Esophageal Stent in Acute Refractory Variceal Bleeding: A Systematic Review and a Meta-Analysis
Abstract
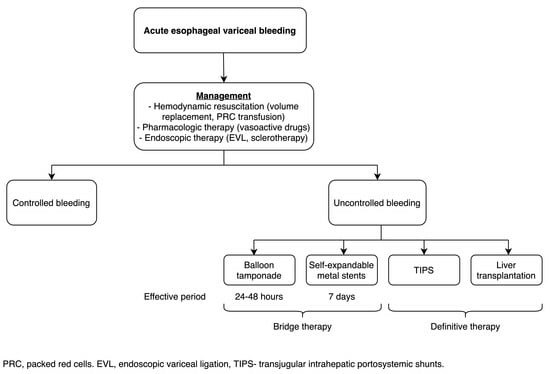
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
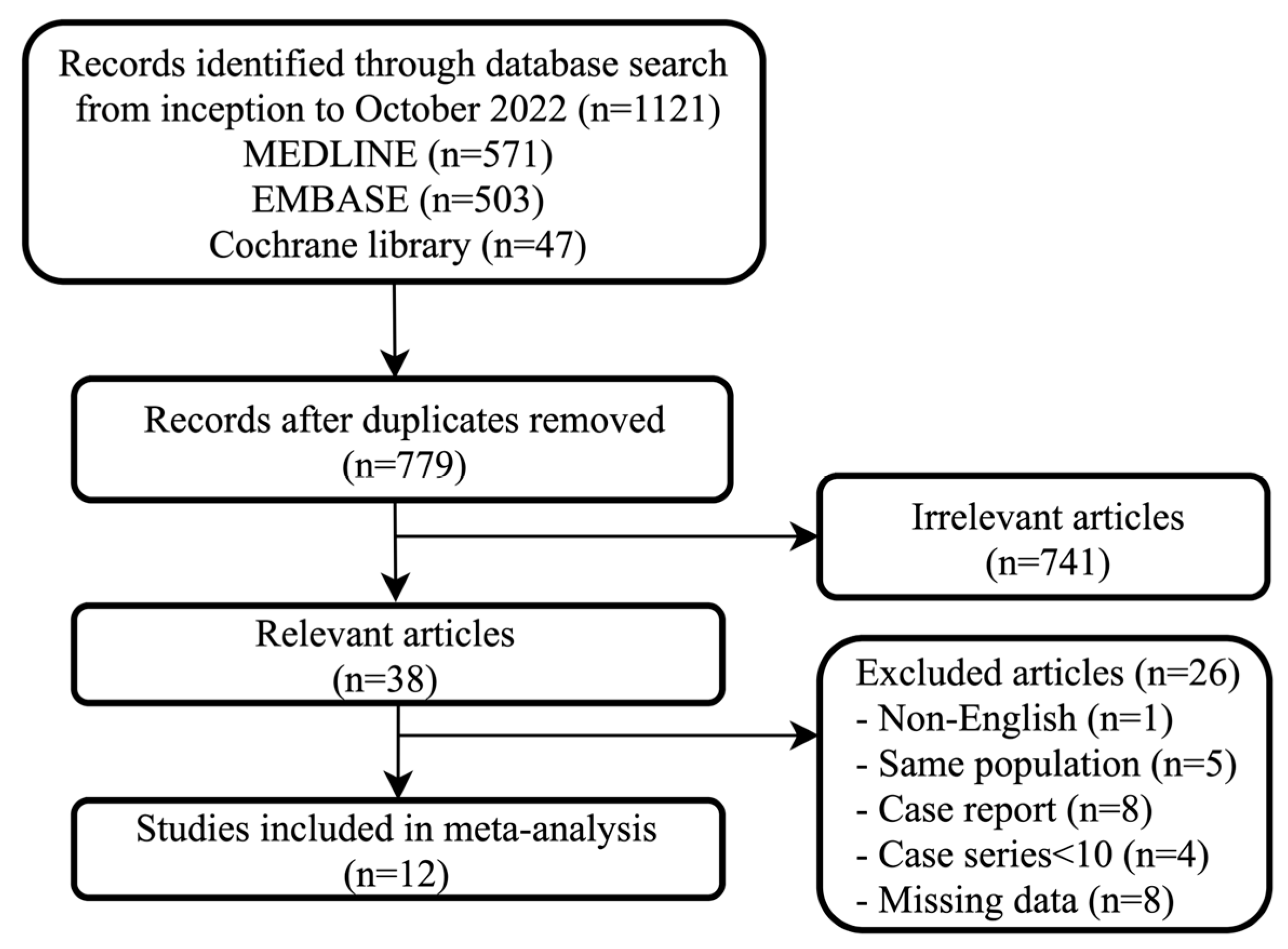
3.1. Search Results and Characteristics of the Included Studies
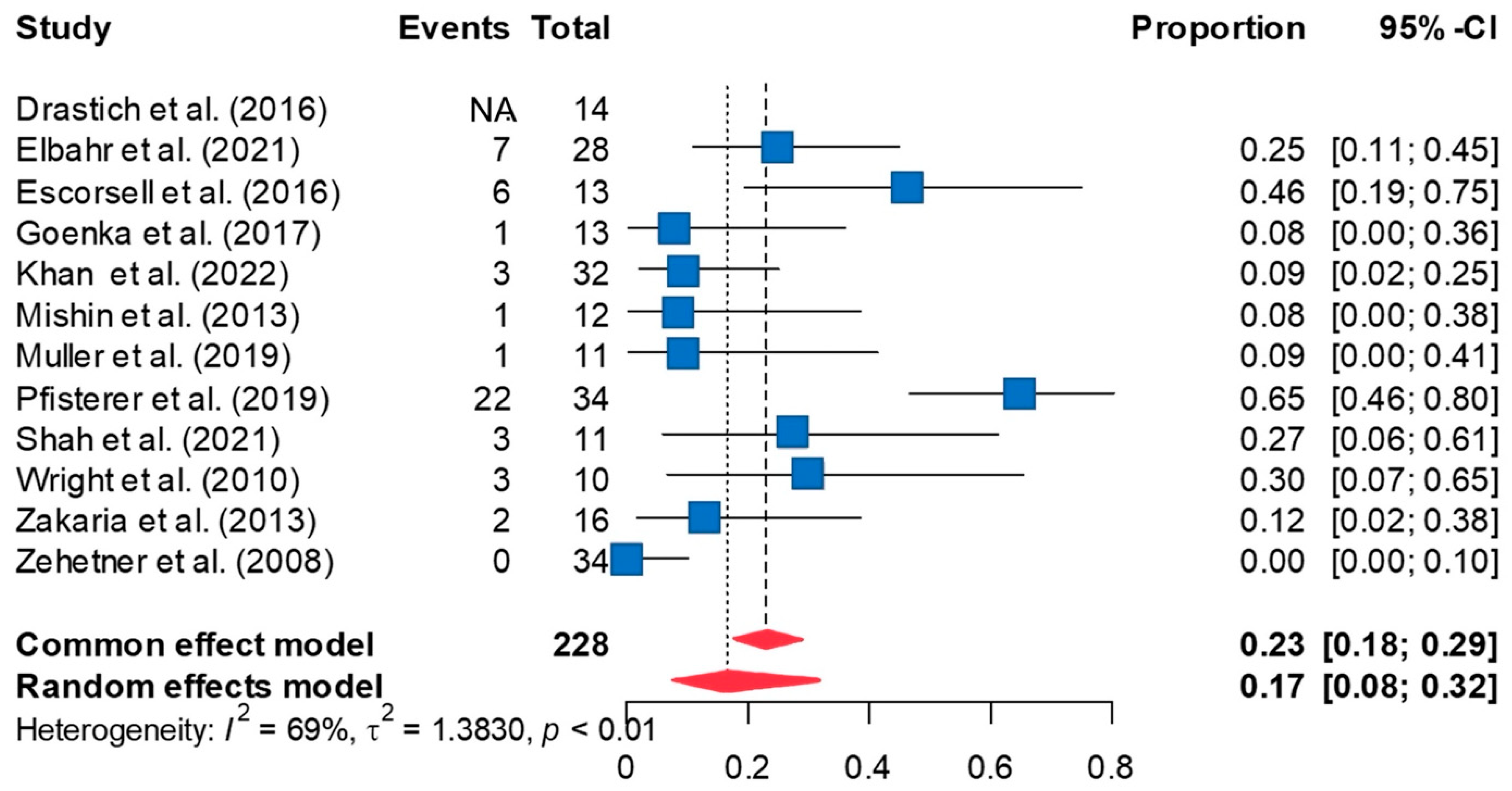
3.2. Successful Controlling of Bleeding
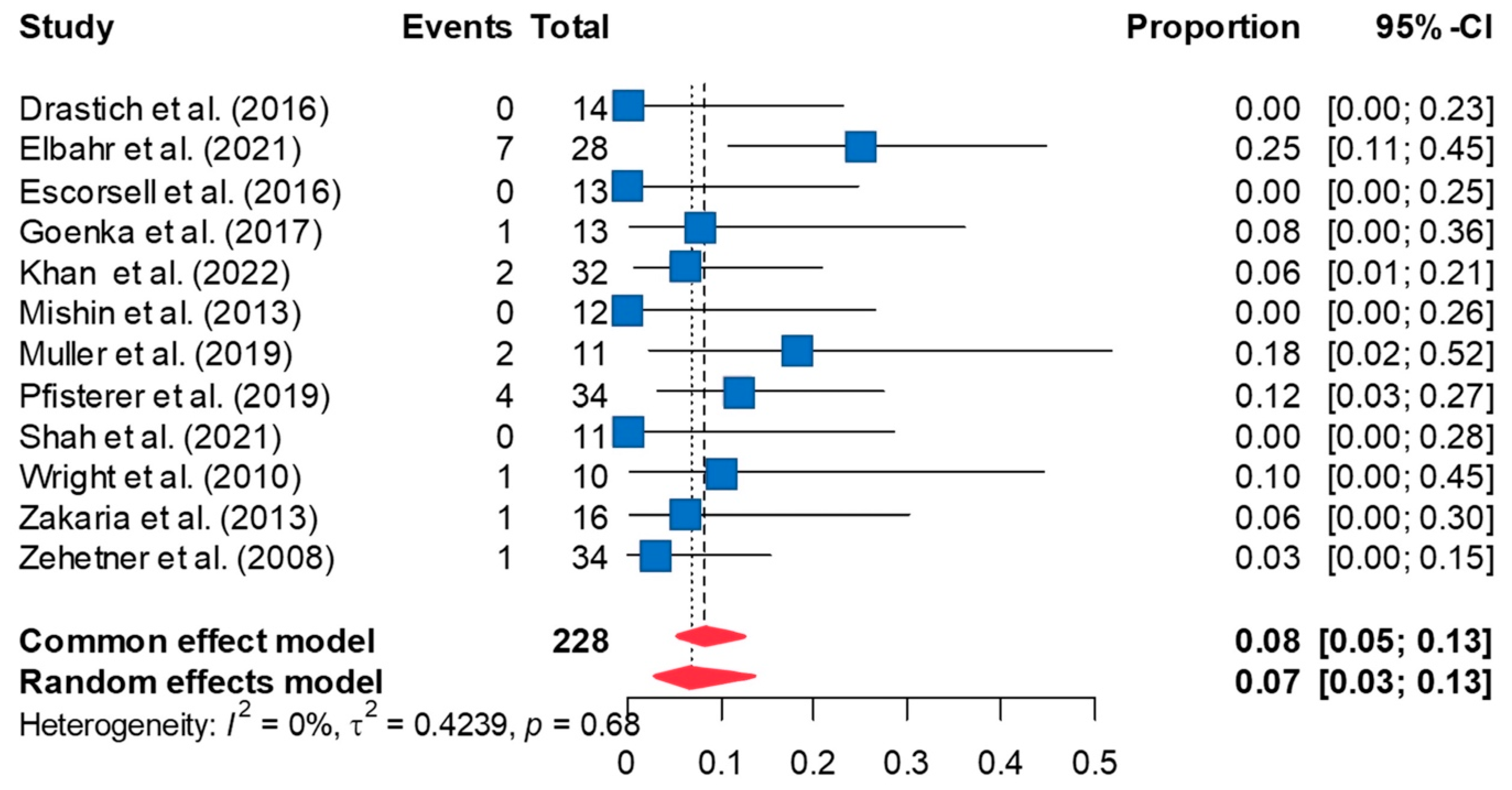
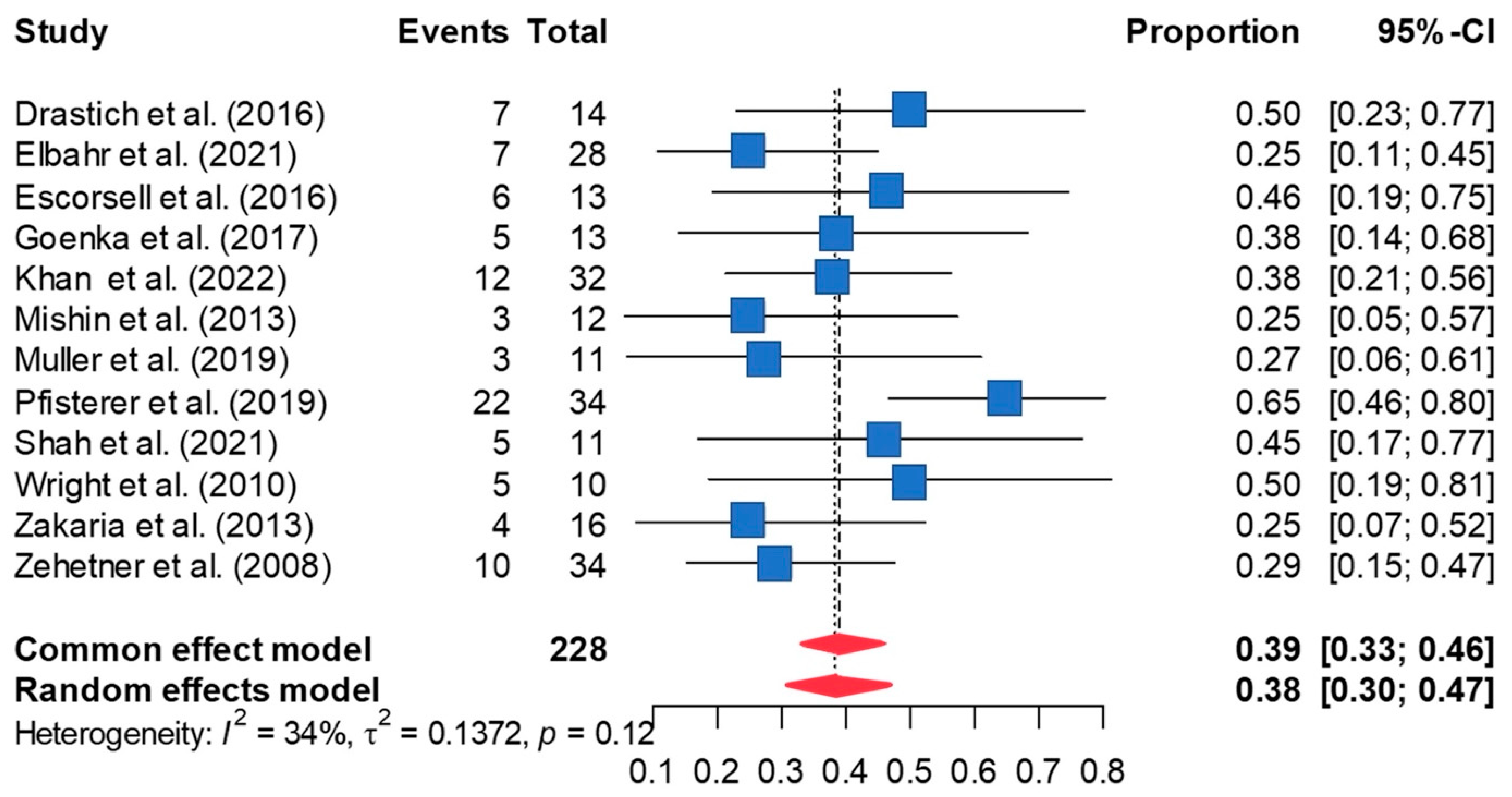
3.3. Rate of Rebleeding
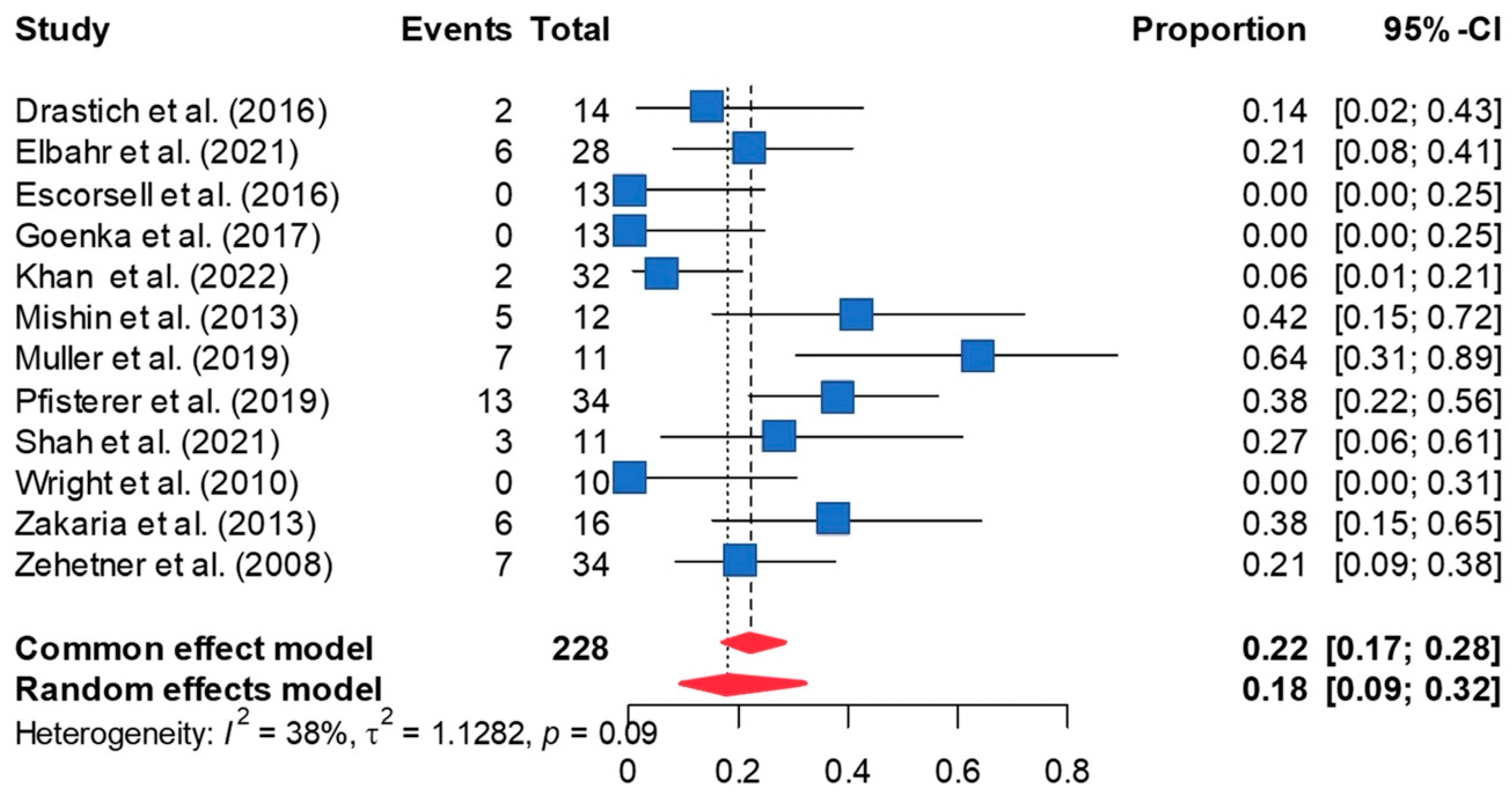
3.4. Rate of Stent Ulceration
3.5. Rate of Stent Migration
3.6. Mortality Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustin, S.; González, A.; Genescà, J. Acute esophageal variceal bleeding: Current strategies and new perspectives. World J. Hepatol. 2010, 2, 261–274. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Hwang, J.H.; Shergill, A.K.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest. Endosc. 2014, 80, 221–227. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Escorsell, À.; Bosch, J. Self-expandable metal stents in the treatment of acute esophageal variceal bleeding. Gastroenterol. Res. Pract. 2011, 2011, 910986. [Google Scholar] [CrossRef] [PubMed]
- Rodge, G.A.; Goenka, U.; Goenka, M.K. Management of Refractory Variceal Bleed in Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Venezia, L.; Michielan, A.; Condino, G.; Sinagra, E.; Stasi, E.; Galeazzi, M.; Fabbri, C.; Anderloni, A. Feasibility and safety of self-expandable metal stent in nonmalignant disease of the lower gastrointestinal tract. World J. Gastrointest. Endosc. 2020, 12, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Escorsell, À.; Pavel, O.; Cárdenas, A.; Morillas, R.; Llop, E.; Villanueva, C.; Garcia-Pagán, J.C.; Bosch, J. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology 2016, 63, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.P.; Chandan, S.; Khan, S.R.; Kotagiri, R.; Olaiya, B.; Ofosu, A.; Adler, D.G. Efficacy of self expanding metal stent (SEMS) in refractory bleeding esophageal varices, is there a mortality benefit? An indirect-comparison meta-analysis to trans-jugular intra-hepatic porto-systemic shunt (TIPS). Am. J. Gastroenterol. 2019, 114, S346–S347. [Google Scholar] [CrossRef]
- Shah, R.; Kesar, V.; Chitnavis, V.; Yeaton, P. Role of self-expandable metal stents in variceal bleeding. Am. J. Gastroenterol. 2021, 116, S311. [Google Scholar] [CrossRef]
- Khan, S.; Gilhotra, R.; Di Jiang, C.; Rowbotham, D.; Chong, A.; Majumdar, A.; White, C.; Huelsen, A.; Brooker, J.; O’Beirne, J.; et al. The role of a novel self-expanding metal stent in variceal bleeding: A multicenter Australian and New Zealand experience. Endosc. Int. Open 2022, 10, E238–E245. [Google Scholar] [CrossRef] [PubMed]
- Elbahr, O.; Saleh, A.; Badra, G.; Elazab, G.; Bedawy, E.; Edrees, A. Self-expandable metal stents (SEMS) for severe bleeding esophageal varices, systematic review and meta-analysis updates. Dig. Endosc. 2020, 32, 86. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. Available online: www.handbook.cochrane.org (accessed on 1 November 2023).
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Drastich, P.; Brezina, J.; Sperl, J.; Frankova, S.; Benes, M.; Spicak, J. Treatment of uncontrollable acute variceal bleeding with self-expanding metal stent: A single center experience. Gastroenterology 2016, 150, S339. [Google Scholar] [CrossRef]
- Goenka, M.K.; Goenka, U.; Tiwary, I.K.; Rai, V. Use of self-expanding metal stents for difficult variceal bleed. Indian J. Gastroenterol. 2017, 36, 468–473. [Google Scholar] [CrossRef]
- Mishin, I.; Zastavnitsky, G.; Ghidirim, G.; Bunic, G. Self-expanding metal stents: A new hemostasis method for bleeding esophageal varices. Hepatol. Int. 2013, 7, S540. [Google Scholar]
- Müller, M.; Seufferlein, T.; Perkhofer, L.; Wagner, M.; Kleger, A. Self-expandable metal stents for persisting esophageal variceal bleeding after band ligation or injection-therapy: A retrospective study. PLoS ONE 2015, 10, e0126525. [Google Scholar] [CrossRef]
- Pfisterer, N.; Riedl, F.; Pachofszky, T.; Gschwantler, M.; König, K.; Schuster, B.; Mandorfer, M.; Gessl, I.; Illiasch, C.; Fuchs, E.M.; et al. Outcomes after placement of a SX-ELLA oesophageal stent for refractory variceal bleeding—A national multicentre study. Liver Int. 2019, 39, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.; Lewis, H.; Hogan, B.; Burroughs, A.; Patch, D.; O’Beirne, J. A self-expanding metal stent for complicated variceal hemorrhage: Experience at a single center. Gastrointest. Endosc. 2010, 71, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.S.; Hamza, I.M.; Mohey, M.A.; Hubamnn, R.G. The first Egyptian experience using new self-expandable metal stents in acute esophageal variceal bleeding: Pilot study. Saudi J. Gastroenterol. 2013, 19, 177–181. [Google Scholar] [PubMed]
- Zehetner, J.; Shamiyeh, A.; Wayand, W.; Hubmann, R. Results of a new method to stop acute bleeding from esophageal varices: Implantation of a self-expanding stent. Surg. Endosc. 2008, 22, 2149–2152. [Google Scholar] [CrossRef]
- Cremers, I.; Ribeiro, S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Ther. Adv. Gastroenterol. 2014, 7, 206–216. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, A.; Angus, P.W.; Baijal, S.S.; Baik, S.K.; Bayraktar, Y.; Chawla, Y.K.; Choudhuri, G.; Chung, J.W.; de Franchis, R.; et al. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol. Int. 2011, 5, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Raman, S.S.; Yu, N.C.; To’o, K.J.; Jutabha, R.; Lu, D.S.K. Esophageal Varices in Cirrhotic Patients: Evaluation with Liver CT. Am. J. Roentgenol. 2007, 188, 139–144. [Google Scholar] [CrossRef]
- Shao, X.D.; Qi, X.S.; Guo, X.Z. Esophageal Stent for Refractory Variceal Bleeding: A Systemic Review and Meta-Analysis. Biomed. Res Int. 2016, 2016, 4054513. [Google Scholar] [CrossRef]
- Sengstaken, R.W.; Blakemore, A.H. Balloon tamponage for the control of hemorrhage from esophageal varices. Ann. Surg. 1950, 131, 781. [Google Scholar] [CrossRef]
- Kumbhari, V.; Saxena, P.; Khashab, M.A. Self-expandable metallic stents for bleeding esophageal varices. Saudi J. Gastroenterol. 2013, 19, 141–143. [Google Scholar]
- Mohan, B.P.; Chandan, S.; Khan, S.R.; Kotagiri, R.; Kassab, L.L.; Olaiya, B.; Ponnada, S.; Ofosu, A.; Adler, D.G. Self-expanding metal stents versus TIPS in treatment of refractory bleeding esophageal varices: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E291–E300. [Google Scholar] [CrossRef]

| Author | Year | Country | Publication Type | Study Type | Number of Patients | Mean Age | Male | Child–Pugh Score | MELD Score | Etiology of Cirrhosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Drastich [20] | 2016 | Czech | Abstract | Retrospective | 14 | 52.9 | 7 | NR | NR | NR |
| Elbahr [12] | 2021 | Egypt | Full paper | Retrospective | 28 | 57.8 | 24 | A (3), B (15), C (10) | 15.7 ± 6.3 | HBV (24), HCV (4) |
| Escorsell [8] | 2016 | Spain | Full paper | Prospective | 13 | 69.0 * | 13 | A (3), B and C (10) | 16.5 (9–32) * | Alcohol (8), HCV (3), others (2) |
| Goenka [21] | 2017 | India | Full paper | Retrospective | 12 | 53.0 | 11 | NR | 20.2 ± 6.0 (14–35) | Alcohol (4), HBV (1), HCV (3), NASH (1), others (3) |
| Khan [11] | 2022 | Australia | Full paper | Retrospective | 30 | 53.3 * | 20 | NR | 20.3 (7–40) | Alcohol (15), HBV (4), alcohol and HCV (3), HCV (1), NASH (3), others (4) |
| Mishin [22] | 2013 | Moldova | Abstract | Retrospective | 12 | 46.9 | 8 | NR | NR | NR |
| Muller [23] | 2015 | Germany | Full paper | Retrospective | 11 | 64.2 | 8 | A (1), B (6), C (3) | 16.8 (8–36) | Alcohol (9), HBV (1), others (2) |
| Pfisterer [24] | 2019 | Austria | Full paper | Retrospective | 34 | 55.5 | 28 | A (1), B (10), C (8) | 18 * IQR 10 | Alcohol (16), viral hepatitis (8), alcohol and viral (4), others (6) |
| Shah [10] | 2021 | USA | Abstract | Retrospective | 11 | 58.8 | 8 | NR | NR | Alcohol (5), HCV (1), NASH (4), other (1) |
| Wright [25] | 2010 | UK | Full paper | Case series | 10 | 49.4 | 9 | NR | 32 (23–39) * | Alcohol (6), alcohol and HCV (2), others (2) |
| Zakaria [26] | 2013 | Egypt | Full paper | Prospective | 16 | 55.6 | 14 | A (2), B (8), C (6) | NR | NR |
| Zehetner [27] | 2008 | Austria | Full paper | Retrospective | 34 | 56.0 | 33 | A (0), B (13), C (21) | NR | Alcohol (26), viral hepatitis (4), others (4) |
| Author | Year | SEMS Type | Number of Patients | Successful Control of Bleeding | Stent Migration | Ulceration | Rebleeding | Mortality | Success of Deployment | Follow-Up(Days) | Stent Indwell Time (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drastich [20] | 2016 | SX-ELLA Danis | 14 | 11 | 2 | 0 | NR | 7 | 9 | 180 | 9.5 |
| Elbahr [12] | 2021 | NITI-S Mega stents-Tae Wong-S Korea | 28 | 23 | 6 | 7 | 7 | 7 | NR? | 42 | NR |
| Escorsell [8] | 2016 | SX-ELLA Danis | 13 | 11 | 0 | 0 | 6 | 6 | 12 | 42 | 7 |
| Goenka [21] | 2017 | SX-ELLA Danis | 12 * | 13 | 0 | 1 | 1 | 5 | 13 | 30 | 17.5 |
| Khan [11] | 2022 | SX-ELLA Danis | 30 | 31 | 2 | 2 | 3 | 12 | 31 (from 32 stents) | 42 | 6.4 |
| Mishin [22] | 2013 | SX-ELLA Danis | 12 | 12 | 5 | 0 | 1 | 3 | 12 | 30 | NR |
| Muller [23] | 2015 | SX-ELLA Danis | 11 | 11 | 7 | 2 | 1 | 3 | 11 | 42 | 12.1 |
| Pfisterer [24] | 2019 | SX-ELLA Danis | 34 | 27 | 13 | 4 | 22 | 22 | NR | 42 | 5 |
| Shah [10] | 2021 | NR | 11 | 8 | 3 | 0 | 3 | 5 | NR | 42 | 13.4 |
| Wright [25] | 2010 | SX-ELLA Danis | 10 | 7 | 0 | 1 | 3 | 5 | 9 | 42 | 9 |
| Zakaria [26] | 2013 | SX-ELLA Danis | 16 | 14 | 6 | 1 | 2 | 4 | NR | 2–4 | |
| Zehetner [27] | 2008 | SX-ELLA Danis | 34 | 34 | 7 | 1 | 0 | 10 | 34 | 60 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songtanin, B.; Kahathuduwa, C.; Nugent, K. Esophageal Stent in Acute Refractory Variceal Bleeding: A Systematic Review and a Meta-Analysis. J. Clin. Med. 2024, 13, 357. https://doi.org/10.3390/jcm13020357
Songtanin B, Kahathuduwa C, Nugent K. Esophageal Stent in Acute Refractory Variceal Bleeding: A Systematic Review and a Meta-Analysis. Journal of Clinical Medicine. 2024; 13(2):357. https://doi.org/10.3390/jcm13020357
Chicago/Turabian StyleSongtanin, Busara, Chanaka Kahathuduwa, and Kenneth Nugent. 2024. "Esophageal Stent in Acute Refractory Variceal Bleeding: A Systematic Review and a Meta-Analysis" Journal of Clinical Medicine 13, no. 2: 357. https://doi.org/10.3390/jcm13020357