Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum
Abstract
1. Introduction
2. Materials and Methods
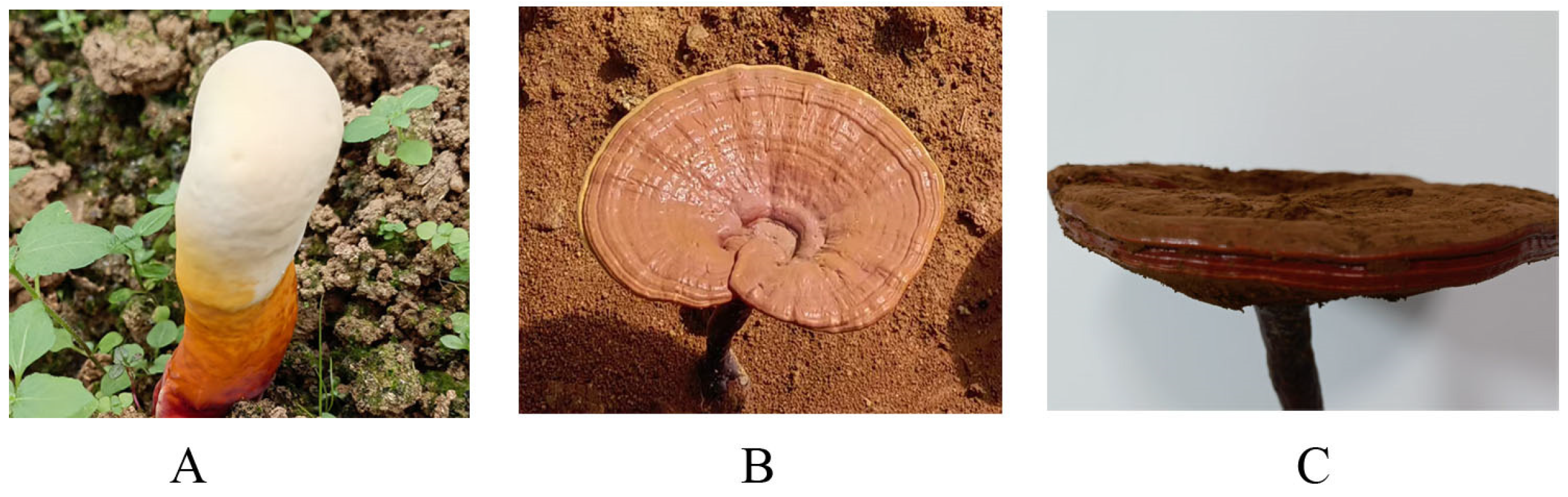
2.1. Samples
2.2. Metabolites Analysis
2.2.1. Metabolites Detection
2.2.2. Metabolites Data Analysis
2.3. Transcriptome Analysis
2.3.1. Transcriptome Sequencing
2.3.2. Transcriptome Data Analysis
2.3.3. Validation of Genes by qRT-PCR
2.3.4. Correlation Analysis
2.4. Statistics Analysis
3. Results and Discussion
3.1. Targeted Metabolomics Analysis
3.1.1. Metabolomic Profiling of G. lucidum at Different Growth Stages
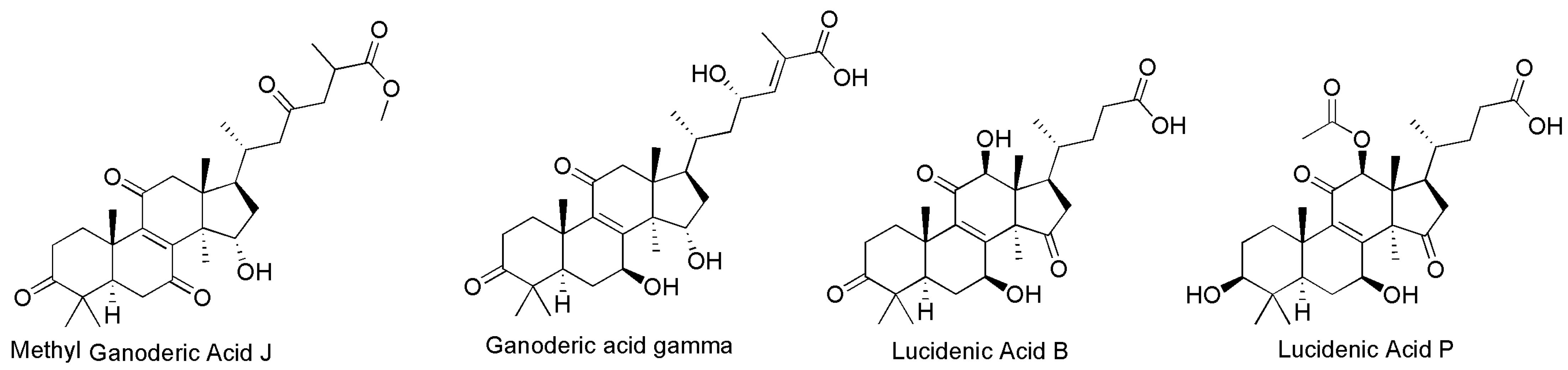
3.1.2. GTs Analysis in G. lucidum at Different Growth Stages
3.1.3. Other Plant Components Analysis of G. lucidum
3.2. Transcriptome Analysis in G. lucidum at Different Growth Stages
3.2.1. Transcriptomic Data
3.2.2. Laccases Associated with the Growth Process
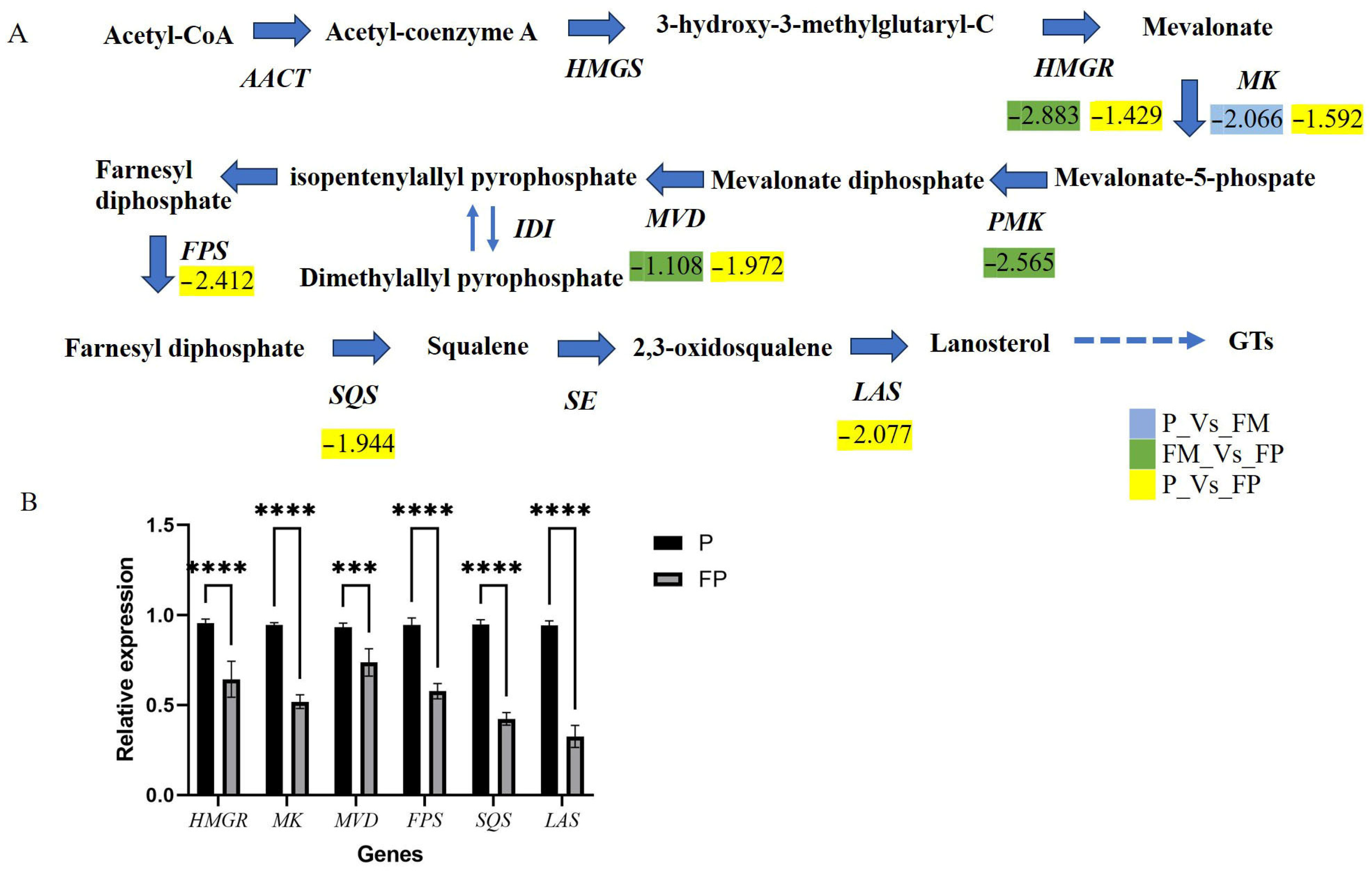
3.2.3. Expression Profile of MVA Pathway Genes
3.2.4. The TFs Related to GTs Synthesis in G. lucidum
3.2.5. CYP450s Related to GTs Synthesis in G. lucidum
3.3. Combined Transcriptome and GTs Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A Review of Chemical Composition and Bioactivity Studies of the Most Promising Species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Hu, Z.; Du, R.; Xiu, L.; Bian, Z.; Ma, C.; Sato, N.; Hattori, M.; Zhang, H.; Liang, Y.; Yu, S.; et al. Protective effect of triterpenes of Ganoderma lucidum on lipopolysaccharide-induced inflammatory responses and acute liver injury. Cytokine 2020, 127, 154917. [Google Scholar] [CrossRef]
- Ryu, D.H.; Cho, J.Y.; Sadiq, N.B.; Kim, J.C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Zeng, X.; Hao, C.; Zhang, Y.; Zhang, M.; Liu, Y.; Li, H.; Li, J.; Zhang, L. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J. Cell. Mol. Med. 2018, 22, 3278–3297. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef]
- Wang, W.F.; Xiao, H.; Zhong, J.J. Biosynthesis of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum. Biotechnol. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, C.; Wang, Q.; Fang, Y.; Wang, J.; Wang, M.; Xiao, H. Biosynthesis of mush-room-derived type II ganoderic acids by engineered yeast. Nat. Commun. 2022, 13, 7740. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Huang, J.J.; Luo, Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar] [CrossRef]
- Wu, F.L.; Zhang, G.; Ren, A.; Dang, Z.H.; Shi, L.; Jiang, A.L.; Zhao, M.W. The pH-responsive transcription factor PacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynthesis in Ganoderma lucidum. Mycologia 2016, 108, 1104–1113. [Google Scholar] [PubMed]
- Wang, S.; Shi, L.; Hu, Y.; Liu, R.; Ren, A.; Zhu, J.; Zhao, M. Roles of the Skn7 response regulator in stress resistance, cell wall integrity and GA biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2018, 114, 12–23. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.; Shi, D.; Zhao, M. Functional analysis of an APSES transcription factor (GlSwi6) involved in fungal growth, fruiting body development and ganoderic-acid biosynthesis in Ganoderma lucidum. Microbiol. Res. 2018, 207, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lian, L.; Xia, J.; Hu, S.; Xu, W.; Zhu, J.; Ren, A.; Shi, L.; Zhao, M.W. Influence of PacC on the environmental stress adaptability and cell wall components of Ganoderma lucidum. Microbiol. Res. 2020, 230, 126348. [Google Scholar] [CrossRef]
- Liang, H.; Zhong, J.J. Role of calcineurin-responsive transcription factor CRZ1 in ganoderic acid biosynthesis by Ganoderma lucidum. Process Biochem. 2020, 95, 166–173. [Google Scholar]
- Wang, Z.; Chen, J.; Ding, J.; Han, J.; Shi, L. GlMPC activated by GCN4 regulates secondary metabolism under nitrogen limitation conditions in Ganoderma lucidum. mBio 2023, 14, e0135623. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, F.; Zhu, Y.; Li, Y.; Zhou, H.; Chen, S.; Ruan, J. Transcriptome profiling of transcription factors in Ganoderma lucidum in response to methyl jasmonate. Front. Microbiol. 2022, 13, 1052377. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of People′s Republic of China, Part 1; China Medical Science and Technology Press: Beijing, China, 2020; p. 195.
- Liu, J.; Kenji, K.; Atsuko, F.; Shuhei, K.; Yoshitaro, S.; Kuniyoshi, S.; Ryuichiro, K. Quantitative determination of the representative triterpenoids in the extracts of Ganoderma lucidum with different growth stages using high-performance liquid chromatography for evaluation of their 5α-reductase inhibitory properties. Food Chem. 2012, 133, 1034–1038. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhang, H.; Zuo, J.H.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Xie, C.; Yan, S.; Zhang, Z.; Gǒng, W.; Zhu, Z.; Zhou, Y.; Li, Y.; Hu, Z.; Ai, L.; Peng, Y. Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidum by untargeted metabolomics. LWT 2020, 129, 109494. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Cassilly, C.D.; Reynolds, T.B. PS, It’s Complicated: The Roles of Phosphatidylserine and Phosphatidylethanolamine in the Pathogenesis of Candida albicans and other Microbial Pathogens. J. Fungi 2018, 4, 28. [Google Scholar] [CrossRef]
- Suzawa, T.; Iwama, R.; Fukuda, R.; Horiuchi, H. Phosphatidylcholine levels regulate hyphal elongation and differentiation in the filamentous fungus Aspergillus oryzae. Sci. Rep. 2024, 14, 11729. [Google Scholar] [CrossRef] [PubMed]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; He, X.; Yang, W.; Song, H.; Yang, J.; Zhang, G.; Yang, Z.; Chen, H.; Liang, Z.; Kollie, L.; et al. Unveiling the distribution of chemical constituents at different body parts and maturity stages of Ganoderma lingzhi by combining metabolomics with desorption electrospray ionization mass spectrometry imaging (DESI). Food Chem. 2024, 436, 137737. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, R.; Chi, X.; Chen, T.; Li, Y.; Xu, Y.; Zhu, B.; Hu, J. Unleashing the power of chlorogenic acid: Exploring its potential in nutrition delivery and the food industry. Food Funct. 2024, 15, 4741–4762. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, N.; Wu, S.; Jiang, C.; Xie, L.; Yang, F.; Yu, Z. A Comparative Analysis of the Chloroplast Genomes of Three Lonicera Medicinal Plants. Genes 2023, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, G.; Zhu, P.; Zhu, W.; Li, Y.; Fan, X.W. Metabolite profiles and antibacterial and antioxidant activities of leaf extracts of five Lonicera species: A comparative study. Chem. Biol. Technol. Agric. 2023, 10, 91. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The effect of chlorogenic acid on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Fang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhou, S.; Li, S.M.; Ran, H.; Song, Z.; Yu, T.; Yin, W.B. A fungal NRPS-PKS enzyme catalyses the formation of the flavonoid naringenin. Nat. Commun. 2022, 13, 6361. [Google Scholar] [CrossRef]
- Amjad, M.; Wang, Y.; Han, S.; Haider, M.Z.; Sami, A.; Batool, A.; Shafiq, M.; Ali, Q.; Dong, J.; Sabir, I.A. Manzoor MA. Genome wide identification of phenylalanine ammonia-lyase (PAL) gene family in Cucumis sativus (cucumber) against abiotic stress. BMC Genom. Data 2024, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhou, R.; Liang, L.; Zang, X.; Lin, J.; Wang, Q.; Wang, L.; Wang, W.; Li, Z.; Ren, P. 4-Coumarate-CoA ligase (4-CL) enhances flavonoid accumulation, lignin synthesis, and fruiting body formation in Ganoderma lucidum. Gene 2024, 899, 148147. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Ma, F.; Xie, S.; Tang, C.; Tang, Q.; Zhang, J. Integrative Analysis of Selected Metabolites and the Fungal Transcriptome during the Developmental Cycle of Ganoderma lucidum Strain G0119 Correlates Lignocellulose Degradation with Carbohydrate and Triterpenoid Metabolism. Appl. Environ. Microbiol. 2021, 87, e0053321. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, A.K.; Mikkelsen, J.D.; Højrup, P.; Meyer, A.S. Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzyme Microb. Technol. 2013, 53, 378–385. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Chen, L.; Shen, Y.; Zhang, X.; Fang, W.; Wang, X.; Bao, X.; Xiao, Y. Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotechnol. Biofuels 2015, 8, 54. [Google Scholar] [CrossRef]
- Zill-e-Huma, A.; Shakil, A. Ganoderma lucidum: A case study for laccase biosynthesis. J. Phytopathol. 2015, 27, 95–103. [Google Scholar]
- Zhou, S.; Zhang, J.; Ma, F.; Tang, C.; Tang, Q.; Zhang, X. Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses. PLoS ONE 2018, 13, e0198404. [Google Scholar] [CrossRef]
- Shang, C.H.; Zhu, F.; Li, N.; Ou-Yang, X.; Shi, L.; Zhao, M.W.; Li, Y.X. Cloning and characterization of a gene encoding hmg-coa reductase from Ganoderma lucidum and its functional identification in yeast. Biosci. Biotechnol. Biochem. 2008, 72, 1333–1339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, Y.X.; Ou-Yang, X.; Shang, C.H.; Ren, A.; Shi, L.; Li, Y.X.; Zhao, M.W. Molecular cloning, characterization, and differential expression of a farnesyl-diphosphate synthase gene from the basidiomycetous fungus Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2008, 72, 1571–1579. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.X.; Ming, W.Z. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef]
- Shi, L.; Qin, L.; Xu, Y.; Ren, A.; Fang, X.; Mu, D.; Tan, Q.; Zhao, M. Molecular cloning, characterization, and function analysis of a mevalonate pyrophosphate decarboxylase gene from Ganoderma lucidum. Mol. Biol. Rep. 2012, 39, 6149–6159. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Ouyang, X.; Shi, L.; Jiang, A.L.; Mu, D.S.; Li, M.J.; Han, Q.; Zhao, M.W. Molecular characterization and expression analysis of glhmgs, a gene encoding hydroxymethylglutaryl-coa synthase from Ganoderma lucidum (ling-zhi) in ganoderic acid biosynthesis pathway. World J. Microbiol. Biotechnol. 2013, 29, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gong, L.; Zhang, X.; Ren, A.; Gao, T.; Zhao, M. The regulation of methyl jasmonate on hyphal branching and GA biosynthesis in Ganoderma lucidum partly via ROS generated by NADPH oxidase. Fungal Genet. Biol. 2015, 81, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, H.; Li, G.; Liu, M.; Yun, Y.; Wang, C.; Ma, Z.; Xu, J.R. The MADS-box transcription factor FgMcm1 regulates cell identity and fungal development in Fusarium graminearum. Environ. Microbiol. 2015, 17, 2762–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, X.; Lu, Z.; Wang, H.; He, Z.; Zhou, G.; Luo, Z.; Zhang, Y. MADS-box transcription factor Mcm1 controls cell cycle, fungal development, cell integrity and virulence in the filamentous insect pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2019, 21, 3392–3416. [Google Scholar] [CrossRef]
- Xu, X.L.; Lai, R.C.; Chen, T.Q.; Shi, L.C.; Chen, S.L. Construction of yeast one-hybrid library and screening of transcription factors regulating FPS expression in Ganoderma lucidum. Chin. Tradit. Herb. Drugs 2020, 44, 3770–3776. [Google Scholar]
- Liu, Y.N.; Wu, F.Y.; Tian, R.Y.; Shi, Y.X.; Xu, Z.Q.; Liu, J.Y.; Huang, J.; Xue, F.F.; Liu, B.Y.; Liu, G.Q. The bHLH-zip transcription factor SREBP regulates triterpenoid and lipid metabolisms in the medicinal fungus Ganoderma lingzhi. Commun. Biol. 2023, 6, 1. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, R.Y.; Lin, J.L.; Zang, X.Z.; Wang, Q.J.; Wang, P.M.; Wang, L.; Li, Z.; Wang, W. Transcriptome and metabolome analyses reveal transcription factors regulating ganoderic acid biosynthesis in Ganoderma lucidum development. Front. Microbiol. 2022, 13, 956421. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Xu, C.; Xu, M.; Li, F.S.; Wada, N.; Mitchell, A.J.; Han, X.L.; Wen, M.L.; Fujita, M.; Weng, J.K. Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat. Commun. 2019, 10, 3206. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Li, C.; Zhou, Z.; Xiao, Y.; Yan, X. Metabolism of ganoderic acids by a Ganoderma lucidum cytochrome P450 and the 3-keto sterol reductase ERG27 from yeast. Phytochemistry 2018, 55, 83–92. [Google Scholar] [CrossRef]
- Syed, K.; Nelson, D.R.; Riley, R.; Yadav, J.S. Genomewide annotation and comparative genomics of cytochrome P450 monooxygenases (P450s) in the polypore species Bjerkandera adusta, Ganoderma sp. and Phlebia brevispora. Mycologia 2013, 105, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Jongsun, P.; Natalie, D.F.A.; Bongsoo, P.; Jaeyoung, C.; Yong, H.L.; Seogchan, K. Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genom. 2012, 13, 525. [Google Scholar]
- Hirofumi, I.; Hiroyuki, W. Heterologous expression and mechanistic investigation of a fungal cytochrome P450 (CYP5150A2): Involvement of alternative redox partners. Arch. Biochem. Biophys. 2012, 518, 8–15. [Google Scholar]
- Lu, W.; Feng, J.; Chen, X.; Bao, Y.J.; Wang, Y.; Wu, Q.Q.; Ma, Y.H.; Zhu, D. Distinct Regioselectivity of Fungal P450 Enzymes for Steroidal Hydroxylation. Appl. Environ. Microbiol. 2019, 85, e01182-e19. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequences(5′→3′) |
|---|---|
| GPD-F | CTCCTTCACGGAGACATT |
| GPD-R | TAACACCCGCAGACGAACA |
| GL18675-las-F | CTTCCGCAAGCACTACCCG |
| GL18675-las-R | AGCAGATGCCCCACGAGCC |
| GL24922-hmgs-F | ACACGACGGAGATTCAGGAG |
| GL24922-hmgs-R | GATGTACTTGCGGCGGTATT |
| GL24088-hmgr-F | GCGTCGGTAACATGATCCTT |
| GL24088-hmgr-R | GACAAGACTCCGCGAATAG |
| GL22068-fps-F | GGTTGGTGTGTCGAGTTCCT |
| GL22068-fps-R | TGACGATCTTTTGGTGCTTG |
| GL17879-mk-F | TTCGCTGTTACGGTCTTA |
| GL17879-mk-R | GTGGTTGTAGTTGCTGATAT |
| GL25304-mvd-F | GCATTAAGGAGATGAAGG |
| GL25304-mvd-R | GAGATGTGAACGGAGTAG |
| GL21690-sqs-F | CTTATTCTACCTGGTGCTA |
| GL21690-sqs-R | TGGAATTGTCGGAGTATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Li, C.; Wu, F.; Zhao, S.; Chen, T.; You, H.; Lin, Y.; Zou, X. Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum. J. Fungi 2025, 11, 57. https://doi.org/10.3390/jof11010057
Xu X, Li C, Wu F, Zhao S, Chen T, You H, Lin Y, Zou X. Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum. Journal of Fungi. 2025; 11(1):57. https://doi.org/10.3390/jof11010057
Chicago/Turabian StyleXu, Xiaolan, Chunxia Li, Fangjing Wu, Shuangshuang Zhao, Tiqiang Chen, Haihong You, Yijie Lin, and Xiaoxing Zou. 2025. "Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum" Journal of Fungi 11, no. 1: 57. https://doi.org/10.3390/jof11010057
APA StyleXu, X., Li, C., Wu, F., Zhao, S., Chen, T., You, H., Lin, Y., & Zou, X. (2025). Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum. Journal of Fungi, 11(1), 57. https://doi.org/10.3390/jof11010057








