Adipose Tissue-Derived Minimally Manipulated Products versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence and Meta-Analysis
Abstract
:1. Introduction
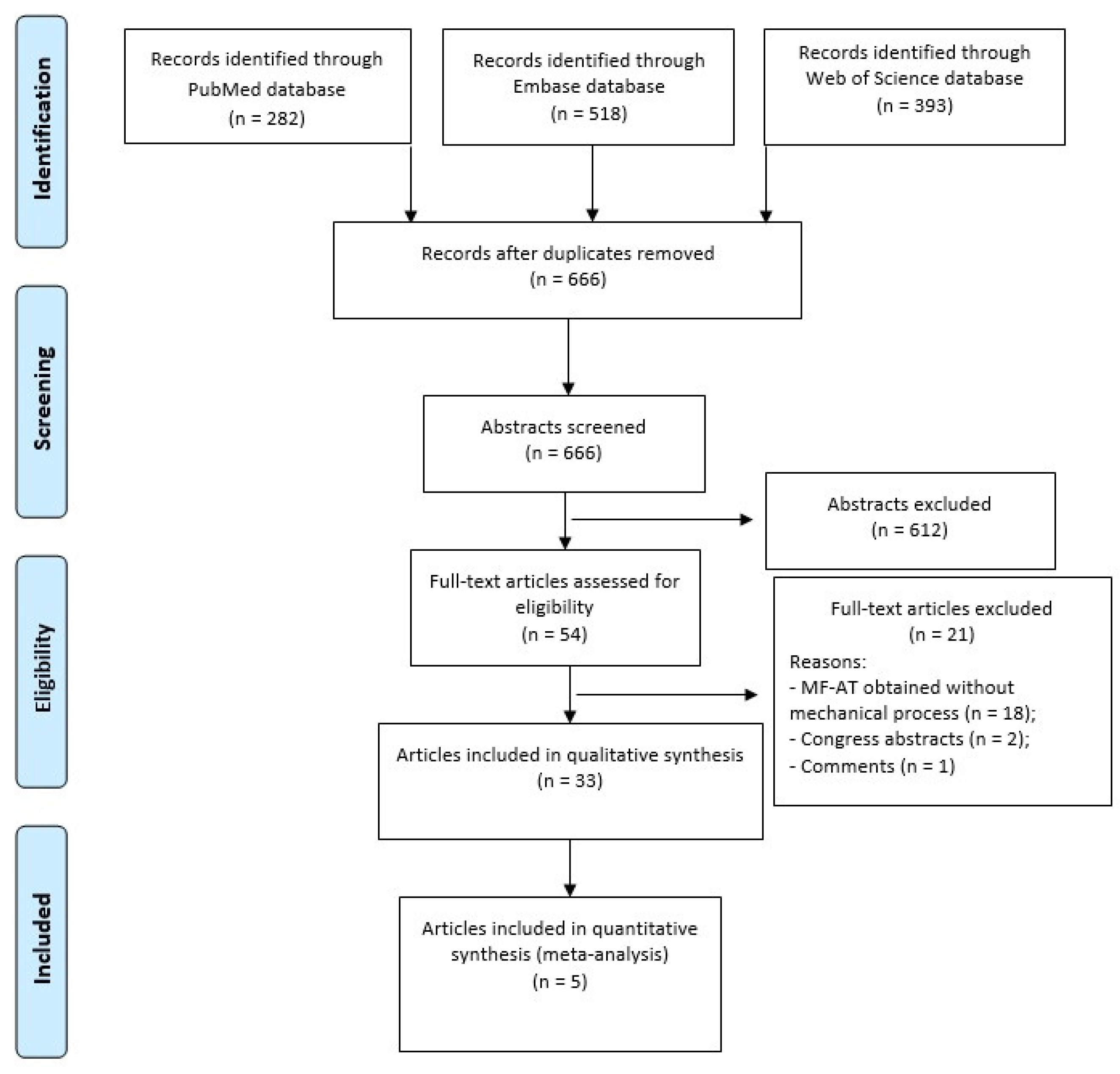
2. Materials and Methods
2.1. Data Source and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias
2.5. Quantitative Synthesis and Statistical Analysis
3. Results
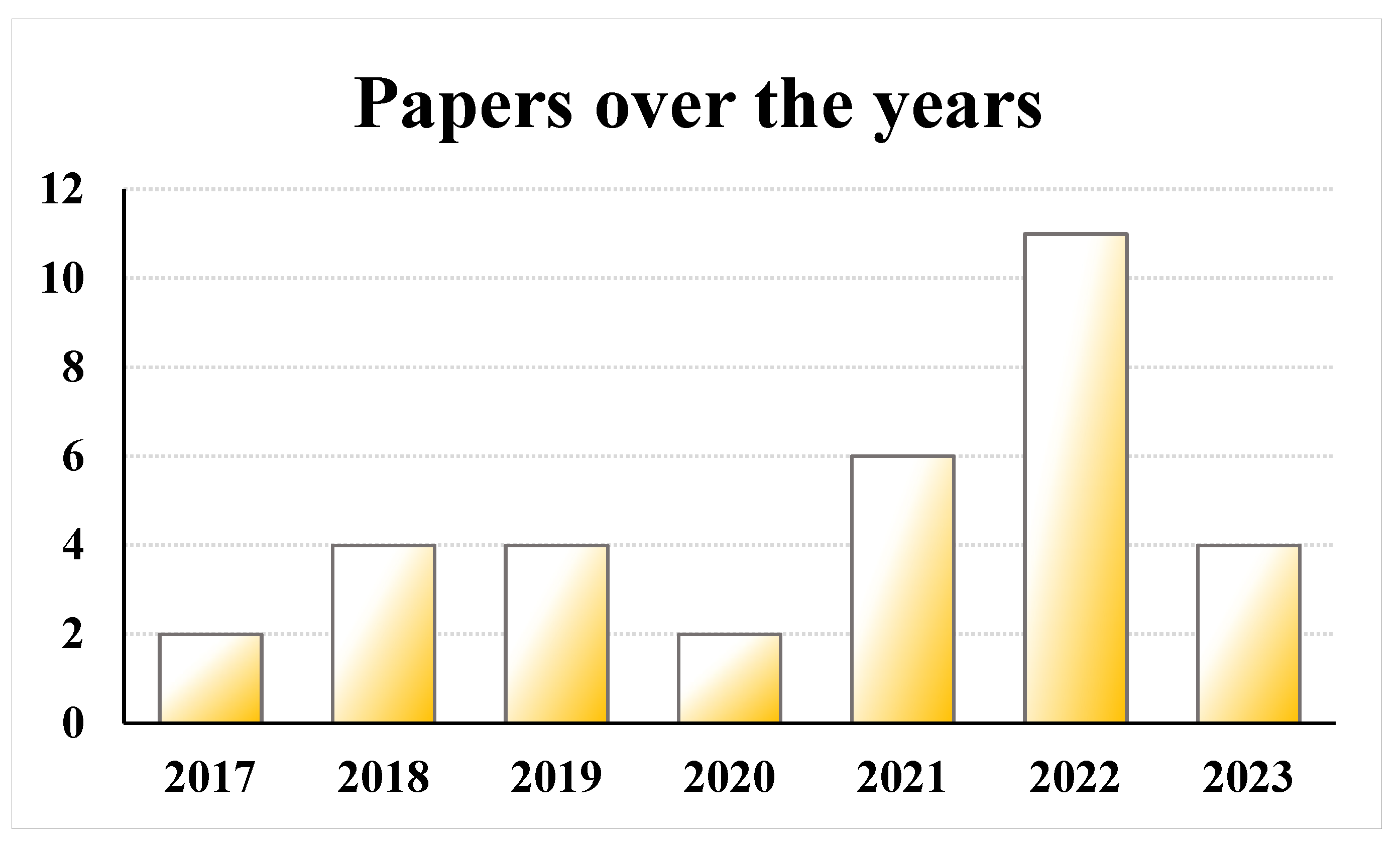
3.1. Study Type
3.2. Patient Characteristics
3.3. MM-AT Characteristics and Treatment
3.4. Safety and Complications
3.5. Qualitative Analysis
3.5.1. Non-Comparative Studies: MM-AT Injection
3.5.2. Comparative Studies: MM-AT Injection vs. BMAC Injection
3.5.3. Comparative Studies: MM-AT Injection Augmentation to Surgical Procedures
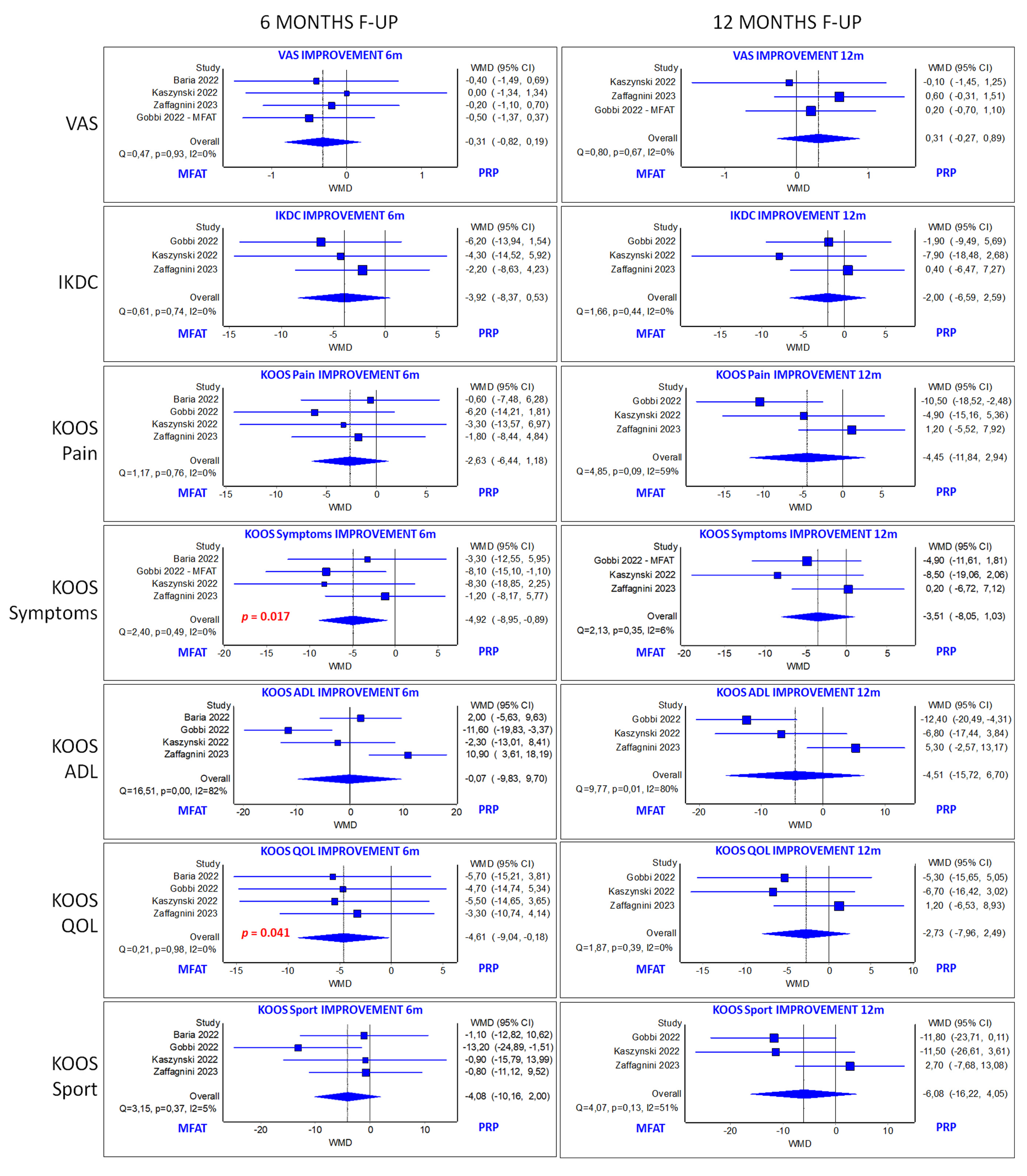
3.6. Quantitative Analysis: MM-AT vs. PRP
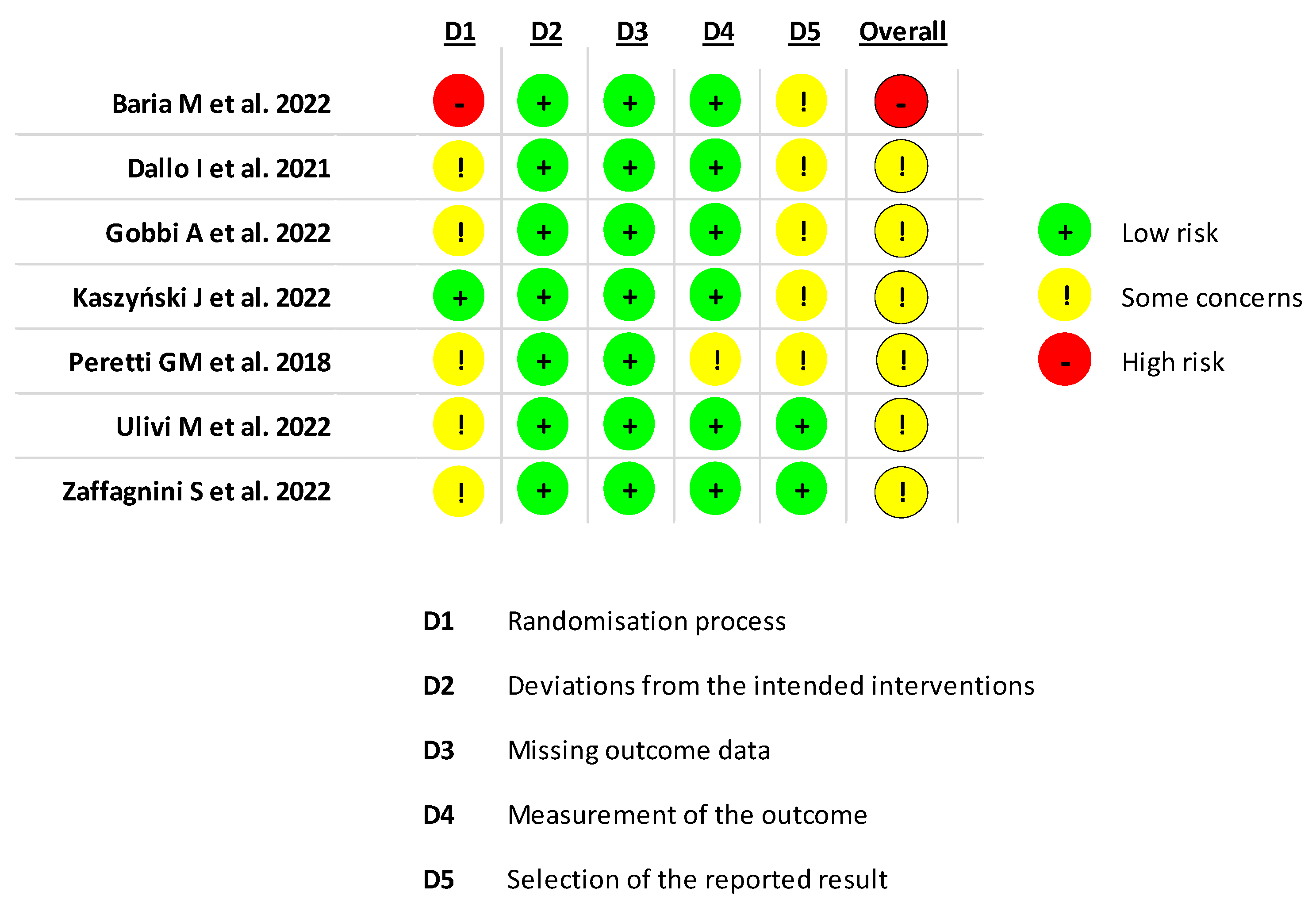
3.7. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagani, S.; Veronesi, F.; Giavaresi, G.; Filardo, G.; Papio, T.; Romandini, I.; Fini, M. Autologous Protein Solution Effect on Chondrogenic Differentiation of Mesenchymal Stem Cells from Adipose Tissue and Bone Marrow in an Osteoarthritic Environment. Cartilage 2021, 13, 225S–237S. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Allaeys, C.; Arnout, N.; Van Onsem, S.; Govaers, K.; Victor, J. Conservative treatment of knee osteoarthritis. Acta Orthop. Belg. 2020, 86, 412–421. [Google Scholar]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef] [PubMed]
- Piontek, T.; Ciemniewska-Gorzela, K.; Szulc, A. All-arthroscopic technique of biological meniscal tear. Pol. Orthop. Traumatol. 2012, 77, 39–45. [Google Scholar]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Wallis, J.A.; Barton, C.J.; Brusco, N.K.; Kemp, J.L.; Sherwood, J.; Young, K.; Jennings, S.; Trivett, A.; Ackerman, I.N. Exploring views of orthopaedic surgeons, rheumatologists and general practitioners about osteoarthritis management. Musculoskelet. Care 2021, 19, 524–532. [Google Scholar] [CrossRef]
- Maheshwer, B.; Polce, E.M.; Paul, K.; Williams, B.T.; Wolfson, T.S.; Yanke, A.; Verma, N.N.; Cole, B.J.; Chahla, J. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: A systematic review and metaanalysis. Arthroscopy 2021, 37, 362–378. [Google Scholar] [CrossRef]
- Ha, C.W.; Park, Y.B.; Kim, S.H.; Lee, H.J. Intra-articular mesenchymal stem cells in osteoarthritis of the knee: A systematic review of clinical outcomes and evidence of cartilage repair. Arthrosc. J. Arthrosc. Relat. Surg. Off. Public Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2019, 35, 277–288.e2. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal stem cells in osteoarthritis therapy: A review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar] [PubMed]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Mesenchymal stromal cell products for intra-articular knee injections for conservative management of osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X21996953. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, Y.G.; Choi, Y.J.; Kim, S.H.; Yoon, D.S.; Lee, M.; Lee, J.W. Characterization of adipose tissue derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; Perucca Orfei, C.; Sourugeon, Y.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; de Girolamo, L.; Filardo, G. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 2: Bone marrow-derived cell-based injectable therapies. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3230–3242. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; Del Romero, A.; Peláez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef]
- Migliorini, F.; Rath, B.; Colarossi, G.; Driessen, A.; Tingart, M.; Niewiera, M.; Eschweiler, J. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: Results at 12-months follow-up: A systematic review of the literature. Arch. Orthop. Trauma. Surg. 2020, 140, 853–868. [Google Scholar] [CrossRef]
- Di Matteo, B.; El Araby, M.M.; D’Angelo, A.; Iacono, F.; Nannini, A.; Vitale, N.D.; Marcacci, M.; Respizzi, S.; Kon, E. Adipose-derived stem cell treatments and formulations. Clin. Sports Med. 2019, 38, 61–78. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Yang, Q.; Liu, X.; Fang, J.; Dai, X. Autologous Micro-Fragmented Adipose Tissue as Stem Cell-Based Natural Scaffold for Cartilage Defect Repair. Cell Transplant. 2019, 28, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Ivone, A.; Fioruzzi, A.; Jannelli, E.; Castelli, A.; Ghiara, M.; Ferranti Calderoni, E.; Fontana, A. Micro-fragmented Adipose Tissue Transplantation (MATT) for the treatment of acetabular delamination. A two years follow up comparison study with microfractures. Acta Biomed. 2019, 90, 69–75. [Google Scholar] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovi’c, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-05724-7. [Google Scholar]
- Aletto, C.; Giordano, L.; Quaranta, M.; Zara, A.; Notarfrancesco, D.; Maffulli, N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Barfod, K.W.; Blønd, L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan. Med. J. 2019, 66, A5565. [Google Scholar]
- Baria, M.; Pedroza, A.; Kaeding, C.; Durgam, S.; Duerr, R.; Flanigan, D.; Borchers, J.; Magnussen, R. Platelet-Rich Plasma Versus Microfragmented Adipose Tissue for Knee Osteoarthritis: A Randomized Controlled Trial. Orthop. J. Sports Med. 2022, 10, 23259671221120678. [Google Scholar] [CrossRef]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Z.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef]
- Cattaneo, G.; De Caro, A.; Napoli, F.; Chiapale, D.; Trada, P.; Camera, A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 176. [Google Scholar] [CrossRef]
- Dallo, I.; Szwedowski, D.; Mobasheri, A.; Irlandini, E.; Gobbi, A. A Prospective Study Comparing Leukocyte-Poor Platelet-Rich Plasma Combined with Hyaluronic Acid and Autologous Microfragmented Adipose Tissue in Patients with Early Knee Osteoarthritis. Stem Cells Dev. 2021, 30, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Grant, R.A.; Whitehead, J.P.; Yewlett, A.; Lee, P.Y.F. An observational study evaluating the efficacy of microfragmented adipose tissue in the treatment of osteoarthritis. Regen. Med. 2023, 18, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Alessio-Mazzola, M.; Sonzogni, B.; Stambazzi, C.; Ursino, C.; Roato, I.; Mussano, F.; Bistolfi, A.; Furlan, S.; Godio, L.; et al. Age and synovitis affect the results of the treatment of knee osteoarthritis with Microfragmented Autologous Fat Tissue. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, A.; Selleri, F.; Zambianchi, F.; Cataldo, G.; Francioni, E.; Catani, F. Autologous micro-fragmented adipose tissue associated with arthroscopy in moderate-severe knee osteoarthritis: Outcome at two year follow-up. BMC Musculoskelet. Disord. 2022, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Dallo, I.; Rogers, C.; Striano, R.D.; Mautner, K.; Bowers, R.; Rozak, M.; Bilbool, N.; Murrell, W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: A multi-centric, international study. Int. Orthop. 2021, 45, 1179–1188. [Google Scholar] [CrossRef]
- Gobbi, A.; Dallo, I.; D’Ambrosi, R. Autologous microfragmented adipose tissue and leukocyte-poor platelet-rich plasma combined with hyaluronic acid show comparable clinical outcomes for symptomatic early knee osteoarthritis over a two-year follow-up period: A prospective randomized clinical trial. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 1895–1904. [Google Scholar]
- Heidari, N.; Borg, T.M.; Olgiati, S.; Slevin, M.; Danovi, A.; Fish, B.; Wilson, A.; Noorani, A. Microfragmented Adipose Tissue Injection (MFAT) May Be a Solution to the Rationing of Total Knee Replacement: A Prospective, Gender-Bias Mitigated, Reproducible Analysis at Two Years. Stem Cells Int. 2021, 9, 9921015. [Google Scholar] [CrossRef]
- Heidari, N.; Noorani, A.; Slevin, M.; Cullen, A.; Stark, L.; Olgiati, S.; Zerbi, A.; Wilson, A. Patient-Centered Outcomes of Microfragmented Adipose Tissue Treatments of Knee Osteoarthritis: An Observational, Intention-to-Treat Study at Twelve Months. Stem Cells Int. 2020, 2020, 8881405. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Z.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef]
- Kaszyński, J.; Bąkowski, P.; Kiedrowski, B.; Stołowski, L.; Wasilewska-Burczyk, A.; Grzywacz, K.; Piontek, T. Intra-Articular Injections of Autologous Adipose Tissue or Platelet-Rich Plasma Comparably Improve Clinical and Functional Outcomes in Patients with Knee Osteoarthritis. Biomedicines 2022, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.A.; Chirichella, P.S.; Hogaboom, N.S.; Capella, T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: A prospective pilot study. Int. Orthop. 2021, 45, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Magnanelli, S.; Screpis, D.; Di Benedetto, P.; Natali, S.; Causero, A.; Zorzi, C. Open-Wedge High Tibial Osteotomy Associated With Lipogems® Intra-Articular Injection For The Treatment Of Varus Knee Osteoarthritis—Retrospective Study. Acta Biomed. 2020, 91, e2020022. [Google Scholar]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.R.; Lee, J.W.; Mistretta, K.L.; Desale, S.; Boucher, H.R. Conversion to Knee Arthroplasty Following Intra-Articular Injection of Microfragmented Adipose Tissue in Patients with Knee Osteoarthritis. Muscles Ligaments Tendons J. MLTJ 2022, 12, 165–172. [Google Scholar] [CrossRef]
- Panchal, J.; Malanga, G.; Sheinkop, M. Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees. Am. J. Orthop. 2018, 47. [Google Scholar]
- Peretti, G.M.; Ulivi, M.; De Girolamo, L.; Meroni, V.; Lombardo, M.D.; Mangiavini, L. Evaluation of the use of autologous micro-fragmented adipose tissue in the treatment of knee osteoarthritis: Preliminary results of a randomized controlled trial. J. Biol. Regul. Homeost. Agents 2018, 32, 193–199. [Google Scholar] [PubMed]
- Pintore, A.; Notarfrancesco, D.; Zara, A.; Oliviero, A.; Migliorini, F.; Oliva, F.; Maffulli, N. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: A prospective comparative clinical trial. J. Orthop. Surg. Res. 2023, 18, 350. [Google Scholar] [CrossRef]
- Priano, V.; Priano, F. Autologous adipose tissue enriched in stromal vascular fraction in knee osteoarthritis. Minerva Orthop. 2022, 73, 416–425. [Google Scholar] [CrossRef]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Orthop. 2017, 4, 33. [Google Scholar] [CrossRef]
- Russo, A.; Screpis, D.; Di Donato, S.L.; Bonetti, S.; Piovan, G.; Zorzi, C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: An update at 3 year follow-up. J. Exp. Orthop. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Santoprete, S.; Marchetti, F.; Rubino, C.; Bedini, M.G.; Nasto, L.A.; Cipolloni, V.; Pola, E. Fresh autologous stromal tissue fraction for the treatment of knee osteoarthritis related pain and disability. Orthop. Rev. (Pavia) 2021, 13, 9161. [Google Scholar] [CrossRef]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, M.; Meroni, V.; Viganò, M.; Colombini, A.; Lombardo, M.D.M.; Rossi, N.; Orlandini, L.; Messina, C.; Sconfienza, L.M.; Peretti, G.M.; et al. Micro-fragmented adipose tissue (mFAT) associated with arthroscopic debridement provides functional improvement in knee osteoarthritis: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3079–3090. [Google Scholar] [CrossRef] [PubMed]
- Van Genechten, W.; Vuylsteke, K.; Martinez, P.R.; Swinnen, L.; Sas, K.; Verdonk, P. Autologous Micro-Fragmented Adipose Tissue (MFAT) to Treat Symptomatic Knee Osteoarthritis: Early Outcomes of a Consecutive Case Series. J. Clin. Med. 2021, 10, 2231. [Google Scholar] [CrossRef] [PubMed]
- Vasso, M.; Corona, K.; Capasso, L.; Toro, G.; Schiavone Panni, A. Intraarticular injection of microfragmented adipose tissue plus arthroscopy in isolated primary patellofemoral osteoarthritis is clinically effective and not affected by age, BMI, or stage of osteoarthritis. J. Orthop. Traumatol. 2022, 23, 7. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, Q.; Li, S.; Liu, M.; Sun, H.; Li, L.; Han, K.; Liu, P. Intra-Articular Injection of Autologous Micro-Fragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: A Prospective Interventional Study. J. Pers. Med. 2023, 13, 504. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Andriolo, L.; Boffa, A.; Poggi, A.; Cenacchi, A.; Busacca, M.; Kon, E.; Filardo, G.; Di Martino, A. Microfragmented Adipose Tissue Versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up. Am. J. Sports Med. 2022, 50, 2881–2892. [Google Scholar] [CrossRef]
- Boffa, A.; Andriolo, L.; Franceschini, M.; Di Martino, A.; Asunis, E.; Grassi, A.; Zaffagnini, S.; Filardo, G. Minimal Clinically Important Difference and Patient Acceptable Symptom State in Patients With Knee Osteoarthritis Treated With PRP Injection. Orthop. J. Sports Med. 2021, 9, 23259671211026242. [Google Scholar] [CrossRef]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2021, 13, 364S–375S. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- Fernández-Pernas, P.; Barrachina, L.; Marquina, M.; Rodellar, C.; Arufe, M.C.; Costa, C. Mesenchymal stromal cells for articular cartilage repair: Preclinical studies. Eur. Cell Mater. 2020, 40, 88–114. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef]
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone marrow concentrate injections for the treatment of osteoarthritis: Evidence from preclinical findings to the clinical application. Int. Orthop. 2021, 45, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Tschon, M.; Perdisa, F.; Brogini, S.; Cavallo, C.; Desando, G.; Giavaresi, G.; Grigolo, B.; Martini, L.; Nicoli Aldini, N.; et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: A comparative preclinical study. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Desando, G.; Bartolotti, I.; Martini, L.; Giavaresi, G.; Nicoli Aldini, N.; Fini, M.; Roffi, A.; Perdisa, F.; Filardo, G.; Kon, E.; et al. Regenerative Features of Adipose Tissue for Osteoarthritis Treatment in a Rabbit Model: Enzymatic Digestion Versus Mechanical Disruption. Int. J. Mol. Sci. 2019, 20, 2636. [Google Scholar] [CrossRef] [PubMed]
- Polancec, D.; Zenic, L.; Hudetz, D.; Boric, I.; Jelec, Z.; Rod, E.; Vrdoljak, T.; Skelin, A.; Plecko, M.; Turkalj, M.; et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, R.P.; Oldweiler, A.; Mickelson, D.T.; Moorman, C.T., 3rd. Adipose derived stem cell transplant technique for degenerative joint disease. Arthrosc. Tech. 2017, 6, e1761–e1766. [Google Scholar] [CrossRef] [PubMed]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: Preliminary results. Front. Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived From Microfragmented Adipose Tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 16, 154–162. [Google Scholar]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Ciliberti, R. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. (Lond.) 2017, 24, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Tierno, R.; Zalduendo, M.; Alkhraisat, M.H. The Effectiveness of Platelet-Rich Plasma as a Carrier of Stem Cells in Tissue Regeneration: A Systematic Review of Pre-Clinical Research. Cells Tissues Organs 2021, 210, 339–350. [Google Scholar] [CrossRef]
- Boffa, A.; Salerno, M.; Merli, G.; De Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4100–4121. [Google Scholar] [CrossRef]
- Haidich, A.B. Meta-analysis in medical research. Hippokratia 2010, 14, 29–37. [Google Scholar]
| Article | Study Type Blinding | Type of Adipose Product (Device) | Injected mL US Guidance | Associated Treatment | Control Group | Pts/Joints | Pts/Joints (Final F-Up) | Age (Mean + SD) | Sex (M/F) | BMI (Mean + SD) | OA Grade | Final F-Up (m) | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aletto, 2022 [27] | Prospective case series no | Micro-filtered AT Lipocell (Tiss’You, RSM) | 10–15 mL no | no | no | 123/123 | 123/123 | 57 | 57/66 | 27 | KL 1–3 | 6 | MM-AT is safe and ameliorates clinical and functional scores in early OA |
| Barfod, 2019 [28] | Prospective case series no | MM-AT (Lipogems International Spa) | 10 mL no | no | no | 20/20 | 20/20 | 49 ± 9 | n.r. | n.r. | n.r. | 12 | MM-AT is safe and improves functional scores |
| Baria, 2022 [29] | RCT no | MM-AT (Lipogems International Spa) | 7.9 ± 3.9 mL yes | no | LR-PRP | 71/71 | 58/58 | 51.9 ± 2.4 (LR-PRP); 56.1 ± 1.7 (MM-AT) | 28/30 | 31.0 ± 0.8 (LR-PRP); 31.0 ± 0.9 (MM-AT) | KL 1–4 | 6 | MM-AT and LR-PRP show same clinical improvements |
| Boric, 2019 [30] | Prospective case series no | MM-AT (Lipogems International Spa) | 4–15 mL no | no | no | 17/32 | 10/18 | 69 ± 12 | 7/3 | n.r. | KL 3–4 | 24 | MM-AT improves GAG content with relevant improvement |
| Cattaneo, 2018 [31] | Retrospective case series no | MM-AT (Lipogems International Spa) | 10 mL no | AS (n: 21) MEN (n: 14) | no | 35/35 | 35/35 | 53 ± 12 (AS) 55 ± 11 (MEN) | 21/14 | 27 ± 4 | KL 1–3 | 12 | MM-AT is safe and adjuvates surgical treatment |
| Dallo, 2021 [32] | RCT single blind (statistician) | MM-AT (Lipogems International Spa) | n.r. no | no | LP-PRP+HA | 50/80 | 50/80 | 61.5 ± 9.5 (MM-AT); 62.5 ± 11.3 (LP-PRP+HA) | 23/27 | 26.3 ± 3.6 (LP-PRP+HA); 25.8 ± 5.1 (MM-AT) | KL 1–2 | 12 | LP-PRP+HA and MM-AT show same clinical improvement |
| Fan, 2023 [33] | Prospective case series no | MM-AT (Lipogems International Spa) | 6–8 mL yes | no | no | 46/50 | 46/50 | 66.9 ± 1.0 | 28/18 | 32.0 ± 1.0 | KL 3–4 | 12 | MM-AT is safe and effective in moderate-to-severe OA |
| Ferracini, 2022 [34] | Prospective case series no | MM-AT (Lipogems International Spa) | 3–50 mL yes | Arthroscopy | no | 101/101 | 91/91 | 62.8 ± 10.1 | 44/47 | 25.3 ± 3.8 | KL 2–3 | 12 | MM-AT, associated with arthroscopy, reduces pain in early/mild OA |
| Giorgini, 2022 [35] | Retrospective case series no | MM-AT (Lipogems International Spa) | 7 mL no | Arthroscopy | no | 49/50 | 45/46 | 52.7 ± 10.0 | 24/25 | n.r. | KL 3–4 | 24 | MM-AT, associated with arthroscopy, is safe and effective |
| Gobbi, 2021 [36] | Retrospective case series no | MM-AT (Lipogems International Spa) | 5–21 mL yes | no | no | 75/120 | 75/120 | Mean 69.6 | 26/49 | Average 28.4 | KL 2–4 | 24 | MM-AT improves clinical, functional, and quality of life |
| Gobbi, 2023 [37] | RCT single blind (clinical assessor) | MM-AT (Lipogems International Spa) | n.r. no | no | LP-PRP+HA | 50/80 | 50/80 | 62.38 ± 11.88 | 39/41 | n.r. | KL 1–2 | 24 | MM-AT and LP-PRP+HA show same functional improvement, and safety, at mid-term f-up |
| Heidari, 2021 [38] | Retrospective case series no | MM-AT (Lipogems International Spa) | 6–8 mL yes | no | no | 220/334 | 25% pts lost to follow-up | n.r. | 125/95 | n.r. | KL 3–4 | 24 | MM-AT improves quality of life and can delay TKR |
| Heidari, 2020 [39] | Prospective case series no | MM-AT (Lipogems International Spa) | 6–8 mL yes | no | no | 110/110 | 110/110 | 42–94 | 60/50 | n.r. | KL 1–4 | 12 | MM-AT improves pain, function, and quality of life |
| Hudetz, 2019 [40] | Prospective case series no | MM-AT (Lipogems International Spa) | 5 mL no | no | no | 20/20 | 17/17 | n.r. | 15/5 | <30 (n: 13); 30–35 (n: 5); >35 (n: 2) | KL 3–4 | 12 | MM-AT shows positive effect in late stages OA |
| Hudetz, 2017 [41] | Prospective case series no | MM-AT (Lipogems International Spa) | 4–15 mL no | no | no | 17/32 | 17/32 | 69 ± 12 | 12/5 | n.r. | KL 3–4 | 12 | MM-AT improves GAG content, pain, and clinical results |
| Kaszynski, 2022 [42] | RCT single blind (clinical assessor) | MM-AT (Lipogems International Spa) | n.r. no | no | PRP | 54/54 | 40/40 | 57 ± 8 (PRP) 55 ± 8 (MM-AT) | n.r. | 26 ± 3 (PRP) 27 ± 3 (MM-AT) | KL 2–3 | 12 | MM-AT and PRP show same improvements in pain, symptoms, and functions |
| Malanga, 2021 [43] | Prospective case series no | MM-AT (Lipogems International Spa) | 7.6 ± 2.3 mL yes | no | no | 20/23 | 20/23 | 59.8 ± 6.5 | 11/9 | 28.6 ± 4.8 | Mild, Moderate, Severe | 12 | MM-AT is safe and effective |
| Magnanelli, 2020 [44] | Retrospective comparative no | MM-AT (Lipogems International Spa) | n.r. no | HTO | HTO | 85/85 | 85/85 | n.r. | n.r. | n.r. | KL 1–3 | 12 | MM-AT, associated with HTO, improves the daily life activity |
| Mautner, 2019 [45] | Retrospective comparative no | MM-AT (Lipogems International Spa) | 9 mL yes | no | BMAC | 76/106 | 76/106 | 59 ± 1 (BMAC); 63 ± 11 (MM-AT) | 36/40 | n.r. | KL 1–4 | 21.6 ± 10.6 (BMAC); 13.1 ± 5.9 (MM-AT) | MM-AT and BMAC show same improvement in pain and function |
| Miles, 2022 [46] | Retrospective case series no | MM-AT (Lipogems International Spa) | 3.5–36 mL n.r. | no | no | 39/56 | 37/53 | 71.1 | 19/20 | 28.4 | KL 1–4 | 22 | MM-AT improves pain, stiffness, and function |
| Panchal, 2018 [47] | Prospective case series no | MM-AT (Lipogems International Spa) | n.r. yes | no | no | 17/26 | 17/26 | 68.27 ± 7.43 | 10/7 | 28.98 ± 4.50 | KL 3–4 | 12 | MM-AT is safe and effective in refractory severe OA |
| Peretti, 2018 [48] | RCT (n.r.) | MM-AT (Lipogems International Spa) | 19.1 ± 8.1 mL n.r. | AD | AD | 39 (of 78 to be included, study ongoing) | 16 (8 cases vs. 8 control) | 56.25 ± 8.396 | 70%/30% | n.r. | KL 3–4 | 6 | MM-AT shows encouraging positive trend |
| Pintore, 2023 [49] | Prospective comparative no | MM-AT (Tulip Soft Harvest GOLD System) | 10 mL no | no | BMAC | 102/102 | 102/102 | 57.64 (BMAC); 61.94 (MM-AT) | 46/56 | 28.76 (BMAC); 26.76 (MM-AT) | KL mean 2.7 (BMAC); KL mean 2.5 (MM-AT) | 6 | BMAC and MM-AT show same improvement in pain and functions |
| Priano, 2022 [50] | Retrospective case series no | Non enzymatic SVF (Hy-tissue SVF Separation System kit) | 8–10 mL no | no | no | 25/25 | 25/25 | 53.2 ± 11.7 | 10/15 | 24.7 ± 2.1 | KL 2–3 | 6 | MM-AT relieves pain and improves stiffness and functions |
| Russo, 2017 [51] | Retrospective case series no | MM-AT (Lipogems International Spa) | 10–15 mL no | ACLR, HTO, MEN (n: 24); arthroscopy (n: 6) | no | 30/30 | 30/30 | Median 43 | 31/9 | Median 26 | ICRS Grade 2–4 | 12 | MM-AT is safe and feasible in degenerative chondral lesions |
| Russo, 2018 [52] | Retrospective case series no | MM-AT (Lipogems International Spa) | 10–15 mL no | ACLR, HTO, MEN (n: 24); arthroscopy (n: 6) | no | 30/30 | 22/22 | Median 43 | 31/9 | Median 26 | ICRS Grade 2–4 | 36 | MM-AT is safe in degenerative chondropathy in the mid-term |
| Santoprete, 2021 [53] | Retrospective case series no | MM-AT (MyStemTM kit) | n.r. no | no | no | 84/102 | 84/102 | 57.3 ± 4.2 | 38/46 | n.r. | KL ≥ 2 | 12 | MM-AT improves pain, stiffness, and ROM |
| Screpis, 2022 [54] | Prospective case series no | MM-AT (Lipogems International Spa) | 8 mL no | no | no | 202/216 | 202/216 | 54.0 ± 9.0 | 97/105 | 26.8 ± 4.2 | KL 1–4 | 24 | MM-AT is safe and effective for symptoms |
| Ulivi, 2022 [55] | RCT single blind (radiologist) | MM-AT (Lipogems International Spa) | 6–8 mL no | AD | AD | 78/78 | 66/66 | 60.7 ± 7.9 | 44/34 | n.r. | KL 3–4 | 13–42 | MM-AT, associated with AD, improves functions and MRI appearance |
| Van Genechten, 2021 [56] | Prospective case series no | MM-AT (Lipogems International Spa) | 8–10 mL yes | no | no | 64 | 56/77 | 54.2 ± 9.1 | 31/33 | 27.2 ± 4.5 | KL 1–4 | 12 | MM-AT shows early clinical improvement but a mediocre response rate |
| Vasso, 2022 [57] | Retrospective case series no | MM-AT (Lipogems International Spa) | 10–15 mL no | AD | no | 23/23 | 23/23 | 58 ± 8 | 8/15 | 28.0 ± 4.8 | ACR criteria 1–3 | 22.1 ± 4.2 | MM-AT, associated with AD, improves clinical and functional scores |
| Yu, 2023 [58] | Prospective case series single blind (clinical assessor) | MM-AT (Lipogems International Spa) | 6–8 mL no | no | no | 20/40 | 20/40 | 54.63 ± 3.90 | 8/12 | 25.5 ± 2.86 | n.r. | 18 | MM-AT improves functions and pain, but not in the long term |
| Zaffagnini, 2022 [59] | RCT single blind (clinical assessor) | MM-AT (Lipogems International Spa) | 5 mL no | no | PRP | 108/108 | 108/108 | 54.5 ± 12.1 (MM-AT); 54.1 ± 10.6 (PRP) | 64/44 | 25.9 ± 4.3 (MM-AT) 28.0 ± 5.5 (PRP) | KL 1–4 | 24 | MM-AT and PRP show same improvements |
| Article | Complications Related to Adipose Tissue Harvesting Procedure | Local or Systemic Complications Related to MM-AT Injection |
|---|---|---|
| Aletto et al., 2022 [27] | / | / |
| Barfod et al., 2019 [28] | Cosmetic changes to the abdominal subcutaneous tissue (5%) | / |
| Baria et al., 2022 [29] | / | / |
| Boric et al., 2019 [30] | n.r. | n.r. |
| Cattaneo et al., 2018 [31] | Temporary and small subcutaneous hematoma (2.9%) | / |
| Dallo et al., 2021 [32] | / | / |
| Fan et al., 2023 [33] | n.r. | n.r. |
| Ferracini et al., 2022 [34] | / | Painful adipose loose bodies (2.2%), recurrent episodes of joint effusion (2.2%) |
| Giorgini et al., 2022 [35] | Hematoma (2.2%) | Knee swelling (8.9%) |
| Gobbi et al., 2021 [36] | Donor site pain (49%), swelling/bruising (28%) | Knee prolonged swelling (13%) |
| Gobbi et al., 2023 [37] | ||
| Heidari et al., 2021 [38] | Donor site bleeding (4.1%), pain (6.4%) | Joint swelling and pain (21.8%), severe reaction requiring wash-out of the joint (0.45%) |
| Heidari et al., 2020 [39] | / | / |
| Hudetz et al., 2019 [40] | / | / |
| Hudetz et al., 2017 [41] | / | / |
| Kaszynski et al., 2022 [42] | n.r. | n.r. |
| Malanga et al., 2021 [43] | Donor site erythema and swelling (1%), soreness (52.5%), and hematoma (15%) | Knee swelling (15%) |
| Magnanelli et al., 2020 [44] | n.r. | n.r. |
| Mautner et al., 2019 [45] | n.r. | n.r. |
| Miles et al., 2022 [46] | / | / |
| Panchal et al., 2018 [47] | / | Knee pain and swelling |
| Peretti et al., 2018 [48] | n.r. | n.r. |
| Pintore et al., 2023 [49] | / | / |
| Priano et al., 2022 [50] | / | Knee crepitus on motion (32%) and effusion (4%) |
| Russo et al., 2017 [51] | Donor site hematoma (6.7%) | Recurrent knee effusions (3.3%) |
| Russo et al., 2018 [52] | / | / |
| Santoprete et al., 2021 [53] | Donor site discomfort and pain (15%) | Knee swelling and pain (7%) |
| Screpis et al., 2022 [54] | / | / |
| Ulivi et al., 2022 [55] | Donor site small hematoma | / |
| Van Genechten et al., 2021 [56] | / | Subjective knee instability (3.6%), muscle aching in the calves (1.8%), gallstones (1.8%), stroke (3.6%), tendinopathy (5.4%) |
| Vasso et al., 2022 [57] | Donor site transitory hematoma (8.7%) | / |
| Yu et al., 2023 [58] | / | / |
| Zaffagnini et al., 2022 [59] | / | MM-AT: mild/moderate knee pain, joint swelling, and/or effusion (18.5%); PRP: knee pain, joint swelling, and/or effusion (11.1%) |
| Article | Reporting | External Validity | Internal Validity Bias | Internal Validity Confounding | Power | Total Score |
|---|---|---|---|---|---|---|
| Aletto C et al., 2022 [27] | 7 | 3 | 5 | 3 | 0 | 18 |
| Barfod KW et al., 2019 [28] | 8 | 3 | 5 | 3 | 0 | 19 |
| Baria M et al., 2022 [29] | 10 | 3 | 5 | 5 | 1 | 24 |
| Boric I et al., 2019 [30] | 9 | 3 | 4 | 3 | 0 | 19 |
| Mautner K et al., 2019 [45] | 8 | 3 | 5 | 4 | 0 | 20 |
| Cattaneo G et al., 2018 [31] | 10 | 3 | 5 | 3 | 0 | 21 |
| Dallo I et al., 2021 [32] | 10 | 3 | 6 | 5 | 1 | 25 |
| Fan F et al., 2023 [33] | 7 | 3 | 6 | 3 | 0 | 19 |
| Ferracini R et al., 2022 [34] | 11 | 3 | 5 | 4 | 1 | 24 |
| Giorgini A et al., 2022 [35] | 11 | 3 | 5 | 4 | 0 | 23 |
| Gobbi A et al., 2023 [37] | 10 | 3 | 6 | 5 | 1 | 25 |
| Gobbi A et al., 2021 [36] | 10 | 3 | 5 | 3 | 1 | 22 |
| Heidari N et al., 2021 [38] | 10 | 3 | 5 | 4 | 0 | 22 |
| Heidari N et al., 2020 [39] | 9 | 3 | 5 | 3 | 0 | 20 |
| Hudetz D et al., 2019 [40] | 9 | 3 | 5 | 3 | 0 | 20 |
| Hudetz D et al., 2017 [41] | 10 | 3 | 5 | 3 | 0 | 21 |
| Kaszyński J et al., 2022 [42] | 9 | 3 | 6 | 4 | 1 | 23 |
| Magnanelli M et al., 2020 [44] | 4 | 2 | 4 | 3 | 0 | 13 |
| Malanga GA et al., 2021 [43] | 10 | 3 | 5 | 3 | 0 | 21 |
| Miles MR et al., 2022 [46] | 11 | 2 | 5 | 4 | 0 | 22 |
| Panchal J et al., 2018 [47] | 7 | 3 | 5 | 2 | 0 | 17 |
| Peretti GM et al., 2018 [48] | 7 | 3 | 4 | 3 | 0 | 17 |
| Priano V et al., 2022 [50] | 9 | 3 | 5 | 3 | 0 | 20 |
| Russo A et al., 2017 [51] | 10 | 3 | 5 | 3 | 0 | 21 |
| Russo A et al., 2018 [52] | 9 | 3 | 5 | 3 | 0 | 20 |
| Santoprete S et al., 2021 [53] | 9 | 3 | 4 | 3 | 0 | 19 |
| Screpis D et al., 2022 [54] | 11 | 3 | 5 | 4 | 0 | 23 |
| Ulivi M et al., 2022 [55] | 10 | 3 | 5 | 4 | 1 | 23 |
| Van Genechten W et al., 2021 [56] | 10 | 3 | 5 | 4 | 0 | 22 |
| Vasso M et al., 2022 [57] | 11 | 2 | 5 | 4 | 0 | 22 |
| Zaffagnini S et al., 2022 [59] | 11 | 3 | 6 | 5 | 1 | 26 |
| Pintore A et al., 2023 [49] | 10 | 2 | 4 | 3 | 0 | 19 |
| Yu Y et al., 2023 [58] | 9 | 2 | 6 | 3 | 1 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Andriolo, L.; Salerno, M.; Boffa, A.; Giavaresi, G.; Filardo, G. Adipose Tissue-Derived Minimally Manipulated Products versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence and Meta-Analysis. J. Clin. Med. 2024, 13, 67. https://doi.org/10.3390/jcm13010067
Veronesi F, Andriolo L, Salerno M, Boffa A, Giavaresi G, Filardo G. Adipose Tissue-Derived Minimally Manipulated Products versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(1):67. https://doi.org/10.3390/jcm13010067
Chicago/Turabian StyleVeronesi, Francesca, Luca Andriolo, Manuela Salerno, Angelo Boffa, Gianluca Giavaresi, and Giuseppe Filardo. 2024. "Adipose Tissue-Derived Minimally Manipulated Products versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence and Meta-Analysis" Journal of Clinical Medicine 13, no. 1: 67. https://doi.org/10.3390/jcm13010067





