Evidence-Based Medicine: Past, Present, Future
Abstract
1. Introduction
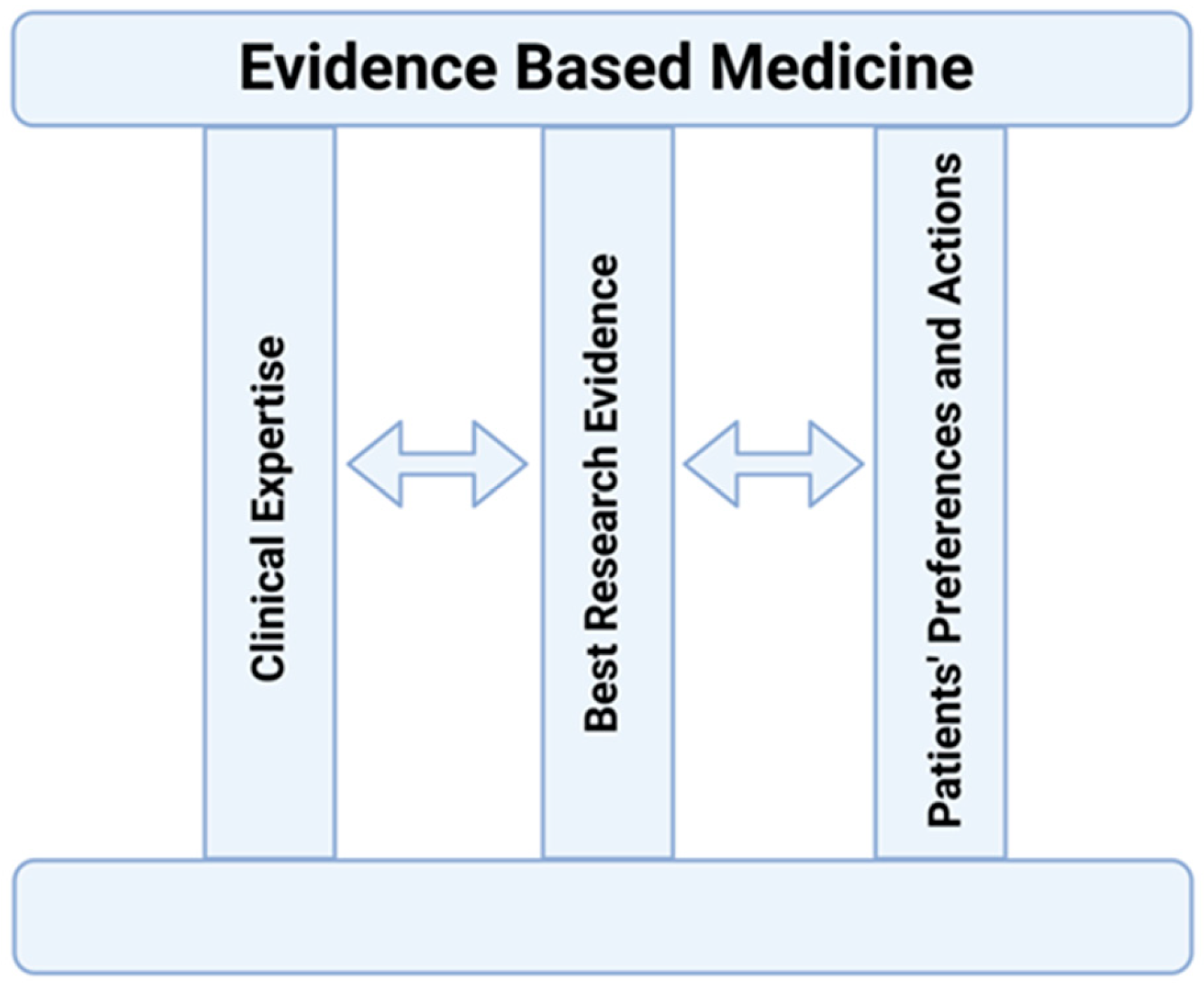
2. The EBM Concept
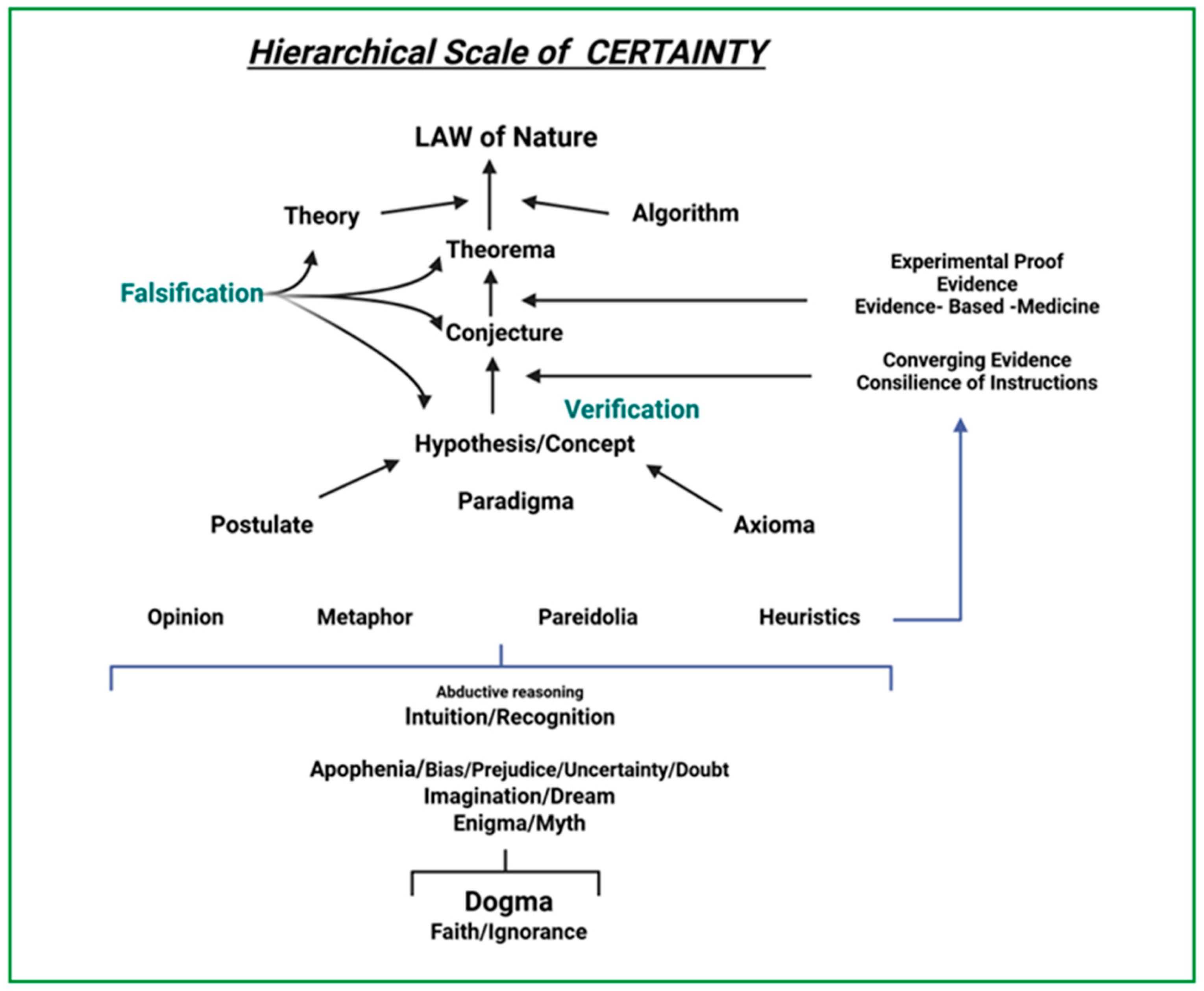
3. EBM as Science
4. Philosophy and EMB
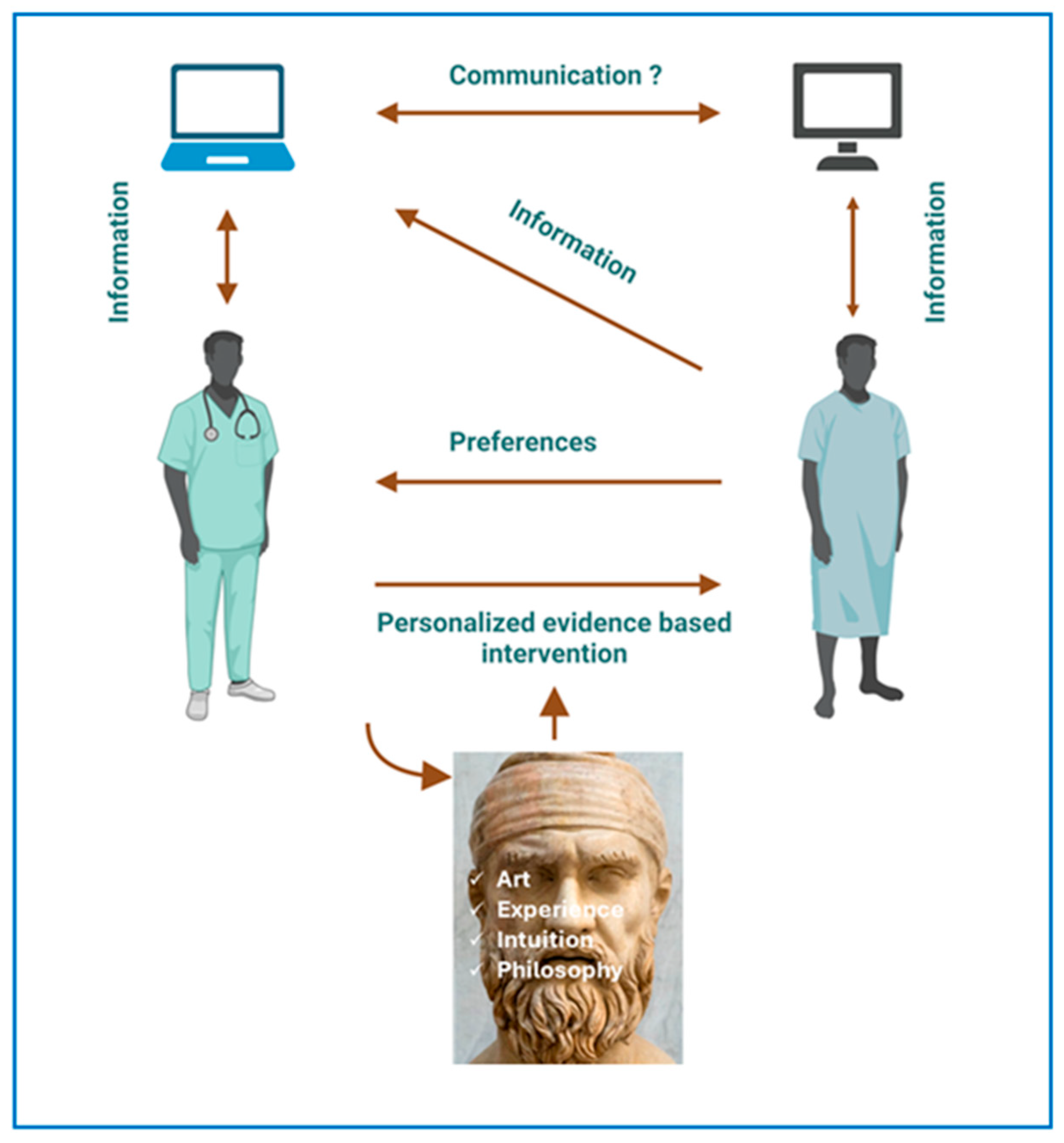
5. Is EBM an Art?
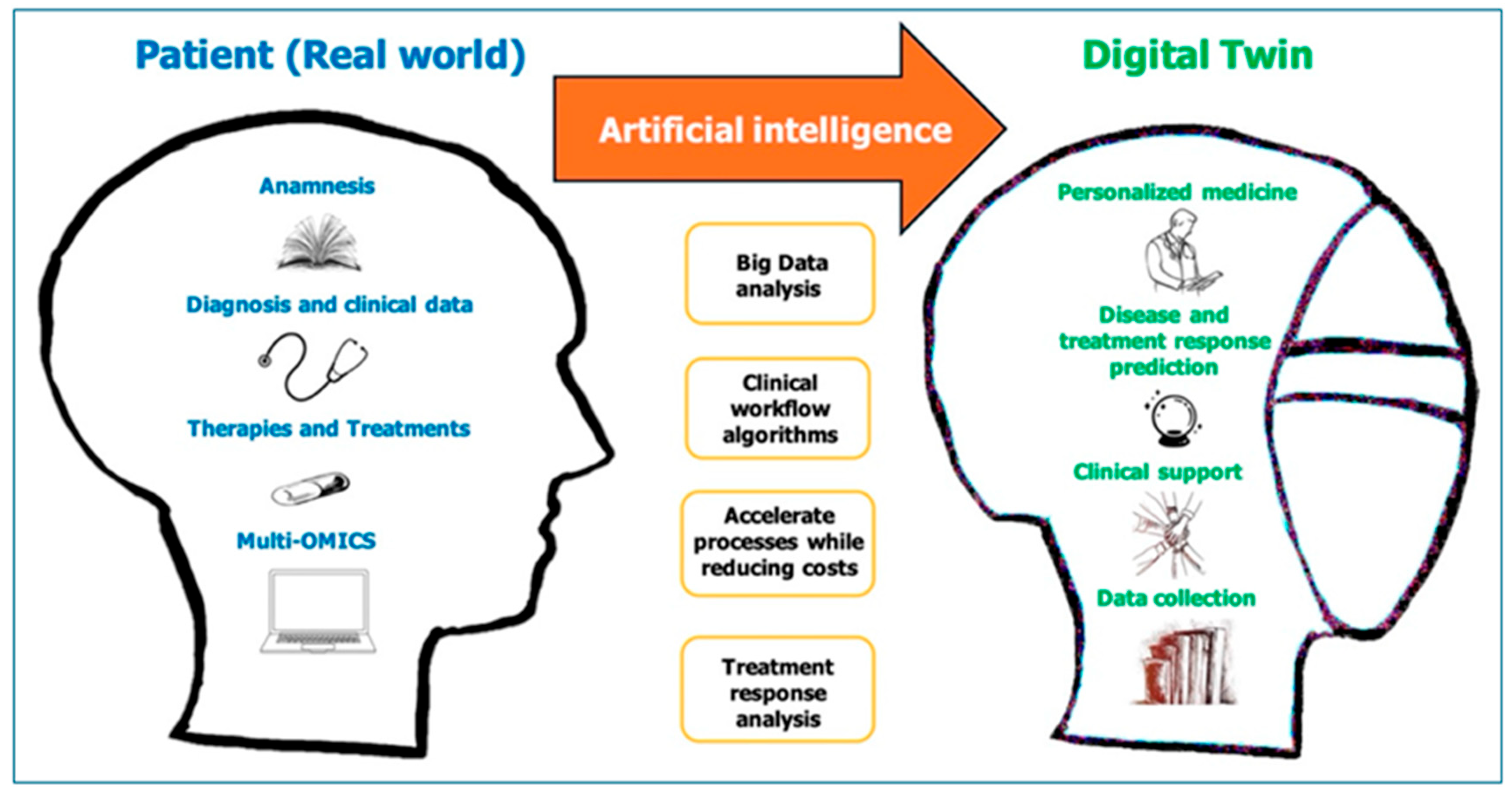
6. EBM in the ERA of Precision Medicine
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heessel, N.P. Diagnosis, Divination and Disease: Towards an Understanding of the Rationale Behind the Babylonian Diagnsostic Handbook. In Magic and Rationality in Ancient Near Eastern and Graeco-Roman Medicine; Horstmanshoff, H.F.J., Stol, M., Van Tilburg, C., Eds.; Brill Publishers: Boston, MA, USA, 2004; pp. 97–116. [Google Scholar]
- Curran, J. The yellow Emperor’s classic of internal medicine. BMJ 2008, 336, 777. [Google Scholar] [CrossRef]
- Mikic, Z. Imhotep—builder, physician, god. Med. Pregl. 2008, 61, 533–538. [Google Scholar]
- Prasad, P.V. General medicine in Atharvaveda with special reference to Yaksma (consumption/tuberculosis). J. Indian. Med. Herit. 2002, 32, 1–14. [Google Scholar]
- Reiser, S.J. What modern physicians can learn from Hippocrates. Cancer 2003, 98, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Kamerow, D. Milestones, tombstones and sex education. BMJ 2007, 334, 0-a. [Google Scholar] [CrossRef]
- Guyatt, G.; Cairns, J.; Churchill, D.; Cook, D.; Haynes, B.; Hirsh, J.; Irvine, J.; Levine, M.; Levine, M.; Nishikawa, J.; et al. Evidence-Based Medicine Working, G. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992, 268, 2420–2425. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Haynes, R.B.; Devereaux, P.J.; Guyatt, G.H. Clinical expertise in the era of evidence-based medicine and patient choice. ACP J. Club 2002, 136, A11-14. [Google Scholar] [CrossRef]
- Weldy, C.S.; Ashley, E.A. Towards precision medicine in heart failure. Nat. Rev. Cardiol. 2021, 18, 745–762. [Google Scholar] [CrossRef]
- Van de Vliet, P.; Sprenger, T.; Kampers, L.F.C.; Makalowski, J.; Schirrmacher, V.; Stucker, W.; Van Gool, S.W. The Application of Evidence-Based Medicine in Individualized Medicine. Biomedicines 2023, 11, 1793. [Google Scholar] [CrossRef]
- Einav, S.; O’Connor, M. The limitations of evidence-based medicine compel the practice of personalized medicine. Intensive Care Med. 2024, 50, 1323–1326. [Google Scholar] [CrossRef]
- Miller, S. Myth-based medicine. Br. J. Gen. Pract. 2015, 65, 313. [Google Scholar] [CrossRef][Green Version]
- Potochnik, A. The diverse aims of science. Stud. Hist. Philos. Sci. 2015, 53, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q. Concepts and Reasoning: A Conceptual Review and Analysis of Logical Issues in Empirical Social Science Research. Integr. Psychol. Behav. Sci. 2024, 58, 502–530. [Google Scholar] [CrossRef] [PubMed]
- Normandin, S. Claude Bernard and an introduction to the study of experimental medicine: “physical vitalism”, dialectic, and epistemology. J. Hist. Med. Allied. Sci. 2007, 62, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Woodcock, J.; LaVange, L.M. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N. Engl. J. Med. 2017, 377, 62–70. [Google Scholar] [CrossRef]
- Fernainy, P.; Cohen, A.A.; Murray, E.; Losina, E.; Lamontagne, F.; Sourial, N. Rethinking the pros and cons of randomized controlled trials and observational studies in the era of big data and advanced methods: A panel discussion. BMC Proc. 2024, 18, 1. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Tang, W.W. How artificial intelligence could redefine clinical trials in cardiovascular medicine: Lessons learned from oncology. Pers. Med. 2019, 16, 83–88. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Kasenda, B.; Briel, M. Meta-analyses: What they can and cannot do. Swiss Med. Wkly. 2012, 142, w13518. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. The scandal of poor medical research. BMJ 1994, 308, 283–284. [Google Scholar] [CrossRef]
- Rovelli, C. The First Scientist: Anaximander and his Legacy; Westholme Publishing: Yardley, PA, USA, 2011. [Google Scholar]
- Sallam, H.N. Aristotle, godfather of evidence-based medicine. Facts Views Vis. Obgyn. 2010, 2, 11–19. [Google Scholar]
- Mamun, A. Science and imagination. J. Pharm. Drug Dev. 2019, 1, 1–2. [Google Scholar]
- Meynell, L. The Scientific Imagination: Philosophical and Psycological Perspectives; Levy, A., Godfrey-Smith, P., Eds.; Oxford University Press: Oxford, UK, 2020; 344p. [Google Scholar]
- Floras, J.S. The 2021 Carl Ludwig Lecture. Unsympathetic autonomic regulation in heart failure: Patient-inspired insights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R338–R351. [Google Scholar] [CrossRef]
- Adler, I. The medical gap: Intuition in medicine. Med. Health Care Philos. 2022, 25, 361–369. [Google Scholar] [CrossRef]
- Buetow, S.A.; Mintoft, B. When should patient intuition be taken seriously? J. Gen. Intern. Med. 2011, 26, 433–436. [Google Scholar] [CrossRef]
- Van den Brink, N.; Holbrechts, B.; Brand, P.L.P.; Stolper, E.C.F.; Van Royen, P. Role of intuitive knowledge in the diagnostic reasoning of hospital specialists: A focus group study. BMJ Open 2019, 9, e022724. [Google Scholar] [CrossRef]
- Miller, F.G.; Kaptchuk, T.J. The power of context: Reconceptualizing the placebo effect. J. R. Soc. Med. 2008, 101, 222–225. [Google Scholar] [CrossRef]
- Walters, R.K.; Durrant, F.G.; Nguyen, S.A.; Meyer, T.A.; Lambert, P.R. The Placebo Effect on Tinnitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Otol. Neurotol. 2024, 45, e263–e270. [Google Scholar] [CrossRef]
- Monaghan, N.P.; Duckett, K.A.; Nguyen, S.A.; Massey, A.A.; Rathi, V.; Soler, Z.M.; Schlosser, R.J. The placebo effect of sham rhinologic procedures in randomized controlled trials: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2024, 14, 1929–1933. [Google Scholar] [CrossRef]
- Antman, E.M.; Loscalzo, J. Precision medicine in cardiology. Nat. Rev. Cardiol. 2016, 13, 591–602. [Google Scholar] [CrossRef]
- Park, J.J.H.; Siden, E.; Zoratti, M.J.; Dron, L.; Harari, O.; Singer, J.; Lester, R.T.; Thorlund, K.; Mills, E.J. Systematic review of basket trials, umbrella trials, and platform trials: A landscape analysis of master protocols. Trials 2019, 20, 572. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational approaches streamlining drug discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Dickson, D.; Johnson, J.; Bergan, R.; Owens, R.; Subbiah, V.; Kurzrock, R. The Master Observational Trial: A New Class of Master Protocol to Advance Precision Medicine. Cell 2020, 180, 9–14. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; McHenry, L.B.; Jureidini, J.N. Industry-corrupted psychiatric trials. Psychiatr. Pol. 2017, 51, 993–1008. [Google Scholar] [CrossRef]
- Batko, K.; Slezak, A. The use of Big Data Analytics in healthcare. J. Big Data 2022, 9, 3. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial Intelligence in Precision Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Bourazana, A.; Xanthopoulos, A.; Briasoulis, A.; Magouliotis, D.; Spiliopoulos, K.; Athanasiou, T.; Vassilopoulos, G.; Skoularigis, J.; Triposkiadis, F. Artificial Intelligence in Heart Failure: Friend or Foe? Life 2024, 14I, 145. [Google Scholar] [CrossRef]
- Corballis, M.C. The evolution of language. Ann. N. Y. Acad. Sci. 2009, 1156, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Bero, L. Managing risk from conflicts of interest in guideline development committees. BMJ 2022, 379, e072252. [Google Scholar] [CrossRef] [PubMed]
- Popper, K.R. Sources of knowledge and ignorance. Philos. Phenomenol. Res. 1962, 23, 292–293. [Google Scholar] [CrossRef]
- Idan, D.; Einav, S. Primer on large language models: An educational overview for intensivists. Crit. Care 2025, 29, 238. [Google Scholar] [CrossRef]
- Lee, P.; Bubeck, S.; Petro, J. Benefits, Limits, and Risks of GPT-4 as an AI Chatbot for Medicine. Reply. N. Engl. J. Med. 2023, 388, 2399–2400. [Google Scholar] [CrossRef]
- Peng, W.; Feng, Y.; Yao, C.; Zhang, S.; Zhuo, H.; Qiu, T.; Zhang, Y.; Tang, J.; Gu, Y.; Sun, Y. Evaluating AI in medicine: A comparative analysis of expert and ChatGPT responses to colorectal cancer questions. Sci. Rep. 2024, 14, 2840. [Google Scholar] [CrossRef]
- Preiksaitis, C.; Ashenburg, N.; Bunney, G.; Chu, A.; Kabeer, R.; Riley, F.; Ribeira, R.; Rose, C. The Role of Large Language Models in Transforming Emergency Medicine: Scoping Review. JMIR Med. Inform. 2024, 12, e53787. [Google Scholar] [CrossRef]
- Goh, E.; Gallo, R.J.; Strong, E.; Weng, Y.; Kerman, H.; Freed, J.A.; Cool, J.A.; Kanjee, Z.; Lane, K.P.; Parsons, A.S.; et al. GPT-4 assistance for improvement of physician performance on patient care tasks: A randomized controlled trial. Nat. Med. 2025, 31, 1233–1238. [Google Scholar] [CrossRef]
- Heinz, M.V.; Mackin, D.M.; Trudeau, B.M.; Bhattacharya, S.; Wang, Y.; Banta, H.; Jewett, A.D.; Salzhauer, A.J.; Griffin, T.J.; Jacobson, N.C. Randomized trial of a generative AI chatbot for mental health treatment. NEJM AI 2025, 2, AIoa2400802. [Google Scholar] [CrossRef]
- Aydin, S.; Karabacak, M.; Vlachos, V.; Margetis, K. Large language models in patient education: A scoping review of applications in medicine. Front. Med. 2024, 11, 1477898. [Google Scholar] [CrossRef]
- Ardila, C.M.; Gonzalez-Arroyave, D.; Ramirez-Arbelaez, J. Advancing large language models as patient education tools for inflammatory bowel disease. World J. Gastroenterol. 2025, 31, 105285. [Google Scholar] [CrossRef] [PubMed]
- Chelli, M.; Descamps, J.; Lavoue, V.; Trojani, C.; Azar, M.; Deckert, M.; Raynier, J.L.; Clowez, G.; Boileau, P.; Ruetsch-Chelli, C. Hallucination Rates and Reference Accuracy of ChatGPT and Bard for Systematic Reviews: Comparative Analysis. J. Med. Internet Res. 2024, 26, e53164. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, C.; Redjdal, A.; Seroussi, B. Generative-AI-Based Approaches for Information Extraction from Clinical Notes: A Scoping Review. Stud. Health Technol. Inform. 2025, 328, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Laubenbacher, R.; Mehrad, B.; Shmulevich, I.; Trayanova, N. Digital twins in medicine. Nat. Comput. Sci. 2024, 4, 184–191. [Google Scholar] [CrossRef]
- Tortora, M.; Pacchiano, F.; Ferraciolli, S.F.; Criscuolo, S.; Gagliardo, C.; Jaber, K.; Angelicchio, M.; Briganti, F.; Caranci, F.; Tortora, F.; et al. Medical Digital Twin: A Review on Technical Principles and Clinical Applications. J. Clin. Med. 2025, 14, 324. [Google Scholar] [CrossRef]
- Sadee, C.; Testa, S.; Barba, T.; Hartmann, K.; Schuessler, M.; Thieme, A.; Church, G.M.; Okoye, I.; Hernandez-Boussard, T.; Hood, L.; et al. Medical digital twins: Enabling precision medicine and medical artificial intelligence. Lancet Digit. Health 2025, 100864. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Lee, T.H. Lost in Thought—The Limits of the Human Mind and the Future of Medicine. N. Engl. J. Med. 2017, 377, 1209–1211. [Google Scholar] [CrossRef]
- Lee, P.; Bubeck, S.; Petro, J. Benefits, Limits, and Risks of GPT-4 as an AI Chatbot for Medicine. N. Engl. J. Med. 2023, 388, 1233–1239. [Google Scholar] [CrossRef]
- Yu, K.H.; Healey, E.; Leong, T.Y.; Kohane, I.S.; Manrai, A.K. Medical Artificial Intelligence and Human Values. N. Engl. J. Med. 2024, 390, 1895–1904. [Google Scholar] [CrossRef]
- Stone, L.; Gordon, J. A is for aphorism—‘Wherever the art of medicine is loved there is also a love of humanity’. Aust. Fam. Physician 2013, 42, 824–825. [Google Scholar]
- Moslehpour, M.; Shalehah, A.; Rahman, F.F.; Lin, K.H. The Effect of Physician Communication on Inpatient Satisfaction. Healthcare 2022, 10, 463. [Google Scholar] [CrossRef]
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triposkiadis, F.; Brutsaert, D.L. Evidence-Based Medicine: Past, Present, Future. J. Clin. Med. 2025, 14, 5094. https://doi.org/10.3390/jcm14145094
Triposkiadis F, Brutsaert DL. Evidence-Based Medicine: Past, Present, Future. Journal of Clinical Medicine. 2025; 14(14):5094. https://doi.org/10.3390/jcm14145094
Chicago/Turabian StyleTriposkiadis, Filippos, and Dirk L. Brutsaert. 2025. "Evidence-Based Medicine: Past, Present, Future" Journal of Clinical Medicine 14, no. 14: 5094. https://doi.org/10.3390/jcm14145094
APA StyleTriposkiadis, F., & Brutsaert, D. L. (2025). Evidence-Based Medicine: Past, Present, Future. Journal of Clinical Medicine, 14(14), 5094. https://doi.org/10.3390/jcm14145094







