202–209. [Google Scholar] [CrossRef]
- Watts, D.D.; Trask, A.; Soeken, K.; Perdue, P.; Dols, S.; Kaufmann, C. Hypothermic Coagulopathy in Trauma: Effect of Varying Levels of Hypothermia on Enzyme Speed, Platelet Function, and Fibrinolytic Activity. J. Trauma Acute Care Surg. 1998, 44, 846. [Google Scholar] [CrossRef]
- Gale, E.A.M.; Bennett, T.; Green, J.H.; MacDonald, I.A. Hypoglycaemia, Hypothermia and Shivering in Man. Clin. Sci. 1981, 61, 463–469. [Google Scholar] [CrossRef]
- Strapazzon, G.; Nardin, M.; Zanon, P.; Kaufmann, M.; Kritzinger, M.; Brugger, H. Respiratory Failure and Spontaneous Hypoglycemia During Noninvasive Rewarming From 24.7 °C (76.5 °F) Core Body Temperature After Prolonged Avalanche Burial. Ann. Emerg. Med. 2012, 60, 193–196. [Google Scholar] [CrossRef]
- Savides, E.P.; Hoffbrand, B.I. Hypothermia, thrombosis, and acute pancreatitis. Br. Med. J. 1974, 1, 614. [Google Scholar] [CrossRef]
- Castellani, J.W.; Young, A.J.; Ducharme, M.B.; Giesbrecht, G.G.; Glickman, E.; Sallis, R.E.; American College of Sports Medicine. American College of Sports Medicine position stand: Prevention of cold injuries during exercise. Med. Sci. Sports Exerc. 2006, 38, 2012–2029. [Google Scholar] [CrossRef]
- Maclean, D.; Murison, J.; Griffiths, P.D. Acute Pancreatitis and Diabetic Ketoacidosis in Accidental Hypothermia and Hypothermic Myxoedema. BMJ 1973, 4, 757–761. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Thurman, J. EMS Management of Traumatic and Medical Disorders in a Wilderness Environment; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK553188/ (accessed on 15 August 2023).
- Dow, J.; Giesbrecht, G.G.; Danzl, D.F.; Brugger, H.; Sagalyn, E.B.; Walpoth, B.; Auerbach, P.S.; McIntosh, S.E.; Némethy, M.; McDevitt, M.; et al. Wilderness Medical Society Clinical Practice Guidelines for the Out-of-Hospital Evaluation and Treatment of Accidental Hypothermia: 2019 Update. Wilderness Environ. Med. 2019, 30, S47–S69. [Google Scholar] [CrossRef]
- Masè, M.; Micarelli, A.; Falla, M.; Regli, I.B.; Strapazzon, G. Insight into the use of tympanic temperature during target temperature management in emergency and critical care: A scoping review. J. Intensiv. Care 2021, 9, 43. [Google Scholar] [CrossRef]
- Hasper, D.; Nee, J.; Schefold, J.C.; Krueger, A.; Storm, C. Tympanic temperature during therapeutic hypothermia. Emerg. Med. J. 2010, 28, 483–485. [Google Scholar] [CrossRef]
- Childs, C.; Harrison, R.; Hodkinson, C. Tympanic membrane temperature as a measure of core temperature. Arch. Dis. Child. 1999, 80, 262–266. [Google Scholar] [CrossRef]
- Duong, H.; Patel, G. Hypothermia; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545239/ (accessed on 17 August 2023).
- Reed, R.L.; Johnston, T.D.; Hudson, J.D.; Fischer, R.P. The disparity between hypothermic coagulopathy and clotting studies. J. Trauma Inj. Infect. Crit. Care 1992, 33, 465–470. [Google Scholar] [CrossRef]
- Chhabra, L.; Devadoss, R.; Liti, B.; Spodick, D.H. Electrocardiographic Changes in Hypothermia: A Review. Ther. Hypothermia Temp. Manag. 2013, 3, 54–62. [Google Scholar] [CrossRef]
- Levis, J.T. ECG Diagnosis: Hypothermia. Perm. J. 2010, 14, 73. [Google Scholar] [CrossRef]
- Vassallo, S.U.; Delaney, K.A.; Hoffman, R.S.; Slater, W.; Goldfrank, L.R. A prospective evaluation of the electrocardiographic mani-festations of hypothermia. Acad. Emerg. Med. 1999, 6, 1121–1126. [Google Scholar] [CrossRef]
- Su, Y.-J. Hypothermic lung edema after accidental hypothermia with out of hospital cardiac arrest. Heart Lung Vessel. 2015, 7, 328–329. [Google Scholar]
- Morales, C.F.; Strollo, P.J. Noncardiogenic Pulmonary Edema Associated with Accidental Hypothermia. Chest 1993, 103, 971–973. [Google Scholar] [CrossRef]
- Stecker, M.M.; Cheung, A.T.; Pochettino, A.; Kent, G.P.; Patterson, T.; Weiss, S.J.; E Bavaria, J. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann. Thorac. Surg. 2001, 71, 14–21. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Macedonio, S.; Gerosa, S.; Belliato, M.; Luzzi, S.; Lucifero, A.G.; Manzoni, F.; Ricevuti, G.; Bressan, M.A. Major Trauma in Elderly Patients: Worse Mortality and Outcomes in an Italian Trauma Center. J. Emerg. Trauma Shock 2021, 14, 98–103. [Google Scholar] [CrossRef]
- Rasouli, H.R.; Esfahani, A.A.; Nobakht, M.; Eskandari, M.; Mahmoodi, S.; Goodarzi, H.; Farajzadeh, M.A. Outcomes of Crowding in Emergency Departments; a Systematic Review. Arch. Acad. Emerg. Med. 2019, 7, e52. [Google Scholar]
- Darraj, A.; Hudays, A.; Hazazi, A.; Hobani, A.; Alghamdi, A. The Association between Emergency Department Overcrowding and Delay in Treatment: A Systematic Review. Healthcare 2023, 11, 385. [Google Scholar] [CrossRef]
- Johnson, K.D.; Winkelman, C. The effect of emergency department crowding on patient outcomes: A literature review. Adv. Emerg. Nurs. J. 2011, 33, 39–54. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Gri, N.; Piccini, G.B.; Longhitano, Y.; Zanza, C.; Piccioni, A.; Esposito, C.; Ricevuti, G.; Bressan, M.A. Emergency Department Overcrowding: Understanding the Factors to Find Corresponding Solutions. J. Pers. Med. 2022, 12, 279. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Novelli, V.; Ricevuti, G.; Bressan, M.A.; Oddone, E. How the coronavirus disease 2019 pandemic changed the patterns of healthcare utilization by geriatric patients and the crowding: A call to action for effective solutions to the access block. Intern. Emerg. Med. 2022, 17, 503–514. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Khalemsky, A.; Li, D.; Zoubeidi, T.; Lauque, D.; Alsabri, M.; Boudi, Z.; Kumar, V.A.; Paxton, J.; et al. The Association between Emergency Department Length of Stay and In-Hospital Mortality in Older Patients Using Machine Learning: An Observational Cohort Study. J. Clin. Med. 2023, 12, 4750. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Manzoni, F.; Ricevuti, G.; Bressan, M.A.; Oddone, E. Role of a Brief Intensive Observation Area with a Dedicated Team of Doctors in the Management of Acute Heart Failure Patients: A Retrospective Observational Study. Medicina 2020, 56, 251. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Novara, E.; Persiano, T.; Grulli, F.; Ricevuti, G.; Bressan, M.A.; Oddone, E. Brief intensive observation areas in the management of acute heart failure in elderly patients leading to high stabilisation rate and less admissions. J. Gerontol. Geriatr. 2021, 69, 87–97. [Google Scholar] [CrossRef]
- Hofman, M.R.; Hanenberg, F.V.D.; Sierevelt, I.N.; Tulner, C.R. Elderly patients with an atypical presentation of illness in the emergency department. Neth. J. Med. 2017, 75, 241–246. [Google Scholar]
- Limpawattana, P.; Phungoen, P.; Mitsungnern, T.; Laosuangkoon, W.; Tansangworn, N. Atypical presentations of older adults at the emergency department and associated factors. Arch. Gerontol. Geriatr. 2016, 62, 97–102. [Google Scholar] [CrossRef]
- Tavazzi, G.; Boffi, A.; Savioli, G.; Greco, A.; Pavesi, C.; Klersy, C.; Guida, S.; Iotti, G.; Mojoli, F.; Ghio, S.; et al. Right ventricular total isovolumic time: Reference value study. Echocardiography 2019, 36, 1234–1240. [Google Scholar] [CrossRef]
- Barbier, P.; Mirea, O.; Cefalu, C.; Maltagliati, A.; Savioli, G.; Guglielmo, M. Reliability and feasibility of longitudinal AFI global and segmental strain compared with 2D left ventricular volumes and ejection fraction: Intra- and inter-operator, test–retest, and inter-cycle reproducibility. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Savioli, G.; Ceresa, I.F.; Maggioni, P.; Lava, M.; Ricevuti, G.; Manzoni, F.; Oddone, E.; Bressan, M.A. Impact of ED Organization with a Holding Area and a Dedicated Team on the Adherence to International Guidelines for Patients with Acute Pulmonary Embolism: Experience of an Emergency Department Organized in Areas of Intensity of Care. Medicines 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, I.F.; Savioli, G.; Angeli, V.; Novelli, V.; Muzzi, A.; Grugnetti, G.; Cobianchi, L.; Manzoni, F.; Klersy, C.; Lago, P.; et al. Preparing for the Maximum Emergency with a Simulation: A Table-Top Test to Evaluate Bed Surge Capacity and Staff Compliance with Training. Open Access Emerg. Med. 2020, 12, 377–387. [Google Scholar] [CrossRef]
- Kanzenbach, T.L.; Dexter, W.W. Cold injuries. Protecting your patients from the dangers of hypothermia and frostbite. Postgrad. Med. 1999, 105, 72–78. [Google Scholar] [CrossRef]
- Clift, J.; Munro-Davies, L. Is defibrillation effective in accidental severe hypothermia in adults? Emerg. Med. J. 2007, 24, 50–51. [Google Scholar] [CrossRef]
- American Heart Association. Part 10.4: Hypothermia. Circulation 2005, 112, IV-136–IV-138. [Google Scholar]
- Tveita, T. Pharmacodynamics in hypothermia. Crit. Care 2012, 16, A6. [Google Scholar] [CrossRef]
- Han, W.-Z.; Ning, M.; Huang, J.-H.; Liu, W.; Zhang, Y.-F.; Cui, W.-Y.; Wang, H. The effect of hypothermia on the vasoconstriction and vasodilatation and concerned with vasoactive drugs. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014, 30, 204–207. [Google Scholar] [PubMed]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, H.; Rahmani, F.; Golzari, S.E.; Safari, S. Main Complications of Mild Induced Hypothermia after Cardiac Arrest: A Review Article. J. Cardiovasc. Thorac. Res. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Crawford, E.S.; Hess, K.R.; Coselli, J.S.; Raskin, S.; Shenaq, S.A.; Safi, H.J. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J. Thorac. Cardiovasc. Surg. 1993, 106, 19–28. [Google Scholar] [CrossRef]
- Meyer, M.; Pelurson, N.; Khabiri, E.; Siegenthaler, N.; Walpoth, B.H. Sequela-free long-term survival of a 65-year-old woman after 8 hours and 40 minutes of cardiac arrest from deep accidental hypothermia. J. Thorac. Cardiovasc. Surg. 2014, 147, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Forti, A.; Brugnaro, P.; Rauch, S.; Crucitti, M.; Brugger, H.; Cipollotti, G.; Strapazzon, G. Hypothermic Cardiac Arrest With Full Neurologic Recovery After Approximately Nine Hours of Cardiopulmonary Resuscitation: Management and Possible Complications. Ann. Emerg. Med. 2019, 73, 52–57. [Google Scholar] [CrossRef]
- Boue, Y.; Lavolaine, J.; Bouzat, P.; Matraxia, S.; Chavanon, O.; Payen, J.-F. Neurologic Recovery From Profound Accidental Hypothermia After 5 Hours of Cardiopulmonary Resuscitation. Crit. Care Med. 2014, 42, e167–e170. [Google Scholar] [CrossRef]
- Hilmo, J.; Naesheim, T.; Gilbert, M. “Nobody is dead until warm and dead”: Prolonged resuscitation is warranted in arrested hypothermic victims also in remote areas—A retrospective study from northern Norway. Resuscitation 2014, 85, 1204–1211. [Google Scholar] [CrossRef]
- Englum, B.R.; Andersen, N.D.; Husain, A.M.; Mathew, J.P.; Hughes, G.C. Degree of hypothermia in aortic arch surgery—Optimal temperature for cerebral and spinal protection: Deep hypothermia remains the gold standard in the absence of randomized data. Ann. Cardiothorac. Surg. 2013, 2, 184–193. [Google Scholar] [CrossRef]
- Paal, P.; Pasquier, M.; Darocha, T.; Lechner, R.; Kosinski, S.; Wallner, B.; Zafren, K.; Brugger, H. Accidental Hypothermia: 2021 Update. Int. J. Environ. Res. Public Health 2022, 19, 501. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. Corrigendum to “European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances” [Resuscitation 161 (2021) 152–219]. Resuscitation 2021, 167, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Blondin, N.A. Diagnosis and management of periodic hypothermia. Neurol. Clin. Pract. 2013, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Patologia Forense—Umani Ronchi, G.; Bolino, G.; Grande, A.; Marinelli, E. Gruppo Italiano di Patologia Forense. Available online: http://www.gipf.it/2016/10/17/patologia-forense-g-umani-ronchi-g-bolino-a-grande-e-marinelli/ (accessed on 15 August 2023).
- Palmiere, C.; Teresiński, G.; Hejna, P. Postmortem diagnosis of hypothermia. Int. J. Legal Med. 2014, 128, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Saukko, P.; Knight, B. Knight’s Forensic Pathology, 4th ed.; CRC Press: London, UK, 2015; Available online: https://www.taylorfrancis.com/books/9781444165081 (accessed on 15 August 2023).
- Forensic Histopathology|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-1-59745-110-9_13 (accessed on 15 August 2023).
- Medicina Legale e Delle Assicurazioni. PICCIN Nuova Libraria S.P.A. Available online: http://www.piccin.it/es/medicina-legale/1933-medicina-legale-e-delle-assicurazioni-9788829923236.html (accessed on 15 August 2023).
- Hirvonen, J. Necropsy findings in fatal hypothermia cases. Forensic Sci. 1976, 8, 155–164. [Google Scholar] [CrossRef]
- Sacco, M.A.; Abenavoli, L.; Juan, C.; Ricci, P.; Aquila, I. Biological Mechanisms behind Wischnewsky Spots Finding on Gastric Mucosa: Autopsy Cases and Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 3601. [Google Scholar] [CrossRef] [PubMed]
- Çetýn, S.; Ýnanir, N.T.; Eren, F.; Eren, B.; Dokgöz, H. Wischnewsky Spots in Fatal Hypothermia: Case Report. Maedica 2015, 10, 280–282. [Google Scholar]
- Hleșcu, A.A.; Grigoraș, A.; Covatariu, G.; Moscalu, M.; Amalinei, C. The Value of Myocardium and Kidney Histopathological and Immunohistochemical Findings in Accidental Hypothermia-Related Fatalities. Medicina 2022, 58, 1507. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mohammed, S.S.; Tammam, H.G.; Ibrahim Abdel-Karim, R.; Farag, M.M. Histopathological, histochemical and bio-chemical postmortem changes in induced fatal hypothermia in rats. Forensic Sci Res. 2022, 7, 211–227. [Google Scholar] [CrossRef]
| Classification from Etiopathology | ||
|---|---|---|
| Spontaneous hypothermia | Primary hypothermia | Secondary hypothermia |
| Induced hypothermia | Therapeutic hypothermia | Trauma-induced hypothermia |
| Type of Rewarming | |||
|---|---|---|---|
| Passive External | Active External | Active Internal (Core) | |
| When to adopt | Mild hypothermia, in which thermoregulation mechanisms are still functional. | Can be used for moderate-to-severe hypothermia and for patients with mild hypothermia who are unstable, lack physiologic reserve, or fail to respond to passive external rewarming | It can be used alone or combined with active external rewarming in patients with severe hypothermia (<28 °C) or patients with moderate hypothermia who fail to respond to less aggressive measures |
| What to do | After wet clothing is removed, the patient is covered with blankets or other types of insulation. | Relies on the delivery of heat to the surface of the body (some combination of warm blankets, heating pads, radiant heat, warm baths or forced warm air, is applied directly to the patient’s skin). | IV administration of warmed crystalloid (40 to 42 °C) or extracorporeal blood rewarming |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savioli, G.; Ceresa, I.F.; Bavestrello Piccini, G.; Gri, N.; Nardone, A.; La Russa, R.; Saviano, A.; Piccioni, A.; Ricevuti, G.; Esposito, C. Hypothermia: Beyond the Narrative Review—The Point of View of Emergency Physicians and Medico-Legal Considerations. J. Pers. Med. 2023, 13, 1690. https://doi.org/10.3390/jpm13121690
Savioli G, Ceresa IF, Bavestrello Piccini G, Gri N, Nardone A, La Russa R, Saviano A, Piccioni A, Ricevuti G, Esposito C. Hypothermia: Beyond the Narrative Review—The Point of View of Emergency Physicians and Medico-Legal Considerations. Journal of Personalized Medicine. 2023; 13(12):1690. https://doi.org/10.3390/jpm13121690
Chicago/Turabian StyleSavioli, Gabriele, Iride Francesca Ceresa, Gaia Bavestrello Piccini, Nicole Gri, Alba Nardone, Raffaele La Russa, Angela Saviano, Andrea Piccioni, Giovanni Ricevuti, and Ciro Esposito. 2023. "Hypothermia: Beyond the Narrative Review—The Point of View of Emergency Physicians and Medico-Legal Considerations" Journal of Personalized Medicine 13, no. 12: 1690. https://doi.org/10.3390/jpm13121690







