Deep Learning-Based Stroke Volume Estimation Outperforms Conventional Arterial Contour Method in Patients with Hemodynamic Instability
Abstract
1. Introduction
2. Methods
2.1. Data Preparation
2.2. Machine Learning Based SV Estimation
2.3. Convolutional Neural Network (CNN)
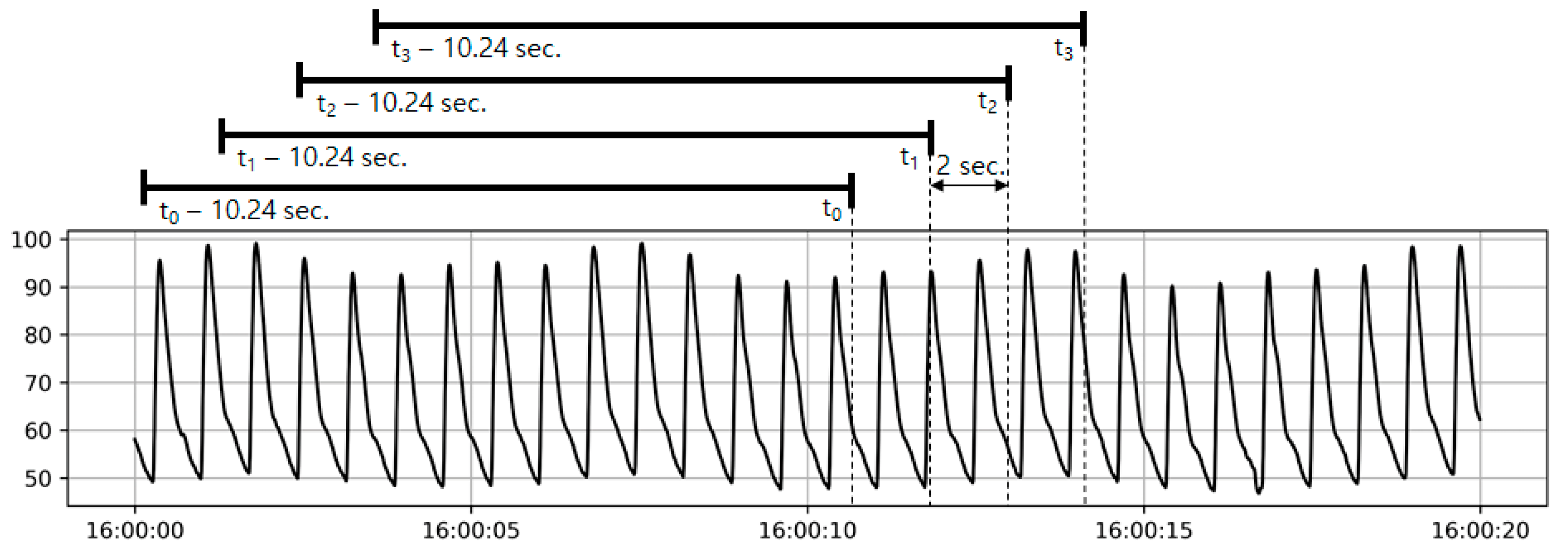
2.4. Interpersonal Scale Variation in Training Data
2.5. Dataset, Model Training, and Post-Processing of Predicted SV Values
2.6. Statistical Analysis
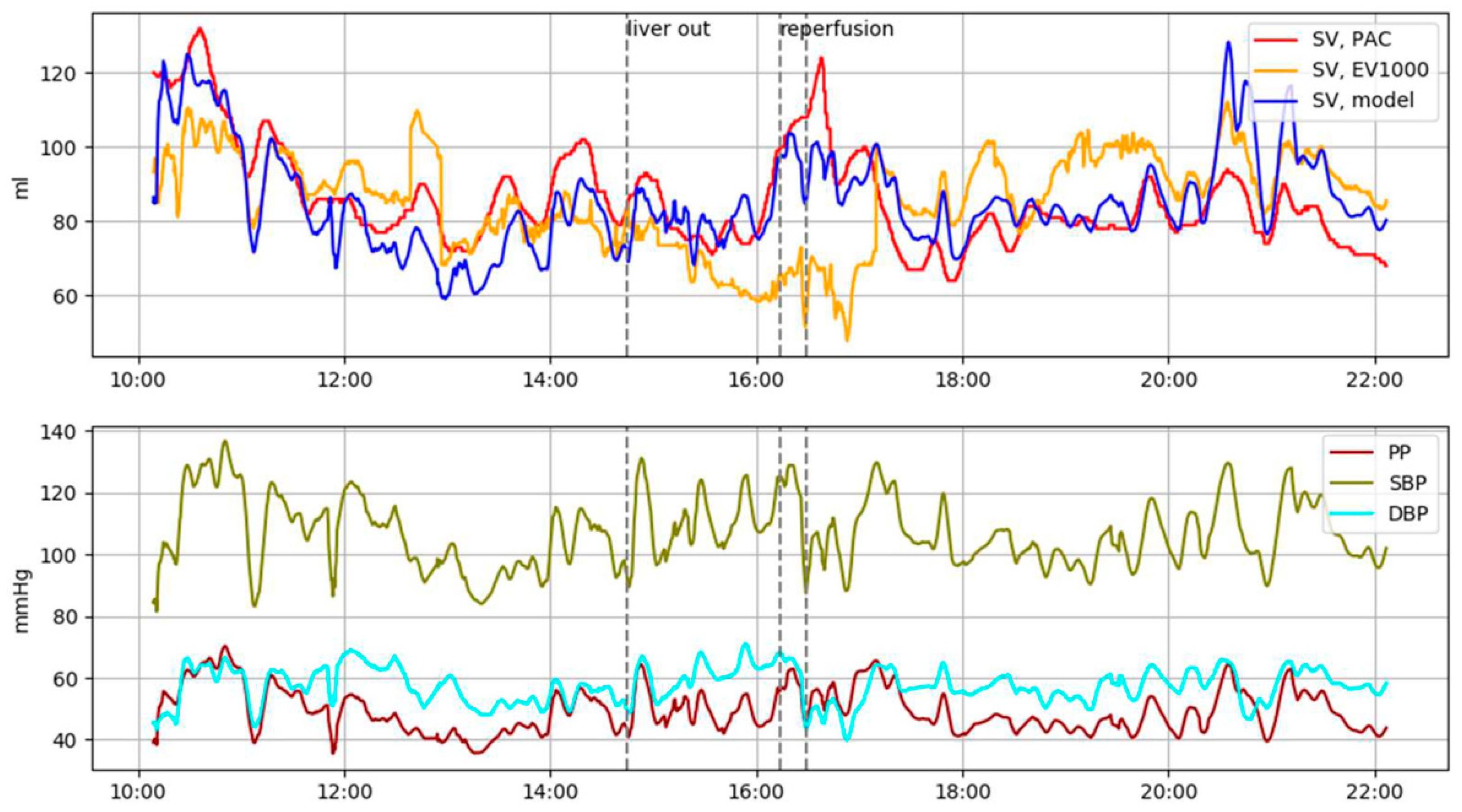
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buhre, W.; Rossaint, R. Perioperative management and monitoring in anaesthesia. Lancet 2003, 362, 1839–1846. [Google Scholar] [CrossRef]
- Park, M.; Han, S.; Kim, G.S.; Gwak, M.S. Evaluation of New Calibrated Pulse-Wave Analysis (VolumeViewTM/EV1000TM) for Cardiac Output Monitoring Undergoing Living Donor Liver Transplantation. PLoS ONE 2016, 11, e0164521. [Google Scholar] [CrossRef][Green Version]
- Bein, B.; Meybohm, P.; Cavus, E.; Renner, J.; Tonner, P.H.; Steinfath, M.; Scholz, J.; Doerges, V. The reliability of pulse contour-derived cardiac output during hemorrhage and after vasopressor administration. Anesth. Analg. 2007, 105, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, S.M.; Coburn, M.; Stoppe, C.; Fries, M.; Simon, T.P.; Reinges, M.H.; Hollig, A.; Rossaint, R.; Marx, G.; Rex, S. Accuracy and precision of calibrated arterial pulse contour analysis in patients with subarachnoid hemorrhage requiring high-dose vasopressor therapy: A prospective observational clinical trial. Crit. Care 2014, 18, R25. [Google Scholar] [CrossRef] [PubMed]
- Biais, M.; Nouette-Gaulain, K.; Cottenceau, V.; Vallet, A.; Cochard, J.F.; Revel, P.; Sztark, F. Cardiac output measurement in patients undergoing liver transplantation: Pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth. Analg. 2008, 106, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Bendjelid, K.; Marx, G.; Kiefer, N.; Simon, T.P.; Geisen, M.; Hoeft, A.; Siegenthaler, N.; Hofer, C.K. Performance of a new pulse contour method for continuous cardiac output monitoring: Validation in critically ill patients. Br. J. Anaesth. 2013, 111, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Camporota, L.; Beale, R. Pitfalls in haemodynamic monitoring based on the arterial pressure waveform. Crit. Care 2010, 14, 124. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. In Proceedings of the 26th Annual Conference on Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; pp. 1097–1105. [Google Scholar]
- Pourbabaee, B.; Roshtkhari, M.J.; Khorasani, K. Deep Convolutional Neural Networks and Learning ECG Features for Screening Paroxysmal Atrial Fibrillation Patients. IEEE Tran. Syst. Man Cybern. Syst. 2018, 48, 2095–2104. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Moon, Y.J.; Lee, S.; Jeong, S.M.; Song, J.G.; Hwang, G.S. Atrioventricular conduction disturbances immediately after hepatic graft reperfusion and their outcomes in patients undergoing liver transplantation. Liver Transplant. 2016, 22, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Kwon, H.M.; Park, Y.S.; Kim, S.H.; Hwang, G.S. Brief Episodes of Newly Developed Intraoperative Atrial Fibrillation Predicts Worse Outcomes in Adult Liver Transplantation. Transplant. Proc. 2018, 50, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Moon, Y.J.; Kim, J.W.; Song, J.G.; Hwang, G.S. Prediction of fluid responsiveness by a non-invasive respiratory systolic time interval variation using heart sound signals in recipients undergoing liver transplantation. Transplant. Proc. 2017, 49, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, G.S.; Kim, S.O.; Kim, Y.K. Is stroke volume variation a useful preload index in liver transplant recipients? A retrospective analysis. Int. J. Med. Sci. 2013, 10, 751–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.C.; Jung, C.W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 2018, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Kim, M.S.; Lim, W.; Park, G.H.; Park, I.; Chang, S.E. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Investig. Dermatol. 2018, 138, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Varvel, J.R.; Donoho, D.L.; Shafer, S.L. Measuring the predictive performance of computer-controlled infusion pumps. J. Pharm. Biopharm. 1992, 20, 63–94. [Google Scholar] [CrossRef]
- Lees, N.; Hamilton, M.; Rhodes, A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit. Care 2009, 13, 231. [Google Scholar] [CrossRef]
- Peyton, P.J.; Chong, S.W. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology 2010, 113, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, O.; Plawiak, P.; Tan, R.S.; Acharya, U.R. Arrhythmia detection using deep convolutional neural network with long duration ECG signals. Comput. Biol. Med. 2018, 102, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Zhang, Q.; Wei, X. Classification of ECG signals based on 1D convolution neural network. In Proceedings of the 2017 IEEE 19th International Conference on e-Health Networking, Applications and Services (Healthcom), Dalian, China, 12–15 October 2017; pp. 1–6. [Google Scholar]
- Rajpurkar, P.; Hannun, A.; Haghpanahi, M.; Bourn, C.; Ng, A. Cardiologist-Level Arrhythmia Detection with Convolutional Neural Networks. Available online: https://stanfordmlgroup.github.io/projects/ecg (accessed on 11 March 2019).
- Wu, Y.; Yang, F.; Liu, Y.; Zha, X.; Yuan, S. A comparison of 1-D and 2-D deep convolutional neural networks in ECG classification. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 17–21 July 2018; pp. 324–327. [Google Scholar]
- Sun, J.X.; Reisner, A.T.; Saeed, M.; Mark, R.G. Estimating cardiac output from arterial blood pressurewaveforms: A critical evaluation using the MIMIC II database. In Proceedings of the Computers in Cardiology, Lyon, France, 25–28 September 2005; pp. 295–298. [Google Scholar]
| Training Set (n = 33) | Testing Set (n = 31) | Total Set (n = 64) | P-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 54.7 ± 9.5 | 55.3 ± 8.1 | 55.0 ± 8.8 | 0.800 |
| Sex (male) | 66.7% | 61.3% | 64.1% | 0.851 |
| Weight (kg) | 64.7 ± 13.7 | 70.2 ± 13.3 | 67.4 ± 13.7 | 0.111 |
| Body mass index (kg/m2) | 23.5 ± 4.0 | 25.7 ± 5.2 | 24.5 ± 4.7 | 0.056 |
| MELD score | 12 (8–15) | 17 (10–21) | 14 (9–21) | 0.333 |
| CTP score | 7 (6–8) | 9 (6–10.5) | 7 (6–9.3) | 0.239 |
| grade A | 42.4% | 29.0% | 35.9% | 0.392 |
| grade B | 36.4% | 38.7% | 37.5% | 1.000 |
| grade C | 21.2% | 32.3% | 26.6% | 0.474 |
| Causes for liver transplantation | ||||
| Hepatitis B virus related liver cirrhosis | 54.5% | 38.7% | 46.9% | 0.309 |
| Hepatitis C virus related liver cirrhosis | 0% | 16.1% | 7.8% | 0.053 |
| Alcoholic liver cirrhosis | 24.2% | 25.8% | 25.0% | 1.000 |
| Hepatocellular carcinoma | 54.5% | 45.2% | 50.0% | 0.617 |
| Others | 15.2% | 12.9% | 14.1% | 1.000 |
| Operation type | ||||
| Living donor | 87.9% | 90.3% | 89.1% | 1.000 |
| Deceased donor | 12.1% | 9.7% | 10.9% | 1.000 |
| Underlying disease | ||||
| Diabetes mellitus | 24.2% | 22.6% | 23.4% | 1.000 |
| Hypertension | 24.2% | 22.6% | 23.4% | 1.000 |
| Medication | ||||
| Beta blocker | 21.2% | 25.8% | 23.4% | 0.890 |
| Diuretics | 42.4% | 48.4% | 45.3% | 0.820 |
| Phases of Liver Transplantation | P for Trend | |||||
|---|---|---|---|---|---|---|
| Pre-Anhepatic | Anhepatic | Reperfusion | Post-Reperfusion | Overall | ||
| Duration (min, %) | 7042 (43.0 %) | 2080 (12.7%) | 295 (1.8%) | 6962 (42.5%) | 16378 (100%) | |
| Blood pressure (mmHg) | ||||||
| Systolic | 110.1 ± 16.3 | 104.4 ± 16.3 | 97.2 ± 17.6 | 105.0 ± 14.2 | 107.0 ± 15.7 | <0.001 |
| Diastolic | 56.0 ± 8.6 | 55.0 ± 7.9 | 48.1 ± 7.1 | 53.2 ± 7.8 | 54.5 ± 8.3 | <0.001 |
| Heart rate (bpm) | 82.8 ± 16.0 | 88.2 ± 18.5 | 86.5 ± 18.4 | 83.4 ± 17.5 | 83.8 ± 17.1 | <0.001 |
| Stroke volume (mL/beat) | ||||||
| SVPAC | 88.5 ± 23.5 | 75.3 ± 24.0 | 85.7 ± 25.2 | 83.8 ± 25.1 | 84.8 ± 24.7 | <0.001 |
| SVEV1000 | 91.5 ± 30.3 | 85.9 ± 33.5 | 91.3 ± 36.5 | 93.2 ± 35.4 | 91.5 ± 33.1 | <0.001 |
| SVDL | 87.9 ± 23.4 | 76.3 ± 22.1 | 83.7 ± 26.4 | 84.2 ± 25.9 | 84.8 ± 24.7 | <0.001 |
| Stroke volume index (mL/beat/m2) | ||||||
| SVIPAC | 50.8 ± 14.8 | 42.6 ± 14.3 | 49.0 ± 14.0 | 47.6 ± 13.4 | 48.4 ± 14.4 | <0.001 |
| SVIEV1000 | 51.8 ± 16.1 | 48.2 ± 18.4 | 51.7 ± 19.2 | 52.9 ± 19.8 | 51.8 ± 18.2 | <0.001 |
| SVIDL | 50.3 ± 14.5 | 43.1 ± 13.4 | 47.7 ± 14.2 | 47.9 ± 14.2 | 48.4 ± 14.4 | <0.001 |
| Systemic vascular resistance (dyne∙s/cm5) | 850.0 ± 331.3 | 910.3 ± 393.0 | 749.9 ± 272.2 | 856.9 ± 290.7 | 858.8 ± 323.6 | <0.001 |
| Stroke volume variation by SVEV1000 (%) | 7.6 ± 4.1 | 10.2 ± 7.0 | 8.4 ± 6.0 | 9.8 ± 5.7 | 8.9 ± 5.4 | <0.001 |
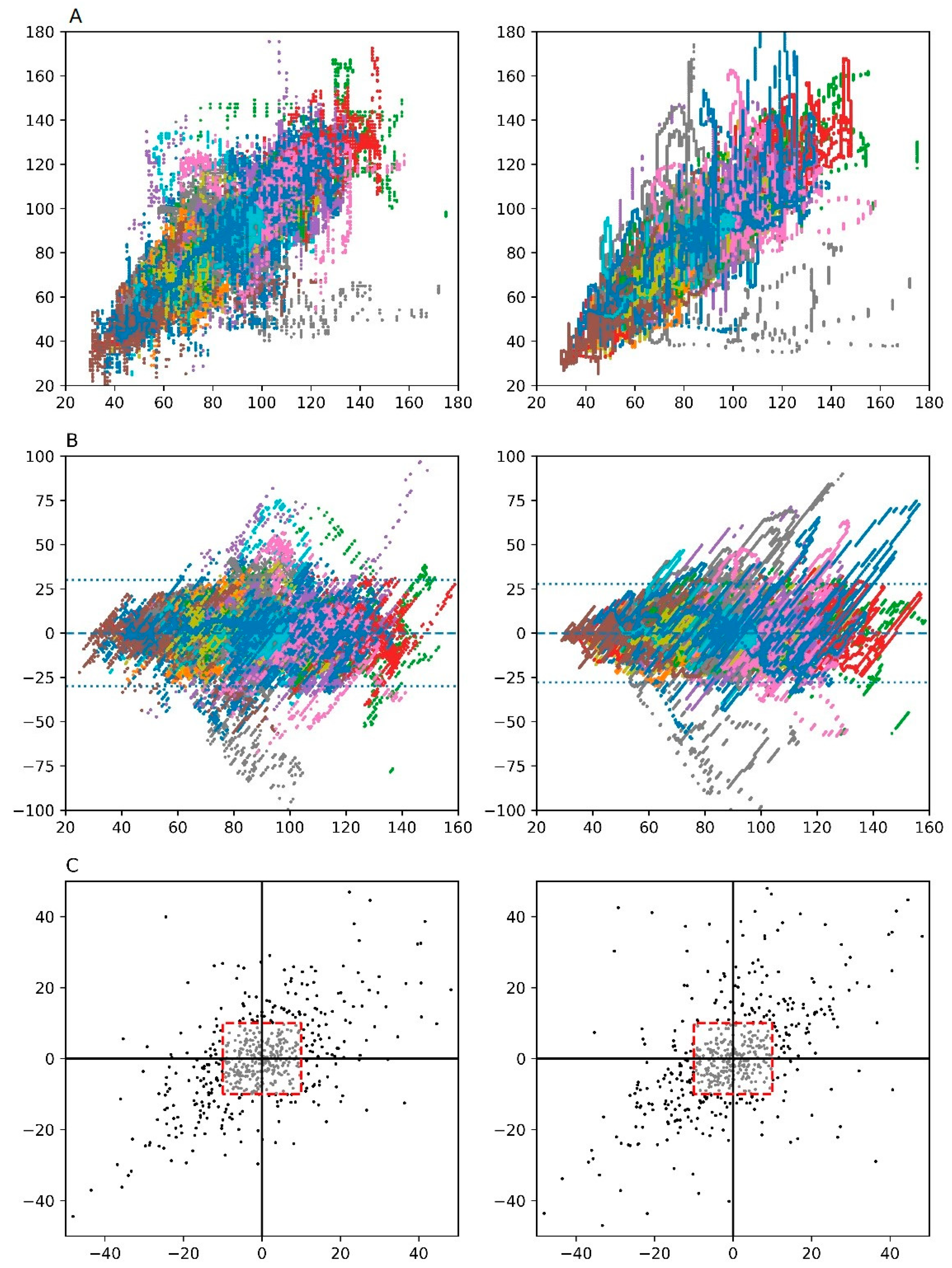
| Phases | Data Records (n) | Linear Regression Analysis | Bland-Altman Analysis | Four-Quadrant Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson Correlation, r (95% CI) | Bias (mL) | 95% Limits of Agreement (mL) | Concordance Rate (%) | |||||||
| Comparison with SVPAC as Standard Reference | ||||||||||
| SVEV1000 | SVDL | P Value | SVEV1000 | SVDL | SVEV1000 | SVDL | SVEV1000 | SVDL | ||
| Overall | 491,353 | 0.813 (0.812–0.814) | 0.840 (0.839–0.841) | <0.001 | Na | Na | −29.52 ~ +29.52 | −27.36 ~ +27.36 | 74.15% | 77.74% |
| Pre-anhepatic | 211,265 | 0.821 (0.820–0.823) | 0.837 (0.836–0.838) | <0.001 | 0.96 | −0.63 | −26.75 ~ +28.67 | −26.87 ~ +25.61 | 75.00% | 75.61% |
| Anhepatic | 62,391 | 0.866 (0.864–0.868) | 0.865 (0.863–0.867) | 0.48 | 2.73 | 0.99 | −21.50 ~ +26.96 | −22.77 ~ +24.75 | 82.14% | 95.65% |
| Reperfusion | 8,841 | 0.570 (0.556–0.584) | 0.861 (0.855–0.866) | <0.001 | −4.66 | −2.01 | −49.13 ~ +39.81 | −28.76 ~ +24.74 | 75.76% | 90.62% |
| Post-reperfusion | 208,856 | 0.795 (0.793–0.797) | 0.828 (0.827–0.829) | <0.001 | −1.59 | 0.43 | −33.03 ~ +29.85 | −28.91 ~ +29.77 | 70.43% | 74.80% |
| SVIEV1000 | SVIDL | P Value | SVIEV1000 | SVIDL | SVIEV1000 | SVIDL | SVIEV1000 | SVIDL | ||
| Overall | 491,353 | 0.827 (0.826-0.828) | 0.848 (0.848–0.849) | <0.001 | Na | Na | −16.58 ~ +16.58 | −15.52 ~ +15.52 | 74.58% | 77.42% |
| Pre-anhepatic | 211,265 | 0.847 (0.846–0.848) | 0.860 (0.859–0.861) | <0.001 | 0.38 | −0.45 | −15.46 ~ +16.22 | −15.64 ~ +14.74 | 75.00 % | 75.61% |
| Anhepatic | 62,391 | 0.882 (0.880–0.884) | 0.878 (0.876–0.880) | 0.002 | 1.49 | 0.55 | −11.94 ~ +14.92 | −12.88 ~ +13.98 | 82.14% | 95.65% |
| Reperfusion | 8,841 | 0.561 (0.546–0.575) | 0.861 (0.856–0.867) | <0.001 | −2.65 | −1.29 | −27.35 ~ +22.05 | −15.70 ~ +13.12 | 75.76% | 91.62% |
| Post-reperfusion | 208,856 | 0.789 (0.788–0.790) | 0.817 (0.816–0.819) | <0.001 | −0.73 | 0.32 | −18.25 ~ +16.79 | −16.10 ~ +16.74 | 71.55% | 74.59% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.-J.; Moon, H.S.; Kim, D.-S.; Kim, J.-M.; Lee, J.-K.; Shim, W.-H.; Kim, S.-H.; Hwang, G.-S.; Choi, J.-S. Deep Learning-Based Stroke Volume Estimation Outperforms Conventional Arterial Contour Method in Patients with Hemodynamic Instability. J. Clin. Med. 2019, 8, 1419. https://doi.org/10.3390/jcm8091419
Moon Y-J, Moon HS, Kim D-S, Kim J-M, Lee J-K, Shim W-H, Kim S-H, Hwang G-S, Choi J-S. Deep Learning-Based Stroke Volume Estimation Outperforms Conventional Arterial Contour Method in Patients with Hemodynamic Instability. Journal of Clinical Medicine. 2019; 8(9):1419. https://doi.org/10.3390/jcm8091419
Chicago/Turabian StyleMoon, Young-Jin, Hyun S. Moon, Dong-Sub Kim, Jae-Man Kim, Joon-Kyu Lee, Woo-Hyun Shim, Sung-Hoon Kim, Gyu-Sam Hwang, and Jae-Soon Choi. 2019. "Deep Learning-Based Stroke Volume Estimation Outperforms Conventional Arterial Contour Method in Patients with Hemodynamic Instability" Journal of Clinical Medicine 8, no. 9: 1419. https://doi.org/10.3390/jcm8091419
APA StyleMoon, Y.-J., Moon, H. S., Kim, D.-S., Kim, J.-M., Lee, J.-K., Shim, W.-H., Kim, S.-H., Hwang, G.-S., & Choi, J.-S. (2019). Deep Learning-Based Stroke Volume Estimation Outperforms Conventional Arterial Contour Method in Patients with Hemodynamic Instability. Journal of Clinical Medicine, 8(9), 1419. https://doi.org/10.3390/jcm8091419





