Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers
Abstract
1. Introduction
1.1. Standard of Care for Oropharyngeal and Nasopharyngeal Cancers
1.2. Therapeutic Cancer Vaccines
1.3. Target Antigens
1.4. Vaccine Delivery Platforms
2. Oropharyngeal Cancer
2.1. Cancer Vaccines for Viral-Induced Oropharyngeal Cancer
2.2. Vaccine-Based Immunotherapy Clinical Trials for HPV-Induced Oropharyngeal Cancer
3. Nasopharyngeal Cancer
3.1. Cancer Vaccines for Viral-Induced Nasopharyngeal Cancer: An Overview
3.2. Vaccine-Based Immunotherapy Clinical Trials for EBV-Induced Nasopharyngeal Cancer
4. Discussion: Clinical Challenges and Ethical Considerations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Pharaon, R.R.; Xing, Y.; Agulnik, M.; Villaflor, V.M. The Role of Immunotherapy to Overcome Resistance in Viral-Associated Head and Neck Cancer. Front. Oncol. 2021, 11, 649963. [Google Scholar] [CrossRef]
- Filippini, D.M.; Carosi, F.; Querzoli, G.; Fermi, M.; Ricciotti, I.; Molteni, G.; Presutti, L.; Foschini, M.P.; Locati, L.D. Rare Head and Neck Cancers and Pathological Diagnosis Challenges: A Comprehensive Literature Review. Diagnostics 2024, 14, 2365. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Gronhoj, C.; Buchwald, C.V. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Evans, M.; Beasley, M.; Chatterjee, S.; Dilkes, M.; Homer, J.; O’Hara, J.; Robinson, M.; Shaw, R.; Sloan, P. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Molteni, G.; Bassani, S.; Arsie, A.E.; Zampieri, E.; Mannelli, G.; Orlandi, E.; Bossi, P.; De Virgilio, A. Role of TORS as De-Escalation Strategy in HPV-Related Oropharyngeal Cancer, What We Need to Know. Healthcare 2024, 12, 1014. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Tao, Y.; Biau, J.; Sun, X.S.; Sire, C.; Martin, L.; Alfonsi, M.; Prevost, J.B.; Modesto, A.; Lafond, C.; Tourani, J.M.; et al. Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015-01 PembroRad): A multicenter, randomized, phase II trial. Ann. Oncol. 2023, 34, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Z.; Zhang, Y.; Bian, C.; Bao, J.; Xin, Y.; Jiang, X. Locally advanced head and neck squamous cell carcinoma treatment efficacy and safety: A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1269863. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Taverna, F.; Alfieri, S.; Romano, R.; Campanini, G.; Marceglia, S.; Giardina, F.; Mazzocchi, A.; Comoli, P.; Gloghini, A.; Quattrone, P.; et al. Comparing BamHI-W and CE-marked assays to detect circulating Epstein-Barr Virus (EBV) DNA of nasopharyngeal cancer patients in a non-endemic area. Oral Oncol. 2022, 135, 106229. [Google Scholar] [CrossRef]
- Wang, L.; Miao, J.; Huang, H.; Chen, B.; Xiao, X.; Zhu, M.; Liang, Y.; Xiao, W.; Huang, S.; Peng, Y.; et al. Long-term Survivals, Toxicities and the Role of Chemotherapy in Early-Stage Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Radiation Therapy: A Retrospective Study with 15-Year Follow-up. Cancer Res. Treat 2022, 54, 118–129. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Lewis, G.D.; Holliday, E.B.; Kocak-Uzel, E.; Hernandez, M.; Garden, A.S.; Rosenthal, D.I.; Frank, S.J. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016, 38 (Suppl. 1), E1886–E1895. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Chan, A.T.; Even, C.; Machiels, J.P.; ESMO Guidelines Committee. ESMO-EURACAN Clinical Practice Guideline update for nasopharyngeal carcinoma: Adjuvant therapy and first-line treatment of recurrent/metastatic disease. Ann. Oncol. 2023, 34, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, S.; Filippini, D.M.; Ottini, A.; Bergamini, C.; Resteghini, C.; Colombo, E.; Lombardo, R.; Nuzzolese, I.; Alfieri, S.; Licitra, L.; et al. Immunotherapy in head and neck squamous cell carcinoma and rare head and neck malignancies. Explor. Target. Anti-Tumor Ther. 2021, 2, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.T.; Trimble, C.L. Current status of therapeutic HPV vaccines. Gynecol. Oncol. 2020, 156, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Prophylactic HPV vaccines. J. Clin. Pathol. 2007, 60, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Hargrave, A.; Mustafa, A.S.; Hanif, A.; Tunio, J.H.; Hanif, S.N.M. Recent Advances in Cancer Immunotherapy with a Focus on FDA-Approved Vaccines and Neoantigen-Based Vaccines. Vaccines 2023, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, C.D.; Wu, X.H. Therapeutic cancer vaccines: From initial findings to prospects. Immunol. Lett. 2018, 196, 11–21. [Google Scholar] [CrossRef]
- Shibata, H.; Xu, N.; Saito, S.; Zhou, L.; Ozgenc, I.; Webb, J.; Fu, C.; Zolkind, P.; Egloff, A.M.; Uppaluri, R. Integrating CD4+ T cell help for therapeutic cancer vaccination in a preclinical head and neck cancer model. Oncoimmunology 2021, 10, 1958589. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K.; Aggarwal, S.; Singh, M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma-A Review. Vaccines 2023, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, X.; Wang, Y.; Zhang, Y. Targeting neoantigens for cancer immunotherapy. Biomark. Res. 2021, 9, 61. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Kim, C.G.; Sang, Y.B.; Lee, J.H.; Chon, H.J. Combining Cancer Vaccines with Immunotherapy: Establishing a New Immunological Approach. Int. J. Mol. Sci. 2021, 22, 8035. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Tureci, O. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Chiang, C.L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef]
- Le, I.; Dhandayuthapani, S.; Chacon, J.; Eiring, A.M.; Gadad, S.S. Harnessing the Immune System with Cancer Vaccines: From Prevention to Therapeutics. Vaccines 2022, 10, 816. [Google Scholar] [CrossRef]
- Perez-Banos, A.; Gleisner, M.A.; Flores, I.; Pereda, C.; Navarrete, M.; Araya, J.P.; Navarro, G.; Quezada-Monras, C.; Tittarelli, A.; Salazar-Onfray, F. Whole tumour cell-based vaccines: Tuning the instruments to orchestrate an optimal antitumour immune response. Br. J. Cancer 2023, 129, 572–585. [Google Scholar] [CrossRef]
- Sheikhlary, S.; Lopez, D.H.; Moghimi, S.; Sun, B. Recent Findings on Therapeutic Cancer Vaccines: An Updated Review. Biomolecules 2024, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef]
- Muthukutty, P.; Yoo, S.Y. Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective. Viruses 2023, 15, 1645. [Google Scholar] [CrossRef]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef]
- Beyaert, S.; Machiels, J.P.; Schmitz, S. Vaccine-Based Immunotherapy for Head and Neck Cancers. Cancers 2021, 13, 6041. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, D.S. Peptide Immunotherapy in Vaccine Development: From Epitope to Adjuvant. Adv. Protein Chem. Struct. Biol. 2015, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Xin, K.Q.; Ooki, T.; Hamajima, K.; Oikawa, T.; Shinoda, K.; Ozaki, T.; Hoshino, Y.; Jounai, N.; Nakazawa, M.; et al. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine 2002, 20, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, L.; Li, X.; Li, J.; Zhao, C.; Zhao, Y.; Zhang, X.; He, P.; Wu, X.; Jiang, S.; et al. Lipid Nanoparticles Outperform Electroporation in Delivering Therapeutic HPV DNA Vaccines. Vaccines 2024, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Vormehr, M.; Tureci, O.; Sahin, U. Harnessing Tumor Mutations for Truly Individualized Cancer Vaccines. Annu. Rev. Med. 2019, 70, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Babala, N.; Melief, C.J.M.; Kastenmuller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Kenter, G.G.; Welters, M.J.; Valentijn, A.R.; Lowik, M.J.; Berends-van der Meer, D.M.; Vloon, A.P.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- Syrjanen, K.; Syrjanen, S.; Pyrhonen, S. Human papilloma virus (HPV) antigens in lesions of laryngeal squamous cell carcinomas. ORL J. Otorhinolaryngol. Relat. Spec. 1982, 44, 323–334. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Sansanaphongpricha, K.; Prince, M.E.P.; Sun, D.; Wolf, G.T.; Lei, Y.L. Engineering Vaccines to Reprogram Immunity against Head and Neck Cancer. J. Dent. Res. 2018, 97, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Rollins, S.; Goloubeva, O.; Morales, R.E.; Tan, M.; Taylor, R.; Wolf, J.S.; Schumaker, L.M.; Cullen, K.J.; Zimrin, A.; et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol. Immunother. 2015, 64, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Voskens, C.J.; Sewell, D.; Hertzano, R.; DeSanto, J.; Rollins, S.; Lee, M.; Taylor, R.; Wolf, J.; Suntharalingam, M.; Gastman, B.; et al. Induction of MAGE-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck 2012, 34, 1734–1746. [Google Scholar] [CrossRef]
- Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16+ cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef] [PubMed]
- Roof, L.; Yilmaz, E. Immunotherapy in HPV-Related Oropharyngeal Cancers. Curr. Treat Options Oncol. 2023, 24, 170–183. [Google Scholar] [CrossRef]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef]
- Chen, Z.; Ozbun, L.; Chong, N.; Wallecha, A.; Berzofsky, J.A.; Khleif, S.N. Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3− and CD8+ T cells. Cancer Immunol. Res. 2014, 2, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Wallecha, A.; French, C.; Petit, R.; Singh, R.; Amin, A.; Rothman, J. Lm-LLO-Based Immunotherapies and HPV-Associated Disease. J. Oncol. 2012, 2012, 542851. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Lalanne, A.; Delord, J.P.; Cassier, P.A.; Rolland, F.; Salas, S.; Limacher, J.M.; Capitain, O.; Lantz, O.; Ekwegbara, C.; et al. Phase Ib/II trial of tipapkinogene sovacivec, a therapeutic human papillomavirus16-vaccine, in combination with avelumab in patients with advanced human papillomavirus16-positive cancers. Eur. J. Cancer 2023, 191, 112981. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients with Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Even, C.; Harrington, K.J.; Massarelli, E.; Klein Hesselink, M.; Visscher, S.; Fury, M.G.; Sanders, F.; Laban, S.; Fayette, J.; Oliva, M.; et al. Results of a randomized, double-blind, placebo-controlled, phase 2 study (OpcemISA) of the combination of ISA101b and cemiplimab versus cemiplimab for recurrent/metastatic (R/M) HPV16-positive oropharyngeal cancer (OPC). J. Clin. Oncol. 2024, 42, 6003. [Google Scholar] [CrossRef]
- Floudas, C.S.; Strauss, J.; Redman, J.M.; Pastor, D.M.; Turkbey, E.B.; Donahue, R.N.; Jochems, C.; McMahon, S.; Lamping, E.; Cordes, L.M.; et al. Phase I evaluation of PRGN-2009 alone and in combination with bintrafusp alfa in patients (pts) with recurrent/metastatic (R/M) HPV-associated cancers (HPV-C). J. Clin. Oncol. 2023, 41, 2628. [Google Scholar] [CrossRef]
- Klinghammer, K.; Saba, N.F.; Castelluci, E.; Colevas, A.D.; Rutkowski, T.; Greil, R.; Thurner, D.; Müller-Richter, U.; Di Giacomo, A.M.; Grewal, J.; et al. 155P BNT113 + pembrolizumab as first-line treatment in patients with unresectable recurrent/metastatic HNSCC: Preliminary safety data from AHEAD-MERIT. Immuno-Oncol. Technol. 2022, 16, 100267. [Google Scholar] [CrossRef]
- Price, K.A.R.; Kaczmar, J.M.; Worden, F.P.; Wood, L.V.; Schaaf, D.T.; Riebel, N.; Chaney, M.F.; Weiss, J. Safety and efficacy of immune checkpoint inhibitor (ICI) naïve cohort from study of PDS0101 and pembrolizumab in HPV16-positive head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2023, 41, 6012. [Google Scholar] [CrossRef]
- Strauss, J.; Floudas, C.S.; Sater, H.A.; Manu, M.; Lamping, E.; Francis, D.C.; Cordes, L.M.; Marte, J.; Donahue, R.N.; Jochems, C. Phase II evaluation of the combination of PDS0101, M9241, and bintrafusp alfa in patients with HPV 16+ malignancies. J. Clin. Oncol. 2022, 40, 2518. [Google Scholar] [CrossRef]
- Aggarwal, C.; Saba, N.F.; Algazi, A.; Sukari, A.; Seiwert, T.Y.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Esser, M.T.; et al. Safety and Efficacy of MEDI0457 plus Durvalumab in Patients with Human Papillomavirus-Associated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2023, 29, 560–570. [Google Scholar] [CrossRef]
- Harrington, K.J.; Kong, A.; Mach, N.; Chesney, J.A.; Fernandez, B.C.; Rischin, D.; Cohen, E.E.W.; Radcliffe, H.S.; Gumuscu, B.; Cheng, J.; et al. Talimogene Laherparepvec and Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (MASTERKEY-232): A Multicenter, Phase 1b Study. Clin. Cancer Res. 2020, 26, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Colevas, A.D.D.; Adkins, D.; Rodriguez, C.P.; Park, J.C.; Gibson, M.K.; Burtness, B.; Johnson, F.M.; Julian, R.A.; Saba, N.F.; et al. A phase 1 dose-escalation and expansion study of CUE-101, a novel HPV16 E7-pHLA-IL2-Fc fusion protein, given as monotherapy and in combination with pembrolizumab in patients with recurrent/metastatic HPV16+ head and neck cancer. J. Clin. Oncol. 2023, 41, 6013. [Google Scholar] [CrossRef]
- Colevas, A.D.; Chung, C.H.; Adkins, D.; Rodriguez, C.P.; Park, J.C.; Gibson, M.K.; Sukari, A.; Worden, F.P.; Johnson, F.M.; Saba, N.F.; et al. A phase 1 dose-escalation and expansion study of CUE-101, given as monotherapy and in combination with pembrolizumab, in patients with recurrent/metastatic HPV16+ head and neck squamous cell cancer (R/M HNSCC). J. Clin. Oncol. 2024, 42, 6004. [Google Scholar] [CrossRef]
- Neparidze, N.; Lacy, J. Malignancies associated with epstein-barr virus: Pathobiology, clinical features, and evolving treatments. Clin. Adv. Hematol. Oncol. 2014, 12, 358–371. [Google Scholar]
- Taylor, G.S.; Steven, N.M. Therapeutic vaccination strategies to treat nasopharyngeal carcinoma. Chin. Clin. Oncol. 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.A.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: A phase I trial in UK patients with EBV-positive cancer. Clin. Cancer Res. 2014, 20, 5009–5022. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Niu, M.; Pan, S.; Zhou, Y.; Shuai, C.; Wang, J.; Peng, S.; Li, G. Cancer stem-like cell: A novel target for nasopharyngeal carcinoma therapy. Stem Cell Res. Ther. 2014, 5, 44. [Google Scholar] [CrossRef]
- Lin, C.L.; Lo, W.F.; Lee, T.H.; Ren, Y.; Hwang, S.L.; Cheng, Y.F.; Chen, C.L.; Chang, Y.S.; Lee, S.P.; Rickinson, A.B.; et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002, 62, 6952–6958. [Google Scholar]
- Li, F.; Song, D.; Lu, Y.; Zhu, H.; Chen, Z.; He, X. Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J. Immunother. 2013, 36, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Si, Y.F.; Lan, G.P.; Wang, Z.; Zhou, L.; Tang, M.Z.; Sj, O.B.; Lan, J.; Zhou, X.Y.; Wang, Y.L.; et al. LMP2-DC Vaccine Elicits Specific EBV-LMP2 Response to Effectively Improve Immunotherapy in Patients with Nasopharyngeal Cancer. Biomed. Environ. Sci. 2020, 33, 849–856. [Google Scholar] [CrossRef]
- Chia, W.K.; Wang, W.W.; Teo, M.; Tai, W.M.; Lim, W.T.; Tan, E.H.; Leong, S.S.; Sun, L.; Chen, J.J.; Gottschalk, S.; et al. A phase II study evaluating the safety and efficacy of an adenovirus-DeltaLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann. Oncol. 2012, 23, 997–1005. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Zhou, L.; Du, H.; Mo, W.; Zeng, Y. Specific cellular immune responses in mice immunized with DNA, adeno-associated virus and adenoviral vaccines of Epstein-Barr virus-LMP2 alone or in combination. Sci. China Life Sci. 2011, 54, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Tsang, J.; Beagley, L.; Chua, D.; Lee, V.; Li, V.; Moss, D.J.; Coman, W.; Chan, K.H.; Nicholls, J.; et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012, 72, 1116–1125. [Google Scholar] [CrossRef]
- Lutzky, V.P.; Corban, M.; Heslop, L.; Morrison, L.E.; Crooks, P.; Hall, D.F.; Coman, W.B.; Thomson, S.A.; Moss, D.J. Novel approach to the formulation of an Epstein-Barr virus antigen-based nasopharyngeal carcinoma vaccine. J. Virol. 2010, 84, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Q.; Zhou, J.; Ma, D.; Xiao, X.; Wang, D.W. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Mol. Cancer Ther. 2009, 8, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Harris, E.; Lorch, J. Vaccination as a therapeutic strategy for Nasopharyngeal carcinoma. Oral Oncol. 2022, 135, 106083. [Google Scholar] [CrossRef]
- Lin, M.C.; Lin, Y.C.; Chen, S.T.; Young, T.H.; Lou, P.J. Therapeutic vaccine targeting Epstein-Barr virus latent protein, LMP1, suppresses LMP1-expressing tumor growth and metastasis in vivo. BMC Cancer 2017, 17, 18. [Google Scholar] [CrossRef]
- Hui, E.P.; Taylor, G.S.; Jia, H.; Ma, B.B.; Chan, S.L.; Ho, R.; Wong, W.L.; Wilson, S.; Johnson, B.F.; Edwards, C.; et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013, 73, 1676–1688. [Google Scholar] [CrossRef]
- Celis, J.J.; Rodriguez, V.; Rodriguez, C. Neoadjuvant treatment with trastuzumab and pertuzumab plus fulvestrant in older patients with HER2-positive, ER-positive early breast cancer. J. Clin. Oncol. 2024, 42, 13. [Google Scholar] [CrossRef]
- Si, Y.; Deng, Z.; Lan, G.; Du, H.; Wang, Y.; Si, J.; Wei, J.; Weng, J.; Qin, Y.; Huang, B.; et al. The Safety and Immunological Effects of rAd5-EBV-LMP2 Vaccine in Nasopharyngeal Carcinoma Patients: A Phase I Clinical Trial and Two-Year Follow-Up. Chem. Pharm. Bull. 2016, 64, 1118–1123. [Google Scholar] [CrossRef]
- Jalilian, H.; Amraei, M.; Javanshir, E.; Jamebozorgi, K.; Faraji-Khiavi, F. Ethical considerations of the vaccine development process and vaccination: A scoping review. BMC Health Serv. Res. 2023, 23, 255. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Marret, G.; Bastien, E.; Sanchez, R.; Borcoman, E.; Le Tourneau, C. Phase I trials of single-agent new drugs in head and neck cancer: A scoping review. Chin. Clin. Oncol. 2024, 13, 73. [Google Scholar] [CrossRef]
- Shukla, R.; Vyas, K.; Khadela, A.; Vora, L.K.; Khatri, D.K. Vaccine safety, efficacy, and ethical considerations. Adv. Vaccin. Technol. Infect. Chronic Dis. 2024, 311–324. [Google Scholar] [CrossRef]
- Fernandes, Q. Precision meets repurposing: Innovative approaches in human papillomavirus and Epstein-Barr virus-driven cancer therapy. Cancer Lett. 2024, 607, 217318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rumgay, H.; Li, M.; Cao, S.; Chen, W. Nasopharyngeal Cancer Incidence and Mortality in 185 Countries in 2020 and the Projected Burden in 2040: Population-Based Global Epidemiological Profiling. JMIR Public Health Surveill. 2023, 9, e49968. [Google Scholar] [CrossRef]
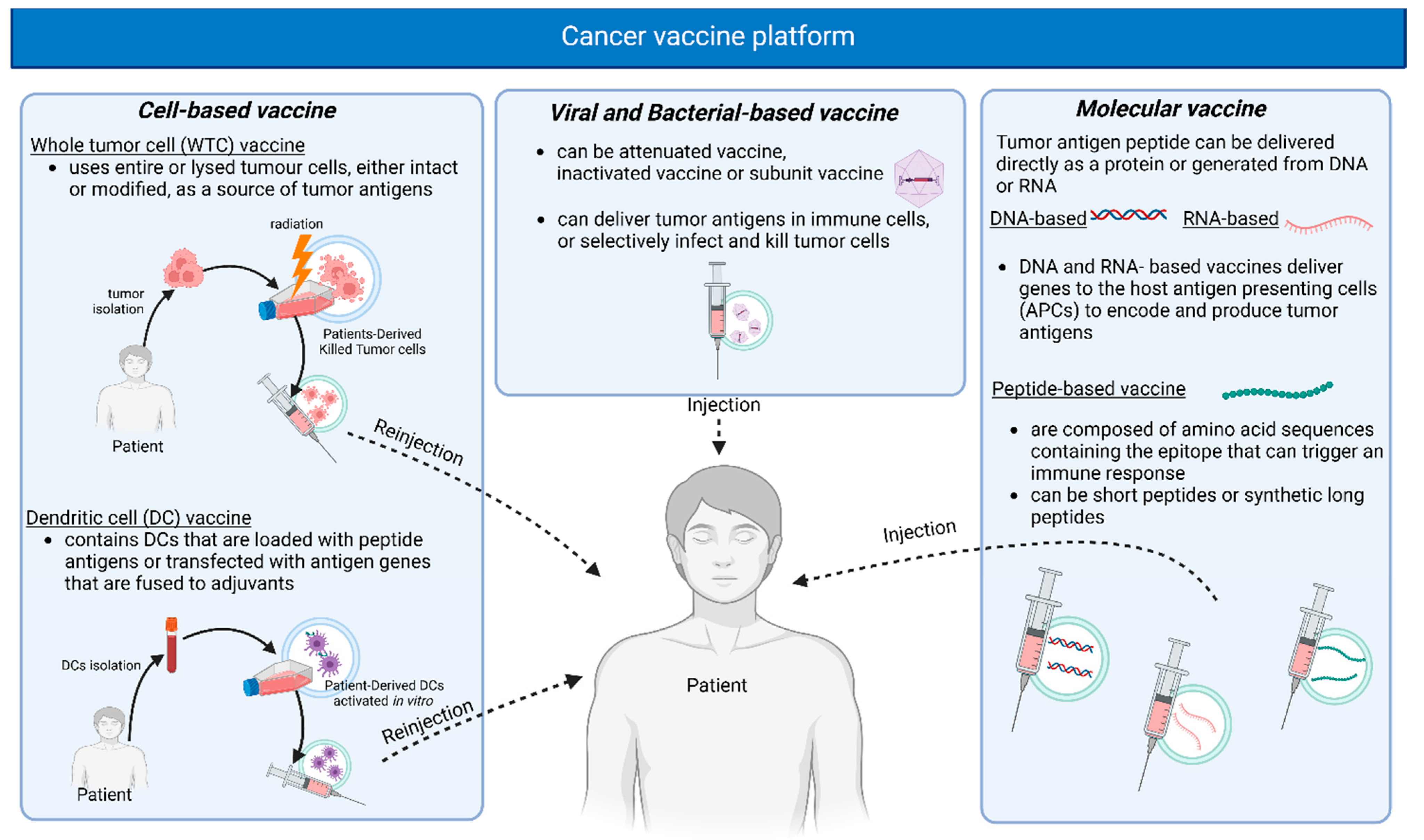
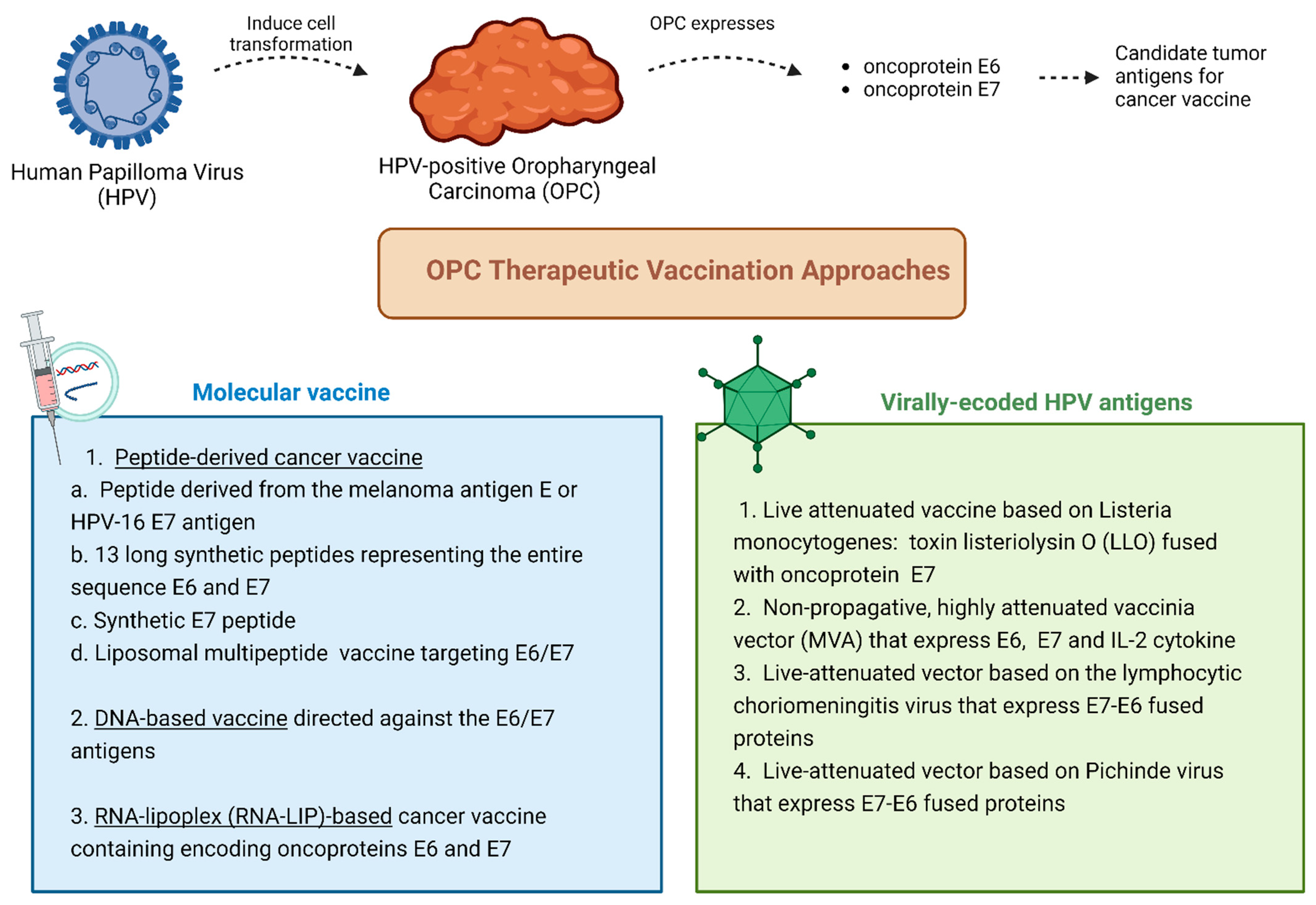
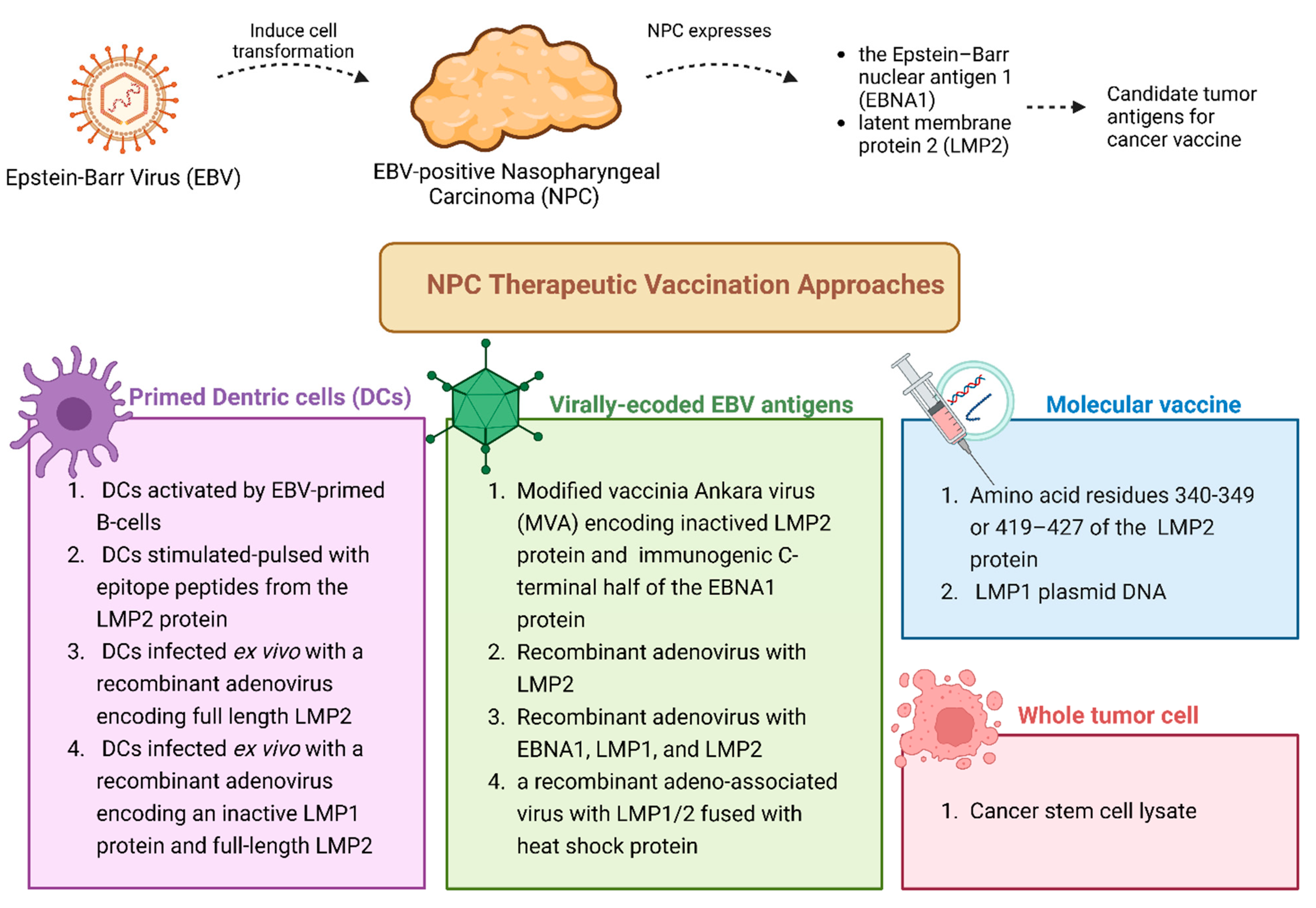
| Cancer Antigen Source | Categories | Target Types | Description | Tumor Specificity | Central Tolerance | Prevalence in Multiple Patients | Examples |
|---|---|---|---|---|---|---|---|
| Tumor cell | Tumor-associated antigens (TAAs) | Differentiation antigens | Antigens expressed during tissue differentiation | Variable | High | High | Melan A, CD19 |
| Overexpressed antigens | Antigens overexpressed on tumor cells compared to normal cells | Variable | High | High | HER2, TROP2 | ||
| Cancer testis antigens | Antigens limitedly expressed on testes, fetal ovaries, and trophoblast | Good | Low | High | MAGE-A3, NY-ESO-1 | ||
| Tumor-specific antigens | Private-personalized neoantigens | Antigens resulting from somatic mutation of patient-specific mutated genes | Ideal | None | Low | Numerous | |
| Shared-public antigens | Antigens resulting from somatic mutation of recurrently mutated genes | Ideal | None | High | KRAS, p53 | ||
| Virus | Oncoviral antigens | Antigens expressed on cancer cells infected with an oncovirus | Ideal | None | High | EBV LMP, HPV E6/7 |
| Class | Target Antigen | Phase | Population/ Setting | Administration | N | ORR | G3/G4 AEs | Other Results | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| ISA101b + CD137 (4-1BB, TNF) agonist utomilumab | Peptide | HPV16 E6/E7 | II | HPV-positive OPCs | sc | 3 | NA | NA | trial closed for infeasibility due to slow accrual | NCT03258008 |
| ISA101b + pembrolizumab + cisplatin+ radiotherapy | Peptide | HPV16 E6/E7 | II | Intermediate risk HPV-positive HNSCC | sc | 50 | NA | NA | NA | NCT04369937 |
| ISA101b + nivolumab | Peptide | HPV16 E6/E7 | II | Incurable HPV16+ cancers | sc | 24 (22 OPC) | 33% | 46% | mPFS: 2.7 mo; mOS 15.3 mo | NCT02426892 [61,67] |
| ISA101b + cemiplimab (vs. cemiplimab) | Peptide | HPV16 E6/E7 | II | First and second line anti-PD-1 naïve HPV16+ OPC patients | sc | 91 (in the combination arm) | 25% | 33% |
In CPS ≥ 20: mOS not reached (28.1, -).
In CPS < 20: shorter OS | NCT03669718 [68] |
| ADXS11-001 | Live attenuated Listeria Monocytogenes | HPV16 E7 | II | Surgically elected HPV+ OPC (Prior to Robot-Assisted Resection) | IV | 15 | NA | 56% | 0.625 number of pts with a >2-fold increase in HPV-specific T-cell response | NCT02002182 |
| PRGN-2009 alone or in combination with bintrafusp alfa | novel gorilla adenovirus | HPV 16 and 18 E6/E7 | I | HPV-positive cancers | sc | 17 | 30% (all cohorts) | 0% (dose escalation, all cohorts) 27% (combination cohort, all cohorts) | mOS: 7.4 mo for dose escalation; mOS: 12.5 mo for combination. | NCT04432597 [69] |
| DPX-E7 alone or with cyclophosphamide | synthetic peptide-based vaccine | HPV16 E711-19 | I/II | HPV-positive OPC, cervical and anal cancers (positive for HLA-A*02) | n.d. | 11 | 0% | 33% (phase Ib) 0% (phase II cohort 1) 50% (phase II cohort 2) | 17% (0.6 to 64.1) changes in responders DLT: 0% | NCT02865135 |
| BNT113 plus pembrolizumab (Vs pembrolizumab alone) | RNA | HPV16 E6/E7 | II | R/M HPV16-positive and PD-L1 expressing HNSCC | IV | 12 | NA | 25% | NA | NCT04534205 [70] |
| PDS0101 plus pembrolizumab | Peptide | HPV16 E6/E7 | II | R/M HPV-positive HNSCC (ICI naive PD-L1 ≥ 1 or ICI-pre treated) | sc | 48 | 26%. (ICI naïve) | 13% (ICI naïve) 4% (ICI pre-treated) | mPFS: 10.4mo, 1-yr OS 87% (ICI naïve) | NCT04260126 (VERSATILE-002) [71] |
| PDS0101+ tumor-targeting interleukin-12 (IL-12) fusion protein M9241 (NHS-IL12) + bintrafusp alfa (Triplet) | Peptide | HPV16 E6/E7 | II | Advanced HPV16-positive cancers | sc | 30 (13 OPC) | 7/8 (88%) (checkpoint naïve ); 6/22 (27%) (checkpoint refractory) | 43% (all cohorts) | 6/8 (75%) pts with checkpoint naïve disease and 17/22 (77%) pts with checkpoint refractory disease are alive after a median of 17 and 12 months follow up respectively. | NCT04287868 [72] |
| MEDI0457 + durvalumab | DNA | HPV16 E6/E7 HPV18 E6/E7 | I/IIa | R/M HNSCC | IM | 35 | 22% | 17% | Tumor-infiltrating CD8+ T cells and peripheral HPV-specific T cells were increased | NCT03162224 [73] |
| Intratumoral talimogene laherparepvec (TVEC, HSV-1) + pembrolizumab | oncolytic | genetically modified herpes simplex virus-1 | IB | R/M HNSCC refractory to platinum-based chemotherapy | intradermal | 36 | 17% | 14% | DCR 38.9%; mDOR: 45.9 mo; mPFS:3.0 mo; mOS:5.8 mo. | NCT02626000 [74] |
| CUE-101 (‘T cell engagers’: HLA-A*0201 complex + E7 epitope + IL-2 molecules) +/− Pembrolizumab | peptide epitope derived from the HPV16 E7 protein and 4 molecules of attenuated human interleukin-2 (IL-2) | HPV16 E7-pHLA-IL2-Fc fusion protein | I | HPV16+ R/M HNSCC | IV | 80 (49 in monotherapy and 31 CUE-101 plus pembrolizumab) | Combo 47% Mono 5.3% | 8% lymphocyte count decreased; 6% anemia; 5% decreased appetite; 5% infusion-related reactions | Combo: mPFS of 5.8 mo Mono: mOS of 20.8 mo | NCT03978689 [75,76] |
| Vaccine + Combined Therapy | Class | Target Antigen | Phase of Study | Population/ Setting | N | Administration | G3/G4 AEs | Other Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MVA-EL | Live (MVA virus) | EBNA 1/LMP2 | I | EBV-induced NPC in CR after first-line treatment | 16 | intradermal | 0% | to assess changes in EBV genome levels and EBNA1-specific antibodies in plasma | NCT01147991 [79] |
| MVA-EL | Live (MVA virus) | EBNA 1/LMP2 | I | EBV-induced NPC in CR or unconfirmed CR | 18 | intradermal | 5% | to determine the dose for subsequent efficacy trials | NCT01256853 [91] |
| MVA-EBNA1/LMP2 | Live (MVA virus) | EBNA 1/LMP2 | Ib | EBV-induced NPC in remission or with current disease for whom no standard therapy is required | 22 | intradermal | NA | NA | NCT01800071 |
| MVA-EBNA1/LMP2 vaccine | Live (MVA virus) | EBNA 1/LMP2 | II | R/M NPC with residual EBV DNA following conventional therapy | 25 | NA | NA | clinical benefit rate | NCT01094405 |
| DC vaccine | Autologous DC | LMP2 | NA | stage II-III NPC | 16 | NA | 0% | lymphocyte subsets, serum cytokines, EBV-DNA levels, the delayed-type hypersensitivity (DTH) responses | [82] |
| LMP-2:340–349 or LMP-2:419–427 | peptide | LMP2 | I/II | locally controlled anaplastic NPC | 99 | intradermal | NA | NA | NCT00078494 |
| mRNA Vaccine | mRNA vaccine | none | I | EBV-positive advanced malignant tumors after failure of second-line standard therapy | 9 | IM | 0% | AEs; PFS; OS | NCT05714748 [92] |
| Ad5F35-LMP1/LMP2-transduced autologous DCs | Autologous DCs transduced with adenovirus | LMP1/LMP2 | II | R/M NPC | 16 | intradermal | 0% | - | [84] |
| rAd5-EBV-LMP2 | Autologous DCs transduced with adenovirus | EBV-antigen | I | regional advanced NPC | 24 | IM | 0% | - | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, D.M.; Broseghini, E.; Liberale, C.; Gallerani, G.; Siepe, G.; Nobili, E.; Ferracin, M.; Molteni, G. Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers. J. Clin. Med. 2025, 14, 1170. https://doi.org/10.3390/jcm14041170
Filippini DM, Broseghini E, Liberale C, Gallerani G, Siepe G, Nobili E, Ferracin M, Molteni G. Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers. Journal of Clinical Medicine. 2025; 14(4):1170. https://doi.org/10.3390/jcm14041170
Chicago/Turabian StyleFilippini, Daria Maria, Elisabetta Broseghini, Carlotta Liberale, Giulia Gallerani, Giambattista Siepe, Elisabetta Nobili, Manuela Ferracin, and Gabriele Molteni. 2025. "Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers" Journal of Clinical Medicine 14, no. 4: 1170. https://doi.org/10.3390/jcm14041170
APA StyleFilippini, D. M., Broseghini, E., Liberale, C., Gallerani, G., Siepe, G., Nobili, E., Ferracin, M., & Molteni, G. (2025). Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers. Journal of Clinical Medicine, 14(4), 1170. https://doi.org/10.3390/jcm14041170







