Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
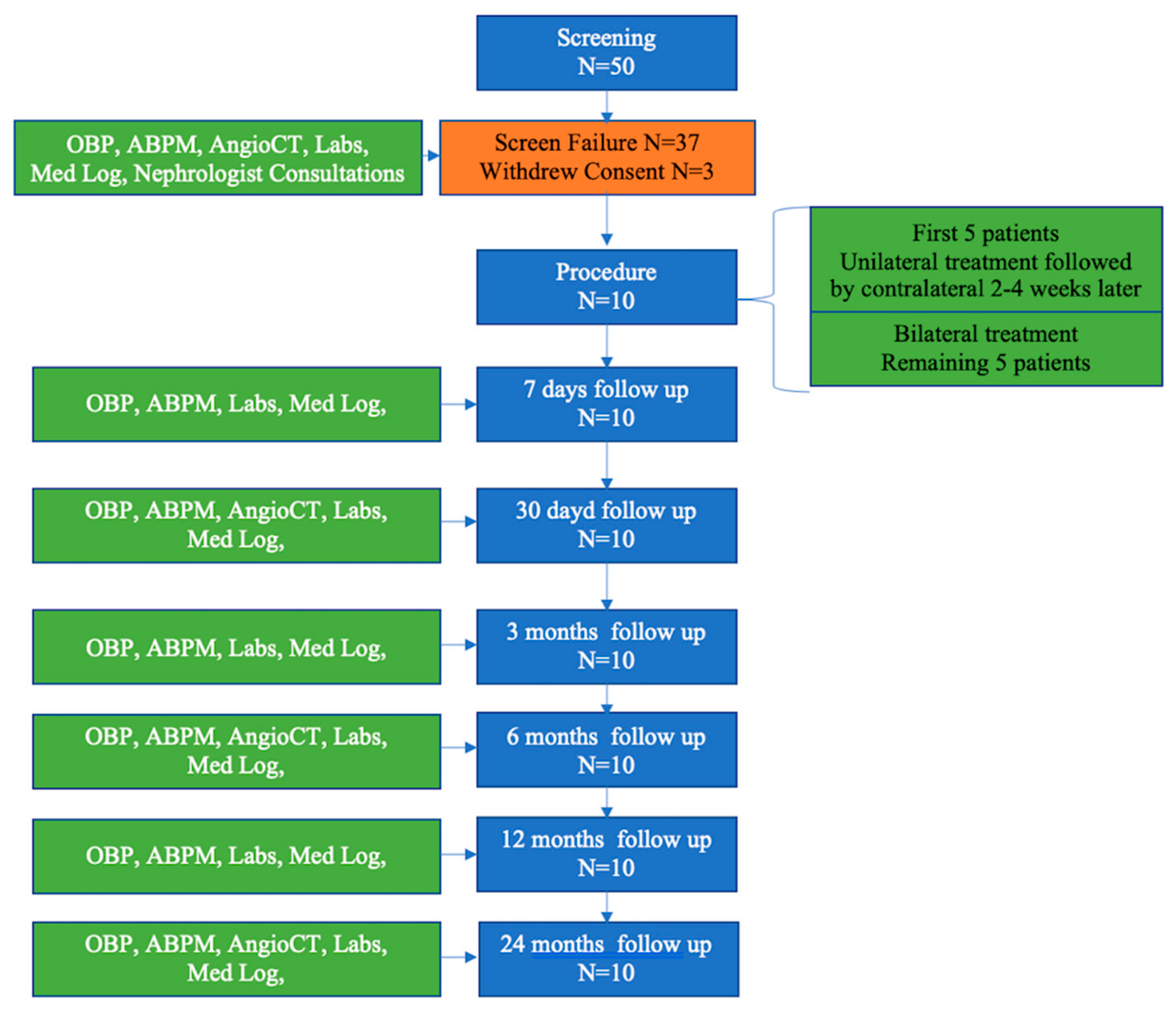
2.2. Study Design
2.3. Study Procedures
2.3.1. Baseline
2.3.2. Renal Denervation Procedure
2.3.3. Pre-Discharge
2.3.4. Follow-Up
2.4. Study Oversight
3. Statistical Analysis
4. Results
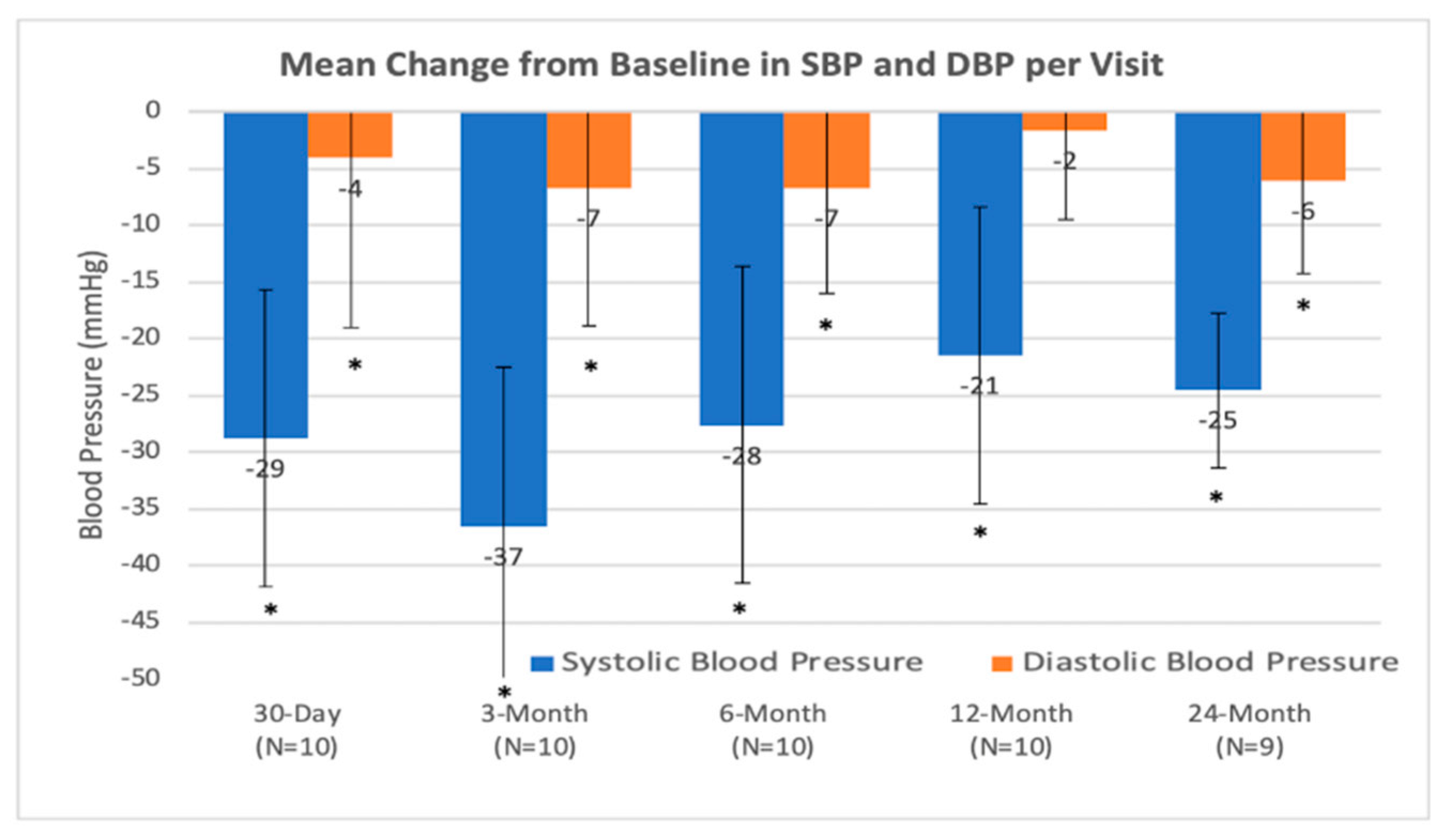
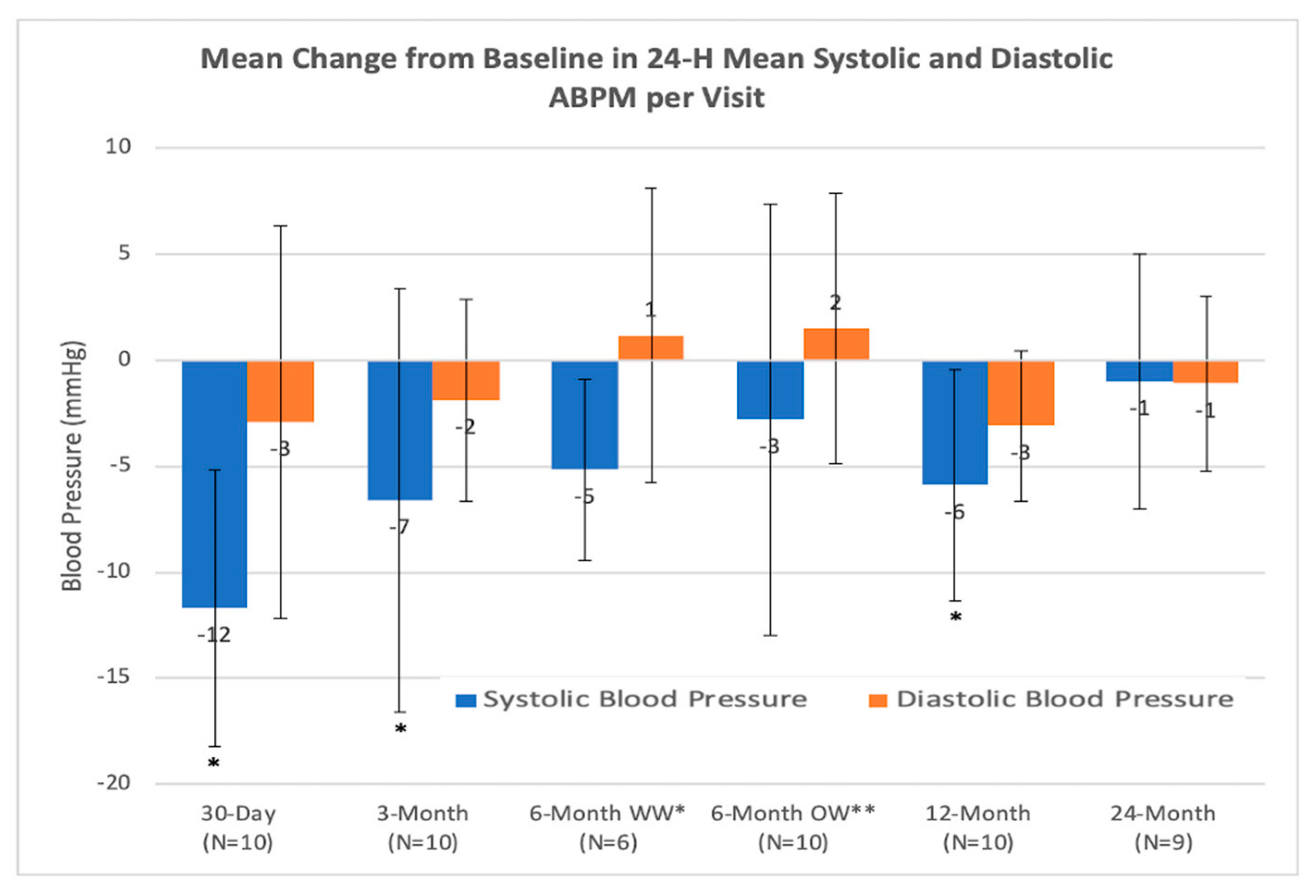
4.1. Safety Objectives
4.2. Efficiency Objectives
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolf-Maier, K.; Cooper, R.S.; Banegas, J.R.; Giampaoli, S.; Hense, H.W.; Joffres, M.; Kastarinen, M.; Poulter, N.; Primatesta, P.; Rodríguez-Artalejo, F.; et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003, 289, 2363–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the american heart association. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Georgianos, P.; Bakris, G.L. Resistant hypertension—Its identification and epidemiology. Nat. Rev. Nephrol. 2012, 9, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Bakris, G.L.; Nathan, S. Renal denervation and left ventricular mass regression: A benefit beyond blood pressure reduction? J. Am. Coll. Cardiol. 2013, 63, 1924–1925. [Google Scholar] [CrossRef] [Green Version]
- DiBona, G.F. Physiology in perspective: The wisdom of the body. Neural control of the kidney. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R633–R641. [Google Scholar] [CrossRef] [Green Version]
- Böhm, M.; Linz, D.; Ukena, C.; Esler, M.; Mahfoud, F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ. Res. 2014, 115, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahfoud, F.; Schlaich, M.P.; Böhm, M.; Esler, M.; Lüscher, T.F. Catheter-based renal denervation: The next chapter begins. Eur. Hear. J. 2018, 39, 4144–4149. [Google Scholar] [CrossRef]
- Townsend, R.R.; Mahfoud, F.; E Kandzari, D.; Kario, K.; Pocock, S.; A Weber, M.; Ewen, S.; Tsioufis, K.; Tousoulis, D.; Sharp, A.S.P.; et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017, 390, 2160–2170. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Böhm, M.; Mahfoud, F.; Townsend, R.R.; A Weber, M.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Fischell, T.A.; Ebner, A.; Gallo, S.; Ikeno, F.; Minarsch, L.; Vega, F.; Haratani, N.; Ghazarossian, V.E. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system. JACC Cardiovasc. Interv. 2016, 9, 589–598. [Google Scholar] [CrossRef]
- Fischell, T.A.; Vega, F.; Raju, N.; Johnson, E.T.; Kent, D.J.; Ragland, R.R.; Fischell, D.R.; Almany, S.L.; Ghazarossian, V.E. Ethanol-mediated perivascular renal sympathetic denervation: Preclinical validation of safety and efficacy in a porcine model. EuroIntervention 2013, 9, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar]
- Krum, H.; Schlaich, M.P.; Sobotka, P.A.; Böhm, M.; Mahfoud, F.; Rocha-Singh, K.; Katholi, R.E.; Esler, M.D. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet 2014, 383, 622–629. [Google Scholar] [CrossRef]
- Esler, M.; Bohm, M.; Sievert, H.; Rump, C.L.; Schmieder, R.E.; Krum, H.; Mahfoud, F.; Schlaich, M.P. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur. Heart J. 2014, 35, 1752–1759. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Sakakura, K.; Ladich, E.; Cheng, Q.; Otsuka, F.; Yahagi, K.; Fowler, D.R.; Kolodgie, F.D.; Virmani, R.; Joner, M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J. Am. Coll. Cardiol. 2014, 64, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Mahfoud, F.; Tunev, S.; Ewen, S.; Cremers, B.; Ruwart, J.; Schulz-Jander, D.; Linz, D.; Davies, J.; Kandzari, D.E.; Whitbourn, R.; et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J. Am. Coll. Cardiol. 2015, 66, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria |
|---|
| Adult patient, aged 18–75, male or female |
| Patient has a clinic systolic blood pressure ≥ 160 mmHg (or ≥150 mmHg in type 2 diabetic patients) based on the average of 3 office/clinic measurements taken manually |
| Patient has a daytime mean systolic pressure ≥ 135 mmHg based on 24 h ambulatory blood pressure monitoring |
| Patient is receiving a stable medication regimen of at least 3 anti-hypertensive medications of different classes (for at least 4 weeks), one of which must be a diuretic, and the medication regimen is not expected to change for at least 1 month |
| Patient has an eGFR ≥ 45 mL/min, based on the Chronic Kidney Disease Epidemiology Colaboration (CKD-EPI) equation |
| Patient has optimal renal artery anatomy (no clear abnormalities) based on Investigator’s evaluation of computed tomography examination and/or angiogram including: Single artery of 5–7 mm in diameter (two arteries are acceptable if diameter of second artery is ≤2 mm) No aneurysms No excessive tortuosity |
| No previous stenting or balloon angioplasty of the renal arteries |
| Patient has provided written informed consent |
| Exclusion Criteria |
|---|
| Patient has known or suspected secondary hypertension |
| Patient has type 1 diabetes mellitus |
| Patient requires chronic oxygen support |
| Patient has primary or secondary pulmonary hypertension |
| Patient has a known bleeding diathesis or is receiving anticoagulant drugs during the 7 days prior to treatment |
| Patient has thrombocytopenia (platelet count <100,000 platelets/µL) |
| Patient is pregnant or nursing |
| Patient has significant imaging-assessed renovascular abnormalities including short length main renal artery and renal artery stenosis > 70% of the normal diameter segment |
| Patient has history of nephrectomy, kidney tumor or hydronephrosis |
| Patient is known to have a unilateral non-functioning kidney or unequal renal size (>2 cm difference in renal length between kidneys) |
| Patient has a renal transplant |
| Patient has a history of kidney stones |
| Patient has a history of heterogeneities in the kidney such as cysts or tumors |
| Patient has a history of pyelonephritis |
| Patient has a history of myocardial infarction, unstable angina pectoris, or cerebrovascular accident within the last six months |
| Patient has hemodynamically significant valvular heart disease |
| Patient has heart failure (NYHA III or IV) or had an ejection fraction ≤ 30% |
| Patient has a known allergy to contrast media |
| Patient has a life expectancy of <12 months |
| Patient is currently enrolled in other potentially confounding research, i.e., another therapeutic or interventional research trial. Patients enrolled in observational registries may still be eligible |
| Characteristic | All Treated Subjects n = 10 |
|---|---|
| Age, years (mean (min; max)) | 59.9 (46;67) |
| Male gender | 50% (5/10) |
| Renal Disease | 0% (0/10) |
| Chronic Kidney Disease | 10% (1/10) |
| Hypertension | 100% (10/10) |
| Diabetes (Type II) | 40% (4/10) |
| Arrhythmia | 0% (0/10) |
| Atrial Fibrillation/Atrial Flutter | 0% (0/10) |
| Prior Stroke or TIA | 10% (1/10) |
| Peripheral Vascular Occlusive Disease | 10% (1/10) |
| Hyperlipidemia/Dyslipidemia | 60% (6/10) |
| Angio-determined Coronary Artery Disease | 30% (3/10) |
| Prior Myocardial Infarction | 20% (2/10) |
| Previous History of Congestive Heart Failure | 0% (0/10) |
| Chronic Obstructive Pulmonary Disease | 0% (0/10) |
| History of Smoking | 30% (3/10) |
| Defined Daily Dose (DDD) of anti-hypertensive | 4.8 ± 1.3 |
| Thiazide Diuretic | 90% (9/10) |
| Loop Diuretics | 30% (3/10) |
| Beta-Blocker | 90% (9/10) |
| Alpha -1 Blocker | 50% (5/10) |
| Centrally Acting Alpha Agonist | 20% (2/10) |
| Direct Acting Vasodilator | 50% (5/10) |
| ACE Inhibitor | 70% (7/10) |
| Calcium Channel Blocker | 50% (5/10) |
| Angiotensin II Receptor Blocker | 50% (5/10) |
| Diuretic Aldosterone Receptor Blockers | 20% (2/10) |
| Parameters | Number of Procedures (n = 15 Procedures) (n = 20 Arteries) Mean ± SD (Min; Max) |
|---|---|
| Time from Insertion of Peregrine Catheter into Renal Artery to Removal from Introducer Sheath, per artery (min) | 8 ± 5 (4; 22) |
| Distance from Ostium to First Bifurcation–Right (mm) | 47.7 ± 10.4 (32.0; 67.0) |
| Distance from Ostium to First Bifurcation–Left (mm) | 33.5 ± 12.0 (12.1; 51.0) |
| Distance to Ostium from Infusion Site–Right (mm) | 25.8 ± 7.3 (9.4; 35.0) |
| Distance to Ostium from Infusion Site–Left (mm) | 16.3 ± 6.8 (8.2; 29.9) |
| Volume of Alcohol Infused, per artery (mL) | 0.3 |
| Total Volume Contrast Used, per artery (mL) | 40 ± 18 (20; 100) |
| Total IV Fluid Used, per artery (mL) | 1003 ± 507 (0; 2058) |
| Fluoroscopic Time, per artery (min) | 6.0 ± 5.4 (2.0; 26.0) |
| Average Hospital Stay (Days)—Median (IQR) | 2 (2; 3) |
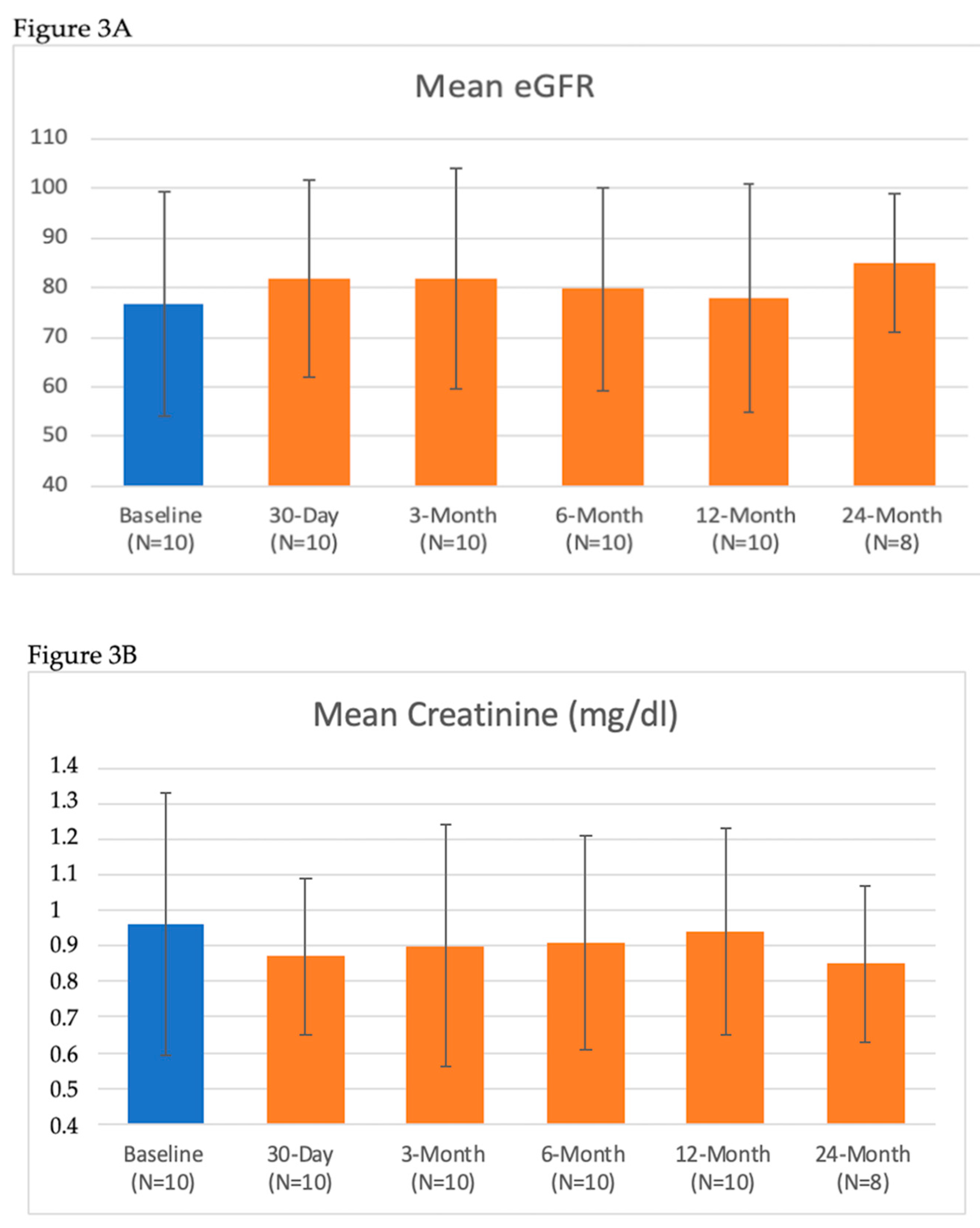
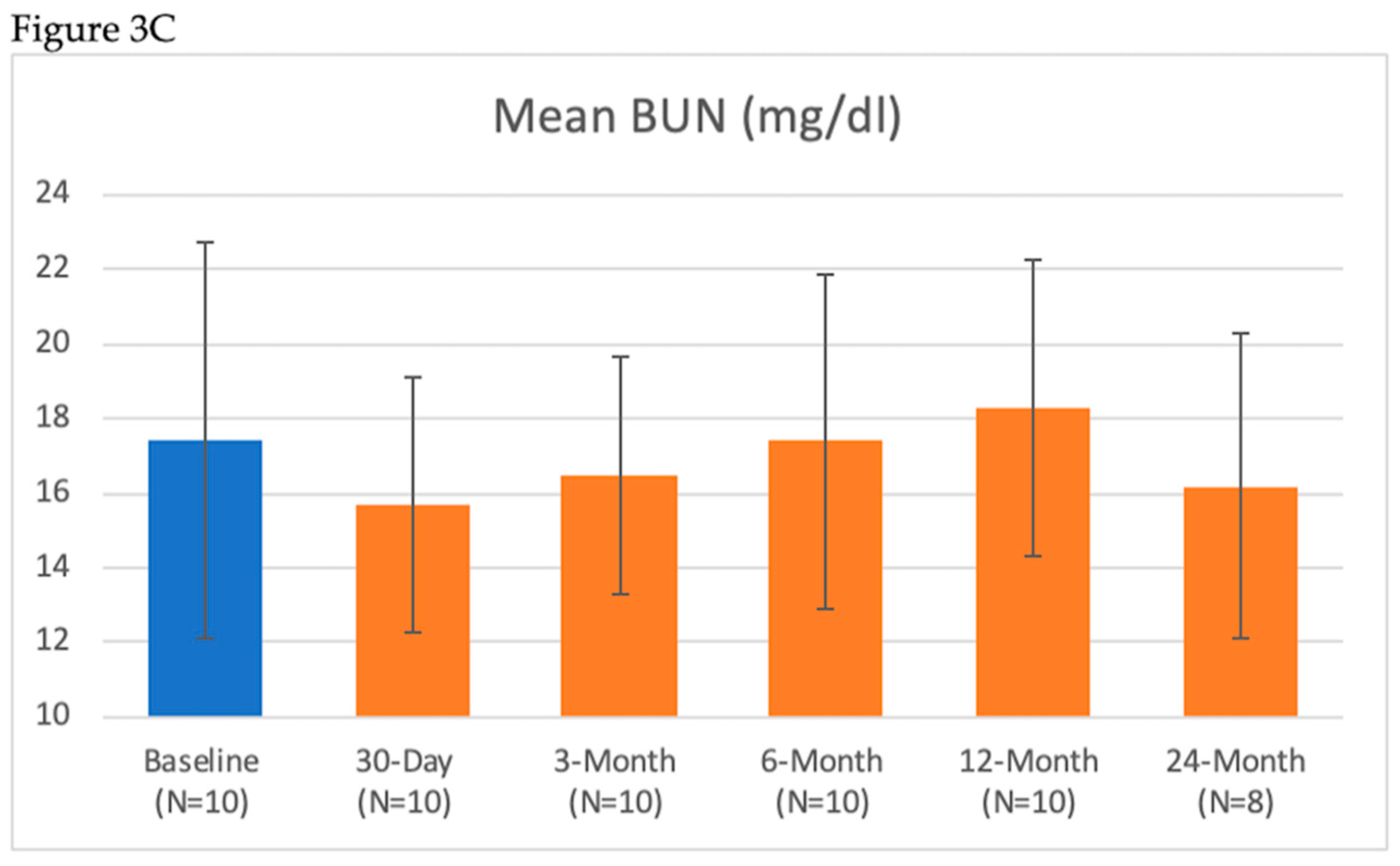
| Renal Lab Parameter | Baseline (Mean ± SD) n = 10 | 7-Day (Mean ± SD) n = 10 | 30-Day (Mean ± SD) n = 10 | 3-Month (Mean ± SD) n = 10 | 6-Month (Mean ± SD) n = 10 | 12-Month (Mean ± SD) n = 10 | 24-Month (Mean ± SD) n = 8 |
|---|---|---|---|---|---|---|---|
| BUN (mg/dL) | 17.4 ± 5.3 | 17.5 ± 3.8 | 15.7 ± 3.4 | 16.5 ± 3.2 | 17.4 ± 4.5 | 18.3 ± 4.0 | 16.2 ± 4.1 |
| Serum Creatinine (mg/dL) | 0.96 ± 0.37 | 0.96 ± 0.37 | 0.87 ± 0.22 | 0.90 ± 0.34 | 0.91 ± 0.30 | 0.94 ± 0.29 | 0.85 ± 0.22 |
| eGFR (mL/min/1.73 m2) | 78 ± 21 | 76 ± 21 | 82 ± 20 | 82 ± 22 | 80 ± 20 | 77 ± 20 | 85 ± 14 |
| ≥25% Reduction in eGFR | 10% (1/10) | 0% (0/10) | 0% (0/10) | 0% (0/10) | 10% (1/10) | 0% (0/8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janas, A.; Król, M.; Hochuł, M.; Jochymczyk, M.; Hayward-Costa, C.; Parise, H.; Haratani, N.; Fischell, T.; Wojakowski, W. Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension. J. Clin. Med. 2020, 9, 1881. https://doi.org/10.3390/jcm9061881
Janas A, Król M, Hochuł M, Jochymczyk M, Hayward-Costa C, Parise H, Haratani N, Fischell T, Wojakowski W. Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension. Journal of Clinical Medicine. 2020; 9(6):1881. https://doi.org/10.3390/jcm9061881
Chicago/Turabian StyleJanas, Adam, Marek Król, Mariusz Hochuł, Monika Jochymczyk, Claudia Hayward-Costa, Helen Parise, Nicole Haratani, Tim Fischell, and Wojciech Wojakowski. 2020. "Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension" Journal of Clinical Medicine 9, no. 6: 1881. https://doi.org/10.3390/jcm9061881





