Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Operational Definition of Cognitive Frailty
2.3. Measurement of the Incidence of Dementia
2.4. Potential Confounding Factors
2.5. Statistical Analysis
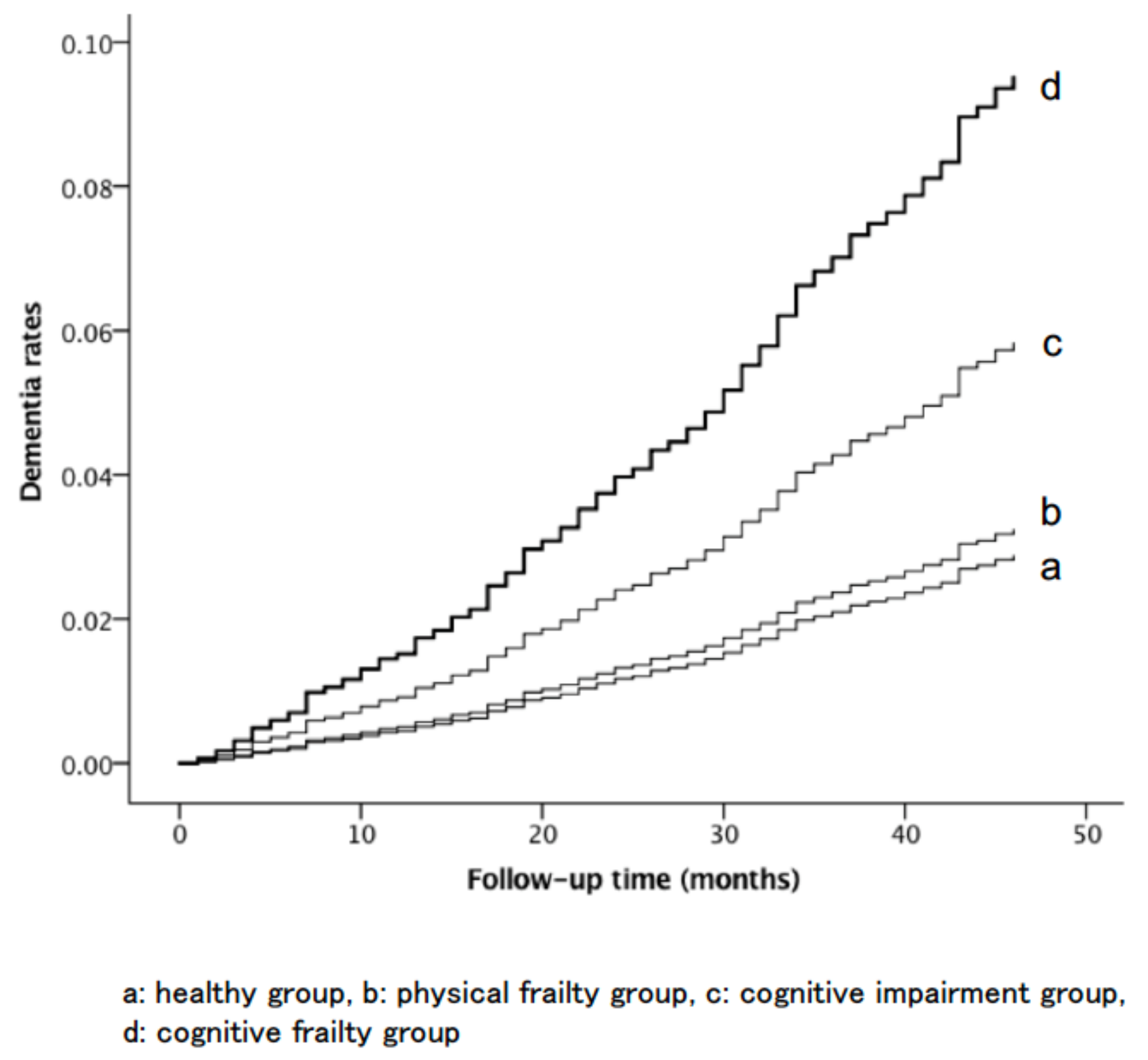
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auyeung, T.W.; Lee, J.S.; Kwok, T.; Woo, J. Physical frailty predicts future cognitive decline—A four-year prospective study in 2737 cognitively normal older adults. J. Nutr. Health Aging 2011, 15, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. The frail brain. J. Am. Med. Dir. Assoc. 2013, 14, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Morris, J.C.; Berg-Weger, M.; Borson, S.; Carpenter, B.D.; Del Campo, N.; Dubois, B.; Fargo, K.; Fitten, L.J.; Flaherty, J.H.; et al. Brain health: The importance of recognizing cognitive impairment: An IAGG consensus conference. J. Am. Med. Dir. Assoc. 2015, 16, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Lee, S.; Doi, T.; Lee, S.; Tsutsumimoto, K.; Harada, K.; Hotta, R.; Bae, S.; Nakakubo, S.; et al. Impact of cognitive frailty on daily activities in older persons. J. Nutr. Health Aging 2016, 20, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Doi, T.; Lee, S.; Suzuki, T. Cognitive frailty and incidence of dementia in older persons. J. Prev. Alzheimers Dis. 2018, 5, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zin Nyunt, M.S.; Gao, Q.; Feng, L.; Yap, K.B.; Ng, T.P. Cognitive frailty and adverse health outcomes: Findings from the Singapore Longitudinal Ageing Studies (SLAS). J. Am. Med. Dir. Assoc. 2017, 18, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Seripa, D.; Lozupone, M.; Imbimbo, B.P.; D’Amato, A.; Tortelli, R.; Schilardi, A.; Galluzzo, L.; Gandin, C.; et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian longitudinal study on aging. J. Am. Med. Dir. Assoc. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef]
- Feng, L.; Nyunt, M.S.; Gao, Q.; Feng, L.; Lee, T.S.; Tsoi, T.; Chong, M.S.; Lim, W.S.; Collinson, S.; Yap, P.; et al. Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the Singapore Longitudinal Ageing Studies. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.M.; Barnes, B.; Speechley, M.; Muir Hunter, S.W.; Doherty, T.J.; Duque, G.; Gopaul, K.; Sposato, L.A.; Casas-Herrero, A.; Borrie, M.J.; et al. Disentangling cognitive-frailty: Results from the gait and brain study. J. Gerontol. A Biomed. Sci. Med. Sci. 2016, 71, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Buracchio, T.; Dodge, H.H.; Howieson, D.; Wasserman, D.; Kaye, J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.B.; Hall, C.B.; Kuslansky, G.; Katz, M.J.; Buschke, H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N. Engl. J. Med. 2002, 347, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Incidence of disability in frail older persons with or without slow walking speed. J. Am. Med. Dir. Assoc. 2015, 16, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Lee, W.J.; Wu, Y.H.; Hwang, A.C.; Lin, M.H.; Shimada, H.; Peng, L.N.; Loh, C.H.; Arai, H.; Chen, L.K. Cognitive frailty and its association with all-cause mortality among community-dwelling older adults in Taiwan: Results from I-lan longitudinal aging study. Rejuv. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Park, H.; Doi, T.; Lee, S. Validity of the national center for geriatrics and gerontology-functional assessment tool and mini-mental state examination for detecting the incidence of dementia in older Japanese adults. Geriatr. Gerontol. Int. 2017, 17, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Makizako, H.; Lee, S.; Harada, K.; Hotta, R.; Bae, S.; Nakakubo, S.; et al. Driving continuity in cognitively impaired older drivers. Geriatr. Gerontol. Int. 2016, 16, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer's Disease, (TA217); NICE Technology Appraisal Guidance: London, UK, 2011; pp. 6–7. ISBN 978-1-4731-1898-0. [Google Scholar]
- Shimada, H.; Suzuki, T.; Suzukawa, M.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; et al. Performance-based assessments and demand for personal care in older Japanese people: A cross-sectional study. BMJ Open 2013, 3, e002424. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Watanabe, S.; Kumagai, S.; Fujiwara, Y.; Amano, H.; Yoshida, H.; Ishizaki, T.; Yukawa, H.; Suzuki, T.; Shibata, H. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000, 29, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Penninx, B.W.; Nicklas, B.J.; Simonsick, E.M.; Newman, A.B.; Tylavsky, F.A.; Brach, J.S.; Satterfield, S.; Bauer, D.C.; et al. Prognostic value of usual gait speed in well-functioning older people—Results from the health, aging and body composition study. J. Am. Geriatr. Soc. 2005, 53, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the health, aging and body composition study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Wallace, D.; Chandler, J.M.; Duncan, P.W.; Rooney, E.; Fox, M.; Guralnik, J.M. Physical performance measures in the clinical setting. J. Am. Geriatr. Soc. 2003, 51, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Newman, A.B.; Visser, M.; Goodpaster, B.; Kritchevsky, S.B.; Rubin, S.; Nevitt, M.C.; Harris, T.B. Mobility limitation in self-described well-functioning older adults: Importance of endurance walk testing. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2008, 63, 841–847. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare of Japan. Annual Health, Labour, and Welfare Report 2011–2012; Ministry of Health Labour and Welfare of Japan: Tokyo, Japan, 2012.
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- De Jager, C.A.; Hogervorst, E.; Combrinck, M.; Budge, M.M. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer’s disease. Psychol. Med. 2003, 33, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.J.; Scheltens, P.; Hodges, J.R. Advances in the early detection of Alzheimer’s disease. Nat. Med. 2004, 10, 34–41. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, C.; Mungas, D.; Harvey, D.; Reed, B.; Weiner, M.; Chui, H.; Jagust, W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004, 63, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.; Singh, V.; Xu, G.; Johnson, S.C. Predictive markers for ad in a multi-modality framework: An analysis of MCI progression in the ADNI population. NeuroImage 2011, 55, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, L.E.; Carle, A.C.; Mackin, R.S.; Harvey, D.; Mukherjee, S.; Insel, P.; Curtis, S.M.; Mungas, D.; Crane, P.K.; Alzheimer’s Disease Neuroimaging Initiative. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012, 6, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wild, K.; Howieson, D.; Webbe, F.; Seelye, A.; Kaye, J. Status of computerized cognitive testing in aging: A systematic review. Alzheimers Dement. 2008, 4, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.S.; Gearing, M.; Snowdon, D.A.; Mori, H.; Markesbery, W.R.; Mirra, S.S. Progression of regional neuropathology in Alzheimer disease and normal elderly: Findings from the nun study. Alzheimer Dis. Assoc. Disord. 1999, 13, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Schneider, J.A.; Leurgans, S.; Bennett, D.A. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology 2008, 71, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Gottdiener, J.S.; McBurnie, M.A.; Hirsch, C.H.; Kop, W.J.; Tracy, R.; Walston, J.D.; Fried, L.P. Associations of subclinical cardiovascular disease with frailty. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M158–M166. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Puts, M.T.; Visser, M.; Twisk, J.W.; Deeg, D.J.; Lips, P. Endocrine and inflammatory markers as predictors of frailty. Clin. Endocrinol. 2005, 63, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Huang, M.H.; Albert, M.; Harris, T.; Rowe, J.W.; Seeman, T.E. Interleukin-6 and risk of cognitive decline: Macarthur studies of successful aging. Neurology 2002, 59, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ehl, C.; Kolsch, H.; Ptok, U.; Jessen, F.; Schmitz, S.; Frahnert, C.; Schlosser, R.; Rao, M.L.; Maier, W.; Heun, R. Association of an interleukin-1beta gene polymorphism at position-511 with Alzheimer’s disease. Int. J. Mol. Med. 2003, 11, 235–238. [Google Scholar] [PubMed]
- Ma, S.L.; Tang, N.L.; Lam, L.C.; Chiu, H.F. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer’s disease. Neurobiol. Aging 2005, 26, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (finger): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Gillette-Guyonnet, S.; Andrieu, S.; Dantoine, T.; Dartigues, J.F.; Touchon, J.; Vellas, B.; MAPT Study Group. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): A new approach to the prevention of Alzheimer’s disease. Alzheimers Dement. 2009, 5, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004, 57, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef] [PubMed]

| Items | Robust (n = 2561) | Physical Frailty (n = 752) | Cognitive Impairment (n = 676) | Cognitive Frailty (n = 412) | p Value |
|---|---|---|---|---|---|
| Age, years * | 70.6 (4.5) | 74.4 (6.3) | 71.0 (4.6) | 75.7 (6.5) | <0.001 |
| Sex, female ** | 1266 (49.4) | 449 (59.7) | 306 (45.3) | 246 (59.7) | <0.001 |
| Education, years * | 11.7 (2.5) | 11.0 (2.5) | 11.3 (2.4) | 10.3 (2.5) | <0.001 |
| Heart disease, yes ** | 400 (15.6) | 146 (19.4) | 105 (15.5) | 92 (22.3) | 0.001 |
| Pulmonary disease, yes ** | 283 (11.1) | 94 (12.5) | 60 (8.9) | 48 (11.7) | 0.170 |
| Hypertension, yes ** | 1098 (42.9) | 388 (51.6) | 311 (46.0) | 231 (56.1) | <0.001 |
| Hyperlipidemia, yes ** | 1091 (42.6) | 331 (44.0) | 264 (39.1) | 161 (39.1) | 0.139 |
| Diabetes, yes ** | 296 (11.6) | 132 (17.6) | 84 (12.4) | 68 (16.5) | <0.001 |
| Osteoarthritis, yes ** | 334 (13.0) | 135 (18.0) | 87 (12.9) | 85 (20.6) | <0.001 |
| Stroke, yes ** | 84 (3.3) | 42 (5.6) | 48 (7.1) | 49 (11.9) | <0.001 |
| Depression, yes ** | 67 (2.6) | 22 (2.9) | 18 (2.7) | 14 (3.4) | 0.819 |
| Medications * | 1.8 (1.9) | 2.6 (2.4) | 2.0 (2.0) | 3.0 (2.4) | <0.001 |
| Living alone, yes ** | 210 (8.2) | 96 (12.8) | 50 (7.4) | 73 (17.7) | <0.001 |
| Current smoking, yes ** | 244 (9.5) | 53 (7.0) | 90 (13.3) | 43 (10.4) | 0.001 |
| Exercise habit, no ** | 1437 (56.1) | 554 (73.7) | 429 (63.5) | 336 (81.6) | <0.001 |
| Engaging in paid work, no ** | 1716 (67.0) | 579 (77.0) | 446 (66.0) | 318 (77.2) | <0.001 |
| Subjective memory complaints, yes ** | 464 (18.1) | 171 (22.7) | 157 (23.2) | 116 (28.2) | <0.001 |
| Geriatric depression scale-15, score * | 2.4 (2.3) | 3.4 (2.8) | 2.8 (2.6) | 4.3 (3.1) | <0.001 |
| Japanese-certified public long-term care insurance system, yes ** | 6 (0.2) | 36 (4.8) | 3 (0.4) | 35 (8.5) | <0.001 |
| Functional limitations, yes ** | 4 (0.2) | 4 (0.5) | 1 (0.1) | 5 (1.2) | 0.003 |
| Items | Participants with Dementia (n = 241) | Participants without Dementia (n = 4160) | p Value |
|---|---|---|---|
| Age, years * | 76.4 (5.9) | 71.5 (5.2) | <0.001 |
| Sex, female ** | 143 (59.3) | 2124 (51.1) | 0.012 |
| Education, years * | 10.6 (2.6) | 11.5 (2.5) | <0.001 |
| Heart disease, yes ** | 57 (23.7) | 686 (16.5) | 0.004 |
| Pulmonary disease, yes ** | 39 (16.2) | 446 (10.7) | 0.008 |
| Hypertension, yes ** | 127 (52.7) | 1901 (45.7) | 0.034 |
| Hyperlipidemia, yes ** | 99 (41.1) | 1748 (42.0) | 0.774 |
| Diabetes, yes ** | 33 (13.7) | 547 (13.1) | 0.808 |
| Osteoarthritis, yes ** | 45 (18.7) | 596 (14.3) | 0.063 |
| Stroke, yes ** | 28 (11.6) | 195 (4.7) | <0.001 |
| Depression, yes ** | 13 (5.4) | 108 (2.6) | 0.010 |
| Medications * | 2.9 (2.5) | 2.0 (2.1) | <0.001 |
| Living alone, yes ** | 34 (14.1) | 395 (9.5) | 0.019 |
| Current smoking, yes ** | 22 (9.1) | 408 (9.8) | 0.730 |
| Exercise habit, no ** | 172 (71.4) | 2584 (62.1) | 0.004 |
| Engaging in paid work, no ** | 194 (80.5) | 2865 (68.9) | <0.001 |
| Subjective memory complaints, yes ** | 92 (38.2) | 816 (19.6) | <0.001 |
| Geriatric depression scale-15, score * | 4.2 (3.1) | 2.7 (2.5) | <0.001 |
| Japanese-certified public long-term care insurance system, yes ** | 19 (7.9) | 61 (1.5) | <0.001 |
| Functional limitations, yes ** | 1 (0.4) | 13 (0.3) | 0.784 |
| Items | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Frail vs. Healthy | 1 | <0.001 |
| Physical frailty | 1.13 (0.76–1.69) | 0.555 |
| Cognitive impairment | 2.06 (1.41–3.02) | <0.001 |
| Cognitive frailty | 3.43 (2.37–4.97) | <0.001 |
| Age, years * | 1.10 (1.08–1.13) | <0.001 |
| Sex, female | 1.35 (1.01–1.81) | 0.042 |
| Education, years * | 1.00 (0.94–1.05) | 0.872 |
| Heart disease, yes | 1.03 (0.75–1.41) | 0.878 |
| Pulmonary disease, yes | 1.26 (0.89–1.78) | 0.201 |
| Hypertension, yes | 0.93 (0.71–1.23) | 0.622 |
| Hyperlipidemia, yes | 0.93 (0.71–1.22) | 0.611 |
| Diabetes, yes | 0.91 (0.62–1.33) | 0.627 |
| Osteoarthritis, yes | 0.83 (0.59–1.18) | 0.299 |
| Stroke, yes | 1.62 (1.07–2.45) | 0.022 |
| Depression, yes | 1.58 (0.88–2.84) | 0.125 |
| Medications * | 1.06 (1.00–1.13) | 0.059 |
| Living alone, yes | 0.81 (0.56–1.19) | 0.279 |
| Current smoking, yes | 1.26 (0.79–1.99) | 0.333 |
| Exercise habit, no | 0.90 (0.67–1.21) | 0.485 |
| Engaging in paid work, no | 1.05 (0.75–1.47) | 0.795 |
| Subjective memory complaints, yes | 1.67 (1.25–2.22) | <0.001 |
| Geriatric depression scale-15, score * | 1.07 (1.02–1.12) | 0.005 |
| Japanese-certified public long-term care insurance system, yes | 0.94 (0.56–1.58) | 0.812 |
| Functional limitations, yes | 0.51 (0.07–3.78) | 0.508 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, H.; Doi, T.; Lee, S.; Makizako, H.; Chen, L.-K.; Arai, H. Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People. J. Clin. Med. 2018, 7, 250. https://doi.org/10.3390/jcm7090250
Shimada H, Doi T, Lee S, Makizako H, Chen L-K, Arai H. Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People. Journal of Clinical Medicine. 2018; 7(9):250. https://doi.org/10.3390/jcm7090250
Chicago/Turabian StyleShimada, Hiroyuki, Takehiko Doi, Sangyoon Lee, Hyuma Makizako, Liang-Kung Chen, and Hidenori Arai. 2018. "Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People" Journal of Clinical Medicine 7, no. 9: 250. https://doi.org/10.3390/jcm7090250
APA StyleShimada, H., Doi, T., Lee, S., Makizako, H., Chen, L.-K., & Arai, H. (2018). Cognitive Frailty Predicts Incident Dementia among Community-Dwelling Older People. Journal of Clinical Medicine, 7(9), 250. https://doi.org/10.3390/jcm7090250







