Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in the Prophylaxis of Graft-Versus-Host Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
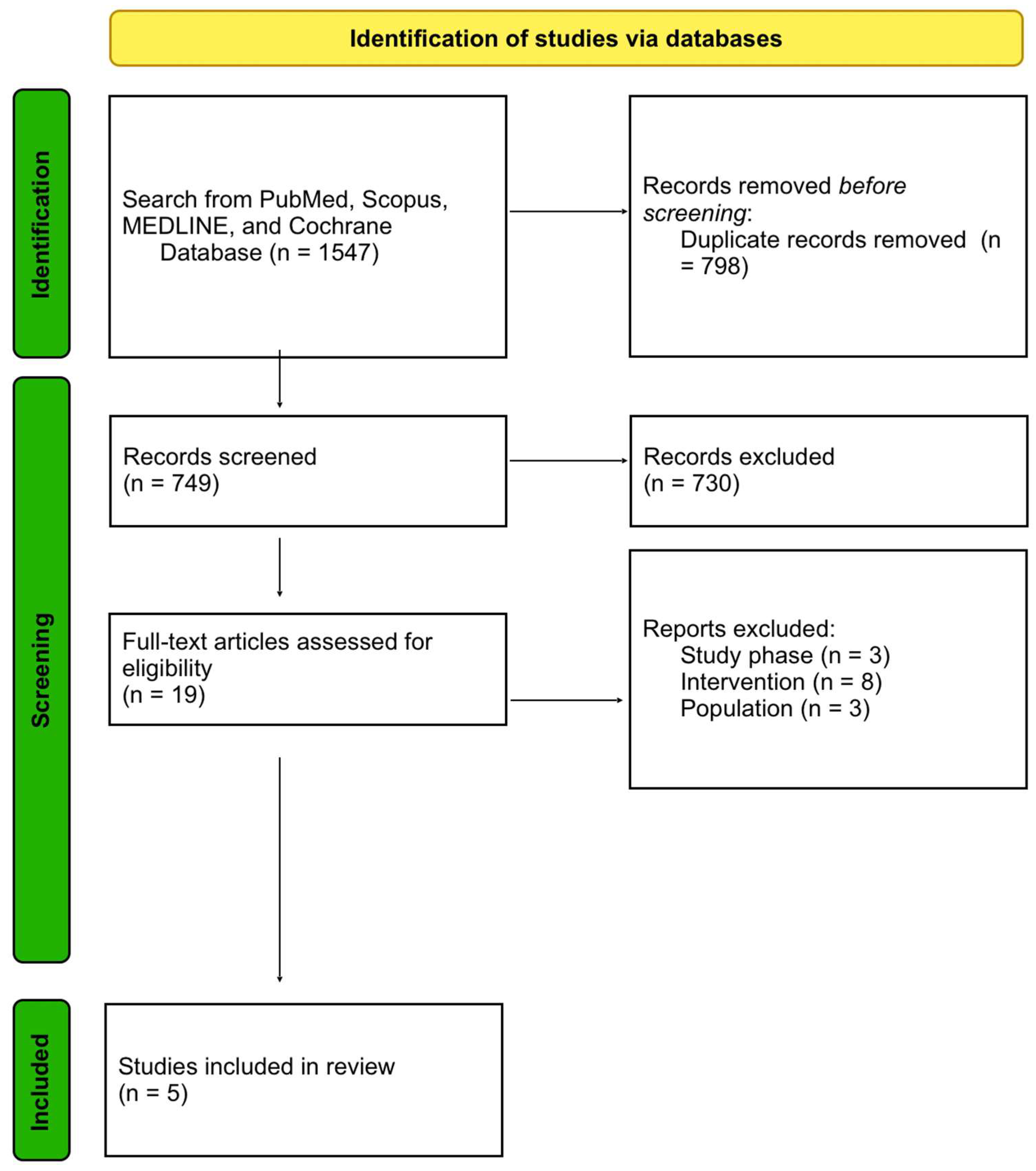
2.1. Systematic Literature Review
2.2. Data Presentation, Extraction and Endpoints
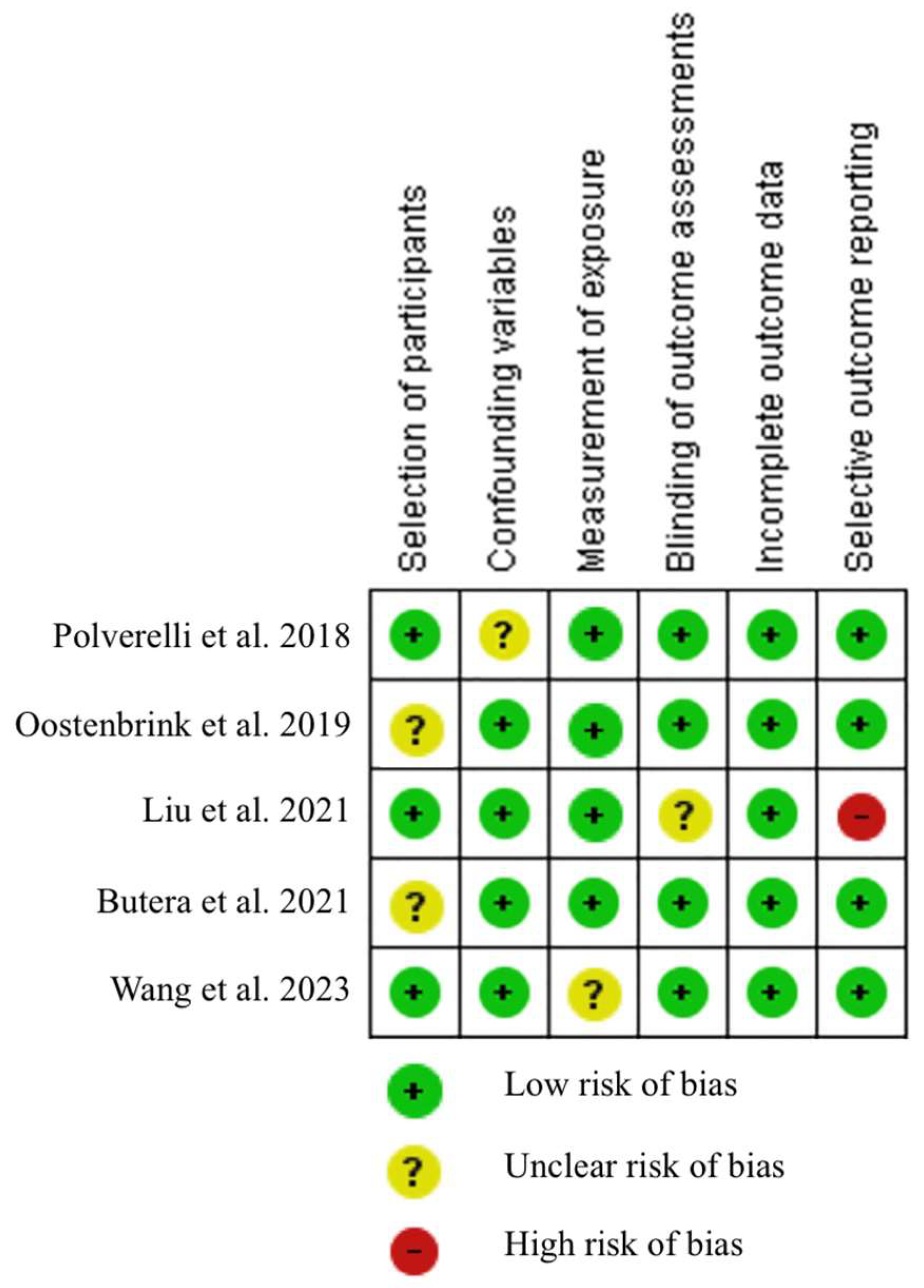
2.3. Risk of Bias Assessment
3. Results
Results—Systematic Literature Review
4. Outcomes
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukaemia |
| ALAL | acute leukaemia with ambiguous lineage |
| ALL | acute lymphoblastic leukaemia |
| allo-HCT | allogeneic hematopoietic stem cell transplantation |
| ATG-G | Grafalon |
| ATG-T | Thymoglobulin |
| h-ATG | horse ATG |
| p-ATG | porcine ATG |
| BM | bone marrow |
| BU | busulfan |
| CIs | confidence intervals |
| CML | chronic myeloid leukaemia |
| CMV | cytomegalovirus |
| CNIs | calcineurin inhibitors |
| CY | cyclophosphamide |
| EBV | Epstein-Barr virus |
| FLU | fludarabine |
| GRFS | graft-versus-host/relapse-free survival |
| GvHD | graft-versus-host disease |
| aGvHD | acute graft-versus-host disease |
| cGvHD | chronic graft-versus-host disease |
| GvL | graft-versus-leukaemia |
| Haplo | haploidentical donor |
| HRs | hazard ratios |
| LFS | leukaemia-free survival |
| MAC | myeloablative conditioning |
| MDS | myelodysplastic syndrome |
| MRD | matched related donor |
| MMRD | mismatched related donor |
| MUD | matched unrelated donor |
| MMUD | mismatched unrelated donor |
| MPNs | myeloproliferative neoplasms |
| MTX | methotrexate |
| NMA | nonmyeloablative conditioning |
| NR | not reported |
| NRM | non-relapse mortality |
| OS | overall survival |
| PBSC | peripheral blood stem cells |
| r-ATG | rabbit anti-thymocyte globulin |
| RCTs | randomised controlled trials |
| RIC | reduced intensity conditioning |
| TRM | transplantation-related mortality |
| URD | unrelated donor |
References
- Wingard, J.R.; Majhail, N.S.; Brazauskas, R.; Wang, Z.; Sobocinski, K.A.; Jacobsohn, D.; Sorror, M.L.; Horowitz, M.M.; Bolwell, B.; Rizzo, J.D.; et al. Long-Term Survival and Late Deaths after Allogeneic Hematopoietic Cell Transplantation. J. Clin. Oncol. 2011, 29, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Counts, G.W.; Appelbaum, F.R.; Lee, S.J.; Sanders, J.E.; Deeg, H.J.; Flowers, M.E.D.; Syrjala, K.L.; Hansen, J.A.; Storb, R.F.; et al. Life Expectancy in Patients Surviving More than 5 Years after Hematopoietic Cell Transplantation. J. Clin. Oncol. 2010, 28, 1011–1016. [Google Scholar] [CrossRef]
- Pidala, J.; Kurland, B.; Chai, X.; Majhail, N.; Weisdorf, D.J.; Pavletic, S.; Cutler, C.; Jacobsohn, D.; Palmer, J.; Arai, S.; et al. Patient-Reported Quality of Life Is Associated with Severity of Chronic Graft-versus-Host Disease as Measured by NIH Criteria: Report on Baseline Data from the Chronic GVHD Consortium. Blood 2011, 117, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.; Williams, K.M. Controversies and Expectations for the Prevention of GVHD: A Biological and Clinical Perspective. Front. Immunol. 2022, 13, 1057694. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Arora, M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar] [CrossRef]
- Flowers, M.E.D.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative Analysis of Risk Factors for Acute Graft-versus-Host Disease and for Chronic Graft-versus-Host Disease According to National Institutes of Health Consensus Criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef]
- Anasetti, C.; Logan, B.R.; Lee, S.J.; Waller, E.K.; Weisdorf, D.J.; Wingard, J.R.; Cutler, C.S.; Westervelt, P.; Woolfrey, A.; Couban, S.; et al. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. N. Engl. J. Med. 2012, 367, 1487–1496. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Ruutu, T.; Van Biezen, A.; Hertenstein, B.; Henseler, A.; Garderet, L.; Passweg, J.; Mohty, M.; Sureda, A.; Niederwieser, D.; Gratwohl, A.; et al. Prophylaxis and Treatment of GVHD after Allogeneic Haematopoietic SCT: A Survey of Centre Strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012, 47, 1459–1464. [Google Scholar] [CrossRef]
- Chen, X.; Wei, J.; Huang, Y.; He, Y.; Yang, D.; Zhang, R.; Jiang, E.; Ma, Q.; Zhai, W.; Yao, J.; et al. Effect of Antithymocyte Globulin Source on Outcomes of HLA-Matched Sibling Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Severe Aplastic Anemia. Biol. Blood Marrow Transplant. 2018, 24, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, C.; Zhuang, J.; Zou, N.; Xu, Y.; Zhang, W.; Li, J.; Duan, M.; Zhu, T.; Cai, H.; et al. Long-Term Follow-up Study of Porcine Anti-Human Thymocyte Immunoglobulin Therapy Combined with Cyclosporine for Severe Aplastic Anemia. Eur. J. Haematol. 2016, 96, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, P.; Nunez, O.; Weinstein, B.; Scheinberg, P.; Biancotto, A.; Wu, C.O.; Young, N.S. Horse versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N. Engl. J. Med. 2011, 365, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Kekre, N.; Zhang, Y.; Zhang, M.J.; Carreras, J.; Ahmed, P.; Anderlini, P.; Atta, E.H.; Ayas, M.; Boelens, J.J.; Bonfim, C.; et al. Effect of Antithymocyte Globulin Source on Outcomes of Bone Marrow Transplantation for Severe Aplastic Anemia. Haematologica 2017, 102, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Baron, F.; Mohty, M.; Blaise, D.; Socié, G.; Labopin, M.; Esteve, J.; Ciceri, F.; Giebel, S.; Gorin, N.C.; Savani, B.N.; et al. Anti-Thymocyte Globulin as Graft-versus-Host Disease Prevention in the Setting of Allogeneic Peripheral Blood Stem Cell Transplantation: A Review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2017, 102, 224–234. [Google Scholar] [CrossRef]
- Mohty, M. Mechanisms of Action of Antithymocyte Globulin: T-Cell Depletion and Beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef]
- Popow, I.; Leitner, J.; Majdic, O.; Kovarik, J.J.; Saemann, M.D.; Zlabinger, G.J.; Steinberger, P. Assessment of Batch to Batch Variation in Polyclonal Antithymocyte Globulin Preparations. Transplantation 2012, 93, 32–40. [Google Scholar] [CrossRef]
- Popow, I.; Leitner, J.; Grabmeier-Pfistershammer, K.; Majdic, O.; Zlabinger, G.J.; Kundi, M.; Steinberger, P. A Comprehensive and Quantitative Analysis of the Major Specificities in Rabbit Antithymocyte Globulin Preparations. Am. J. Transplant. 2013, 13, 3103–3113. [Google Scholar] [CrossRef]
- Servais, S.; Menten-Dedoyart, C.; Beguin, Y.; Seidel, L.; Gothot, A.; Daulne, C.; Willems, E.; Delens, L.; Humblet-Baron, S.; Hannon, M.; et al. Impact of Pre-Transplant Anti-T Cell Globulin (ATG) on Immune Recovery after Myeloablative Allogeneic Peripheral Blood Stem Cell Transplantation. PLoS ONE 2015, 10, e0130026. [Google Scholar] [CrossRef]
- Bosch, M.; Dhadda, M.; Hoegh-Petersen, M.; Liu, Y.; Hagel, L.M.; Podgorny, P.; Ugarte-Torres, A.; Khan, F.M.; Luider, J.; Auer-Grzesiak, I.; et al. Immune Reconstitution after Anti-Thymocyte Globulin-Conditioned Hematopoietic Cell Transplantation. Cytotherapy 2012, 14, 1258–1275. [Google Scholar] [CrossRef]
- Bonifazi, F.; Rubio, M.T.; Bacigalupo, A.; Boelens, J.J.; Finke, J.; Greinix, H.; Mohty, M.; Nagler, A.; Passweg, J.; Rambaldi, A.; et al. Rabbit ATG/ATLG in Preventing Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation: Consensus-Based Recommendations by an International Expert Panel. Bone Marrow Transplant. 2020, 55, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Addition of Anti-Thymocyte Globulin to Standard Graft-versus-Host Disease Prophylaxis versus Standard Treatment Alone in Patients with Haematological Malignancies Undergoing Transplantation from Unrelated Donors: Final Analysis of a Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Haematol. 2020, 7, e100–e111. [Google Scholar] [CrossRef] [PubMed]
- El-Cheikh, J.; Devillier, R.; Dulery, R.; Massoud, R.; Al Chami, F.; Ghaoui, N.; Moukalled, N.; Pagliardini, T.; Marino, F.; Malard, F.; et al. Impact of Adding Antithymocyte Globulin to Posttransplantation Cyclophosphamide in Haploidentical Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Ayuk, F.; Wolschke, C.; Kröger, N. Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in Allogeneic Stem Cell Transplantation: Systematic Literature Review and Network Meta-Analysis. Biol. Blood Marrow Transplant. 2017, 23, 2184–2191. [Google Scholar] [CrossRef]
- Polverelli, N.; Malagola, M.; Turra, A.; Skert, C.; Perucca, S.; Chiarini, M.; Cattina, F.; Rambaldi, B.; Cancelli, V.; Morello, E.; et al. Comparative Study on ATG-Thymoglobulin versus ATG-Fresenius for the Graft-versus-Host Disease (GVHD) Prophylaxis in Allogeneic Stem Cell Transplantation from Matched Unrelated Donor: A Single-Centre Experience over the Contemporary Years. Leuk. Lymphoma 2018, 59, 2700–2705. [Google Scholar] [CrossRef]
- Oostenbrink, L.V.E.; Jol-Van Der Zijde, C.M.; Kielsen, K.; Jansen-Hoogendijk, A.M.; Ifversen, M.; Müller, K.G.; Lankester, A.C.; Van Halteren, A.G.S.; Bredius, R.G.M.; Schilham, M.W.; et al. Differential Elimination of Anti-Thymocyte Globulin of Fresenius and Genzyme Impacts T-Cell Reconstitution After Hematopoietic Stem Cell Transplantation. Front. Immunol. 2019, 10, 315. [Google Scholar] [CrossRef]
- Liu, L.; Xu, G.; Zhang, Y.; Jiao, W.; Lei, M.; Zhou, H.; Wang, Q.; Qiu, H.; Tang, X.; Han, Y.; et al. Comparison of 2 Different Rabbit Anti-Thymocyte Globulin (r-ATG) Preparations: Thymocyte r-ATG versus T Lymphoblast Cell Line r-ATG in Allogeneic Hematopoietic Stem Cell Transplantation for Acquired Severe Aplastic Anemia: Propensity Score-Matched Analysis. Transplant. Cell. Ther. 2021, 27, 186.e1–186.e3. [Google Scholar] [CrossRef]
- Wang, L.; Kong, P.; Zhang, C.; Gao, L.; Zhu, L.; Liu, J.; Gao, S.; Chen, T.; Liu, H.; Yao, H.; et al. Outcomes of Patients with Hematological Malignancies Who Undergo Unrelated Donor Hematopoietic Stem Cell Transplantation with ATG-Fresenius versus ATG-Genzyme. Ann. Hematol. 2023, 102, 1–11. [Google Scholar] [CrossRef]
- Butera, S.; Cerrano, M.; Brunello, L.; Dellacasa, C.M.; Faraci, D.G.; Vassallo, S.; Mordini, N.; Sorasio, R.; Zallio, F.; Busca, A.; et al. Impact of Anti-Thymocyte Globulin Dose for Graft-versus-Host Disease Prophylaxis in Allogeneic Hematopoietic Cell Transplantation from Matched Unrelated Donors: A Multicenter Experience. Ann. Hematol. 2021, 100, 1837–1847. [Google Scholar] [CrossRef]
- Lee, S.J.; Logan, B.; Westervelt, P.; Cutler, C.; Woolfrey, A.; Khan, S.P.; Waller, E.K.; Maziarz, R.T.; Wu, J.; Shaw, B.E.; et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-Term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1583–1589. [Google Scholar] [CrossRef]
- Kumar, A.; Reljic, T.; Hamadani, M.; Mohty, M.; Kharfan-Dabaja, M.A. Antithymocyte Globulin for Graft-versus-Host Disease Prophylaxis: An Updated Systematic Review and Meta-Analysis. Bone Marrow Transplant. 2019, 54, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, N.; Wang, L.; Du, J.; Yang, J.; Wen, Y.; Wei, Y.; Qian, K.; Wang, H.; Jiao, Y.; et al. Reduced Risk of Chronic Graft-Versus-Host Disease (CGVHD) by Rabbit Anti-Thymocyte Globulin (ATG) in Patients Undergoing Matched Sibling Donor Transplantation in Hematological Malignancies. Ann. Transplant. 2022, 27, e937356-1. [Google Scholar] [CrossRef]
- Arcuri, L.J.; Kerbauy, M.N.; Kerbauy, L.N.; de Souza Santos, F.P.; Ribeiro, A.A.F.; Hamerschlak, N. ATG in HLA-Matched, Peripheral Blood, Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Secondary Analysis of a CIBMTR Database. Transplant. Cell. Ther. 2023, 29, 40.e1–40.e4. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Baron, F.; Galimard, J.E.; Labopin, M.; Yakoub-Agha, I.; Niittyvuopio, R.; Kröger, N.; Griskevicius, L.; Wu, D.; Forcade, E.; Richard, C.; et al. Allogeneic Peripheral Blood Stem Cell Transplantation with Anti-Thymocyte Globulin versus Allogeneic Bone Marrow Transplantation without Anti-Thymocyte Globulin. Haematologica 2020, 105, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Hou, C.; Ma, C.; Li, F.; Gao, X.; Huang, W.; Wang, S.; Gao, C.; Yu, L.; Liu, D. Reduced Risk of Chronic GVHD by Low-Dose RATG in Adult Matched Sibling Donor Peripheral Blood Stem Cell Transplantation for Hematologic Malignancies. Ann. Hematol. 2020, 99, 167–179. [Google Scholar] [CrossRef]
- Arai, Y.; Jo, T.; Matsui, H.; Kondo, T.; Takaori-Kondo, A. Efficacy of Antithymocyte Globulin for Allogeneic Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis. Leuk. Lymphoma 2017, 58, 1840–1848. [Google Scholar] [CrossRef]
- Yuan, J.; Pei, R.; Su, W.; Cao, J.; Lu, Y. Meta-Analysis of the Actions of Antithymocyte Globulin in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Oncotarget 2017, 8, 10871–10882. [Google Scholar] [CrossRef]
- Soiffer, R.J.; Kim, H.T.; McGuirk, J.; Horwitz, M.E.; Johnston, L.; Patnaik, M.M.; Rybka, W.; Artz, A.; Porter, D.L.; Shea, T.C.; et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J. Clin. Oncol. 2017, 35, 4003–4011. [Google Scholar] [CrossRef]
- Bonini, C.; Peccatori, J.; Stanghellini, M.T.L.; Vago, L.; Bondanza, A.; Cieri, N.; Greco, R.; Bernardi, M.; Corti, C.; Oliveira, G.; et al. Haploidentical HSCT: A 15-Year Experience at San Raffaele. Bone Marrow Transplant. 2015, 50 (Suppl. 2), S67–S71. [Google Scholar] [CrossRef]
- Di Bartolomeo, P.; Santarone, S.; De Angelis, G.; Picardi, A.; Cudillo, L.; Cerretti, R.; Adorno, G.; Angelini, S.; Andreani, M.; De Felice, L.; et al. Haploidentical, Unmanipulated, G-CSF-Primed Bone Marrow Transplantation for Patients with High-Risk Hematologic Malignancies. Blood 2013, 121, 849–857. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, H.X.; Liu, D.H.; Xu, L.P.; Zhang, X.H.; Chang, Y.J.; Chen, Y.H.; Wang, F.R.; Sun, Y.Q.; Tang, F.F.; et al. Influence of Two Different Doses of Antithymocyte Globulin in Patients with Standard-Risk Disease Following Haploidentical Transplantation: A Randomized Trial. Bone Marrow Transplant. 2014, 49, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hieber, M.; Schwarck, S.; Stroux, A.; Ganepola, S.; Reinke, P.; Thiel, E.; Uharek, L.; Blau, I.W. Immune Reconstitution and Cytomegalovirus Infection after Allogeneic Stem Cell Transplantation: The Important Impact of in Vivo T Cell Depletion. Int. J. Hematol. 2010, 91, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Pretreatment with Anti-Thymocyte Globulin versus No Anti-Thymocyte Globulin in Patients with Haematological Malignancies Undergoing Haemopoietic Cell Transplantation from Unrelated Donors: A Randomised, Controlled, Open-Label, Phase 3, Multicentre Trial. Lancet Oncol. 2016, 17, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Lamparelli, T.; Bruzzi, P.; Guidi, S.; Alessandrino, P.E.; Di Bartolomeo, P.; Oneto, R.; Bruno, B.; Barbanti, M.; Sacchi, N.; et al. Antithymocyte Globulin for Graft-versus-Host Disease Prophylaxis in Transplants from Unrelated Donors: 2 Randomized Studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001, 98, 2942–2947. [Google Scholar] [CrossRef]
- Finke, J.; Bethge, W.A.; Schmoor, C.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Standard Graft-versus-Host Disease Prophylaxis with or without Anti-T-Cell Globulin in Haematopoietic Cell Transplantation from Matched Unrelated Donors: A Randomised, Open-Label, Multicentre Phase 3 Trial. Lancet Oncol. 2009, 10, 855–864. [Google Scholar] [CrossRef]
- Terasako, K.; Sato, K.; Sato, M.; Kimura, S.I.; Nakasone, H.; Okuda, S.; Kako, S.; Tanaka, Y.; Yamazaki, R.; Oshima, K.; et al. The Effect of Different ATG Preparations on Immune Recovery after Allogeneic Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia. Hematology 2010, 15, 165–169. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
| ATG Formulation | Type of Antibodies | Recommended Dose for GvHD Prophylaxis (Total, mg/kg) |
|---|---|---|
| h-ATG | Polyclonal IgG from horses immunised with human thymocytes | - |
| ATG-T | Polyclonal IgG from rabbits immunised with human thymocytes | 2.5–10 |
| ATG-G | Polyclonal IgG from rabbits immunised with human Jurkat T leukaemia cell line | 15–60 |
| Polverelli et al., 2018, [25], (n = 77) | Oostenbrink et al., 2019, [26], (n = 58) | Liu et al., 2021, [27], (n = 214–Total, n = 67–Selected for ATG-T, ATG-G) * | Butera et al., 2021, [29], (n = 395) | Wang et al., 2023, [28], (n = 186) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of ATG utilised | ATG-T | ATG-G | ATG-T | ATG-G | ATG-T | ATG-G | ATG-T | ATG-T | ATG-G | ATG-T |
| Number of patients | n = 31 (40%) | n = 46 (60%) | n = 42 (72%) High-dose n = 24, Low-dose n = 18 | n = 16 (28%) High-dose n = 9 Low-dose n = 7 | n = 44 (66%) | n = 23 (34%) | n = 197 (50%) | n = 198 (50%) | n = 107 (58%) | n = 79 (42%) |
| Age (years), median (range) | 45 (17–61) | 48 (18–66) | 9 (1–18) | 6 (1–17) | 27 (6–50) | 26 (3–52) | 52.4 (20.7–69.4) | 50.4 (20.7–66.8) | 25 (3–59) | 30 (3–65) |
| Sex, (%) Male Female | n = 23 (74%) n = 8 (26%) | n = 29 (63%) n = 19 (37%) | NR | NR | n = 27 (61.36%) n = 17 (38.64%) | n = 13 (56.52%) n = 10 (43.48%) | n = 99 (50%) n = 98 (50%) | n = 117 (59%) n = 81 (41%) | n = 63 (58.9%) n = 44 (41.1%) | n = 50 (63.3%) n = 29 (36.7%) |
| Dose of ATG (total, mg/kg) | 7.5 mg/kg | 30 mg/kg | High-dose 10 mg/kg Low-dose 6–8 mg/kg | High-dose 60 mg/kg Low-dose 45 mg/kg | MRD 12.5 mg/kg Haplo 10 mg/kg | MRD 25 mg/kg Haplo 20 mg/kg | 5 mg/kg | 6–7.5 mg/kg | 20 mg/kg | 10 mg/kg |
| Follow-up (days/months), median (range) | 20 (1–88) months | 22 (2–60) months | NR | NR | 47.65 (0.50–186.78) months | 44.34 (3.0–76.15) months | 81.5 (50.2–119.3) months | 81.5 (50.2–119.3) months | NR | NR |
| Diagnosis | Acute leukaemia n = 17 (56%) MDS n = 1 (3%) MPNs n = 1 (3%) Lymphoproliferative neoplasms n = 11 (35%) Others n = 1 (3%) | Acute leukaemia n = 24 (52%) MDS n = 7 (15%) MPNs n = 2 (5%) Lymphoproliferative neoplasms n = 12 (26%) Others n = 1 (2%) | ALL n = 17 (40%) AML n = 25 (60%) | ALL n = 16 (100%) | Severe aplastic anaemia | Severe aplastic anaemia | ALL n = 23 (11.7%) AML/MDS n = 111 (56.3%) MPN n = 14 (7.1%) LPD n = 49 (24.9%) | ALL n = 29 (14.7%) AML/MDS n = 88 (44.4%) MPN n = 19 (9.6%) LPD n = 62 (31.3%) | ALAL n = 4 (3.7%) ALL n = 29 (27.1%) AML n = 42 (39.3%) CLL n = 1 (0.9%) CML n = 23 (21.5%) MDS n = 7 (6.5%) NHL n = 1 (0.9%) | ALAL n = 4 (5.1%) ALL n = 16 (20.3%) AML n = 43 (54.4%) CLL n = 0 (0%) CML n = 6 (7.6%) MDS n = 6 (7.6%) NHL n = 4 (5.1%) |
| Conditioning regimen | MAC n = 16 (52%) RIC n = 15 (48%) | MAC n = 22 (48%) RIC n = 24 (52%) | NR | NR | FLU + CY 5 n = 15 (34.01%) BU + CY 5 n = 29 (65.91%) | FLU + CY 5 n = 4 (17.39%) BU + CY 5 n = 19 (82.61%) | MAC n = 154 (78.2%) RIC n = 43 (21.8%) | MAC n = 107 (54%) RIC n = 91 (46%) | TBI/CY 1 n = 10 (9.3%) BU/CY 2 n = 60 (56.1%) Haplo 3 n = 30 (28.0%) FB3 4 n = 6 (5.6%) Other n = 1 (0.9%) | TBI/CY 1 n = 3 (3.8%) BU/CY 2 n = 3 (3.8%) Haplo 3 n = 21 (26.6%) FB3 4 n = 6 (7.6%) Other n = 0 (0%) |
| Stem cell source, (%) BM PBSC | BM n = 5 (16%) PBSC n = 26 (84%) | BM n = 5 (11%) PBSC n = 41 (89%) | BM n = 34 (81%) PBSC n = 8 (19%) | BM n = 14 (87%) PBSC n = 2 (13%) | BM + PBSC n = 28 (63.64%) BM n = 10 (22.73%) PBSC n = 6 (13.64%) | BM + PBSC n = 18 (78.26%) BM n = 2 (8.7%) PBSC n = 3 (13.04%) | BM n = 25 (12.7%) PBSC n = 172 (87.3%) | BM n = 30 (15.15%) PBSC n = 168 (84.85%) | NR | NR |
| Donor | MUD | MUD | MUD n = 30 (71%) MMUD n = 12 (29%) | MUD n = 13 (81%) MMUD n = 3 (19%) | MRD n = 13 (29.55%) Haplo n = 28 (63.64%) URD n = 3 (6.82%) | MRD n = 6 (26.09%) Haplo n = 16 (69.57%) URD n = 1 (4.35%) | MUD | MUD | MUD n = 69 (64.5%) MMUD n = 38 (35.5%) | MUD n = 45 (57.0%) MMUD n = 34 (43.0%) |
| Endpoint | Polverelli et al., 2018 [25], (n = 77) | Oostenbrink et al., 2019, [26], (n = 58) | Liu et al., 2021, [27], (n = 214–Total, n = 67–Selected for ATG-T, ATG-G) * | Butera et al., 2021, [29], (n = 395) | Wang et al., 2023, [28], (n = 186) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of ATG | ATG-T | ATG-G | ATG-T | ATG-G | ATG-T | ATG-G | ATG-T (5 mg/kg total) | ATG-T (6–7.5 mg/kg total) | ATG-G | ATG-T |
| Chronic GvHD | n = 8 (31%) p = 0.77 | n = 10 (26%) p = 0.77 | High-dose n = 6 (25%) Low-dose n = 3 (17%) p = 0.97 | n = 2 (13%) p = 0.97 | 26.83% p = 0.704 | 22.73% p = 0.704 | Moderate-severe cGvHD 17.4% p = 0.34 | Moderate-severe cGvHD 20.3% p = 0.34 | 43.9% p = 0.279 | 28.8% p = 0.279 |
| Acute GvHD grade II–IV | n = 13 (42%) | n = 20 (43%) | High-dose n = 2 (8%) Low-dose n = 6 (33%) | n = 6 (38%) | 20.45% p = 0.948 | 21.74% p = 0.948 | 28.6% p = 0.18 | 33.9% p = 0.18 | 8.4% p = 0.583 | 6.3% p = 0.583 |
| Acute GvHD grade III–IV | n = 3 (10%) p = 0.39 | n = 2 (4%) p = 0.39 | High-dose n = 1 (4%) Low-dose n = 4 (22%) p = 0.025 | n= 0 (0%) p = 0.025 | 2.27% p = 0.026 | 17.39% p = 0.026 | 10.2% p = 0.26 | 13.7% p = 0.26 | NR | NR |
| OS | 5-years period n = 35 (43%) p = 0.58 | High-dose 62 months (1–92) Low-dose 33 months (4–53) p = 0.15 | 34 months (4–84) p = 0.15 | 5-year period 86.4% p = 0.245 | 5-year period 95.7% p = 0.245 | 56.6% p = 0.052 | 46.3% p = 0.052 | 75% p = 0.645 | 80.9% p = 0.645 | |
| TRM | 5 years period n = 18 (24.5%) p = 0.54 | High-dose n = 1 Low-dose n = 0 | n = 0 | 11.36% p = 0.614 | 4.35% p = 0.614 | NR | NR | NR | NR | |
| NRM | 5 years period n = 19 (25.65%) 45% | NR | NR | NR | NR | 5-year period 27.9% p = 0.094 | 5-year period 21.5% p = 0.094 | 10.4% p = 0.402 | 15% p = 0.402 | |
| GRFS | 2 years period 41.9% p = 0.042 | 2 years period 67.4% p = 0.042 | NR | NR | GVHD-free, failure-free survival 77.3% p = 0.986 | GVHD-free, failure-free survival 78.3% p = 0.986 | 43.1% p = 0.014 | 32.4% p = 0.014 | 33.5% p = 0.109 | 52.8% p = 0.109 |
| LFS | NR | NR | NR | NR | NR | NR | 46.3% p = 0.051 | 38.6% p = 0.051 | NR | NR |
| Relapse | 2 years period 32% p = 0.41 | 2 years period 38% p = 0.41 | High-dose n = 4 (16%) Low-dose n = 4 (22%) p = 0.54 | n = 3 (18%) p = 0.54 | NR | NR | 5-year period 31.7% p = 0.66 | 5-year period 33.6% p = 0.66 | 33.5% p = 0.153 | 19.4% p = 0.153 |
| CMV reactivation | n = 22 (71%) p = 0.23 | n = 26 (57%) p = 0.23 | High-dose n = 5 Low-dose n = 7 p = 0.62 | n = 4 p = 0.62 | NR | NR | Day 100 32.7% p = 0.3 | Day 100 35.6% p = 0.3 | 29.9% p < 0.001 | 64.6% p < 0.001 |
| EBV reactivation | NR | NR | High-dose n = 7 Low-dose n = 4 p = 0.28 | n = 2 p = 0.28 | NR | NR | 10.7% p = 0.95 | 11.1% p = 0.95 | NR | NR |
| Infections overall | n = 30 (97%) p = 1 | n = 45 (98%) p = 1 | NR | NR | 59.09% p = 0.84 | 56.52% p = 0.84 | NR | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybko, J.; Giordano, U.; Pilch, J.; Mizera, J.; Borkowski, A.; Mordak-Domagała, M. Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in the Prophylaxis of Graft-Versus-Host Disease: A Systematic Review. J. Clin. Med. 2023, 12, 5449. https://doi.org/10.3390/jcm12175449
Dybko J, Giordano U, Pilch J, Mizera J, Borkowski A, Mordak-Domagała M. Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in the Prophylaxis of Graft-Versus-Host Disease: A Systematic Review. Journal of Clinical Medicine. 2023; 12(17):5449. https://doi.org/10.3390/jcm12175449
Chicago/Turabian StyleDybko, Jarosław, Ugo Giordano, Justyna Pilch, Jakub Mizera, Artur Borkowski, and Monika Mordak-Domagała. 2023. "Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in the Prophylaxis of Graft-Versus-Host Disease: A Systematic Review" Journal of Clinical Medicine 12, no. 17: 5449. https://doi.org/10.3390/jcm12175449





