The Efficacy of Platelet-Rich Plasma Augmentation in Microfracture Surgery Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction Process
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
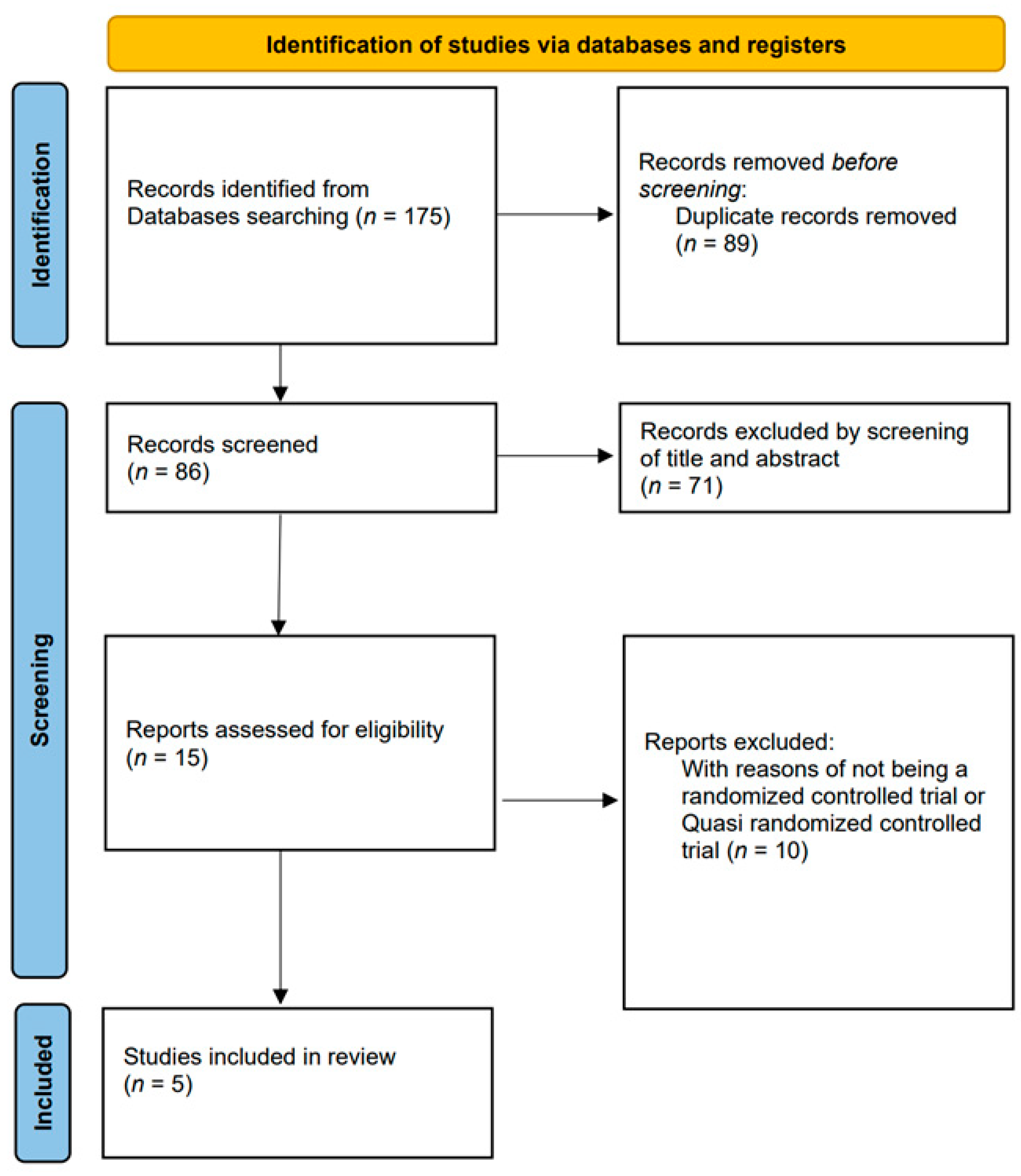
3.1. Study Selection and Characteristics of the Studies
3.2. Surgical Techniques of the Studies
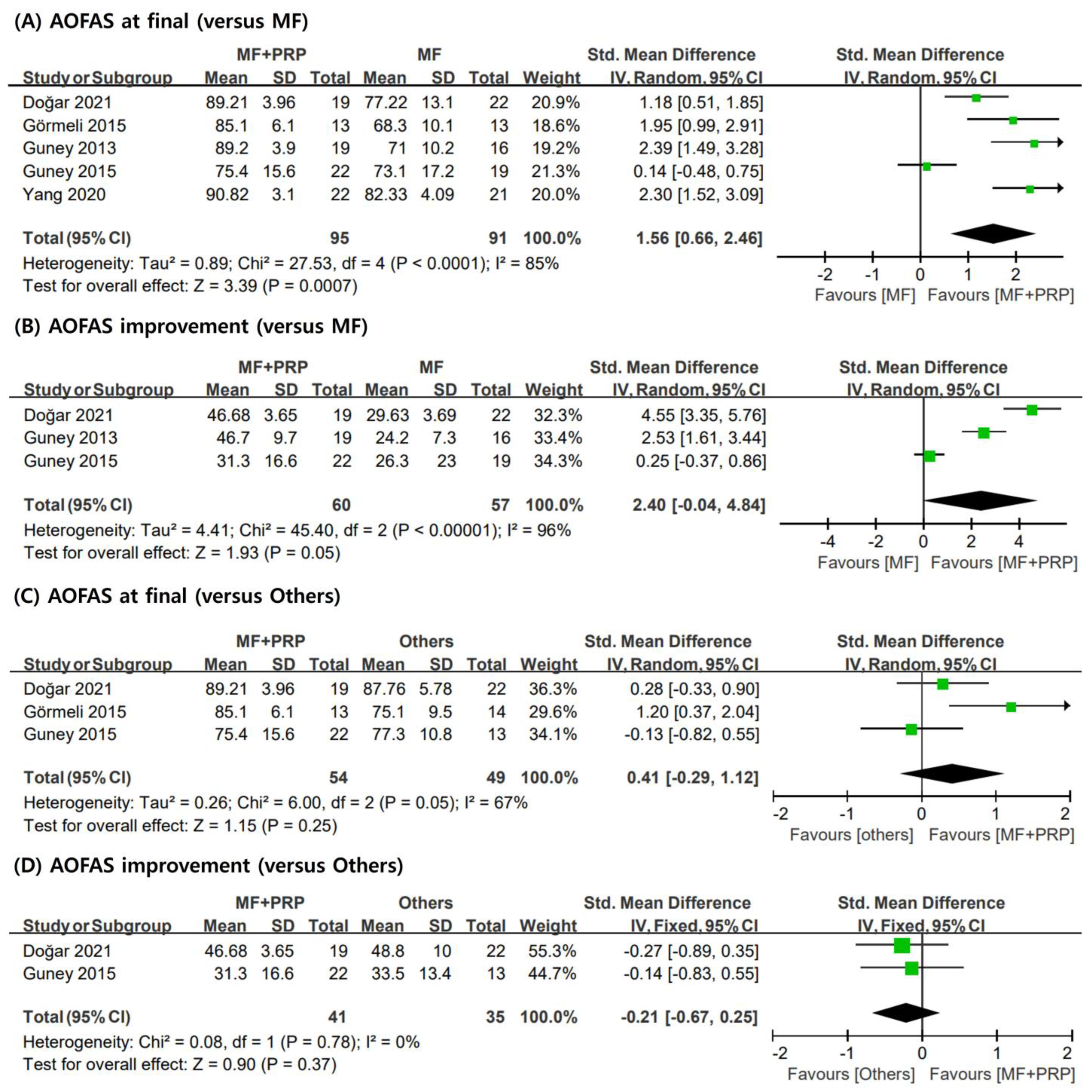
3.3. AOFAS
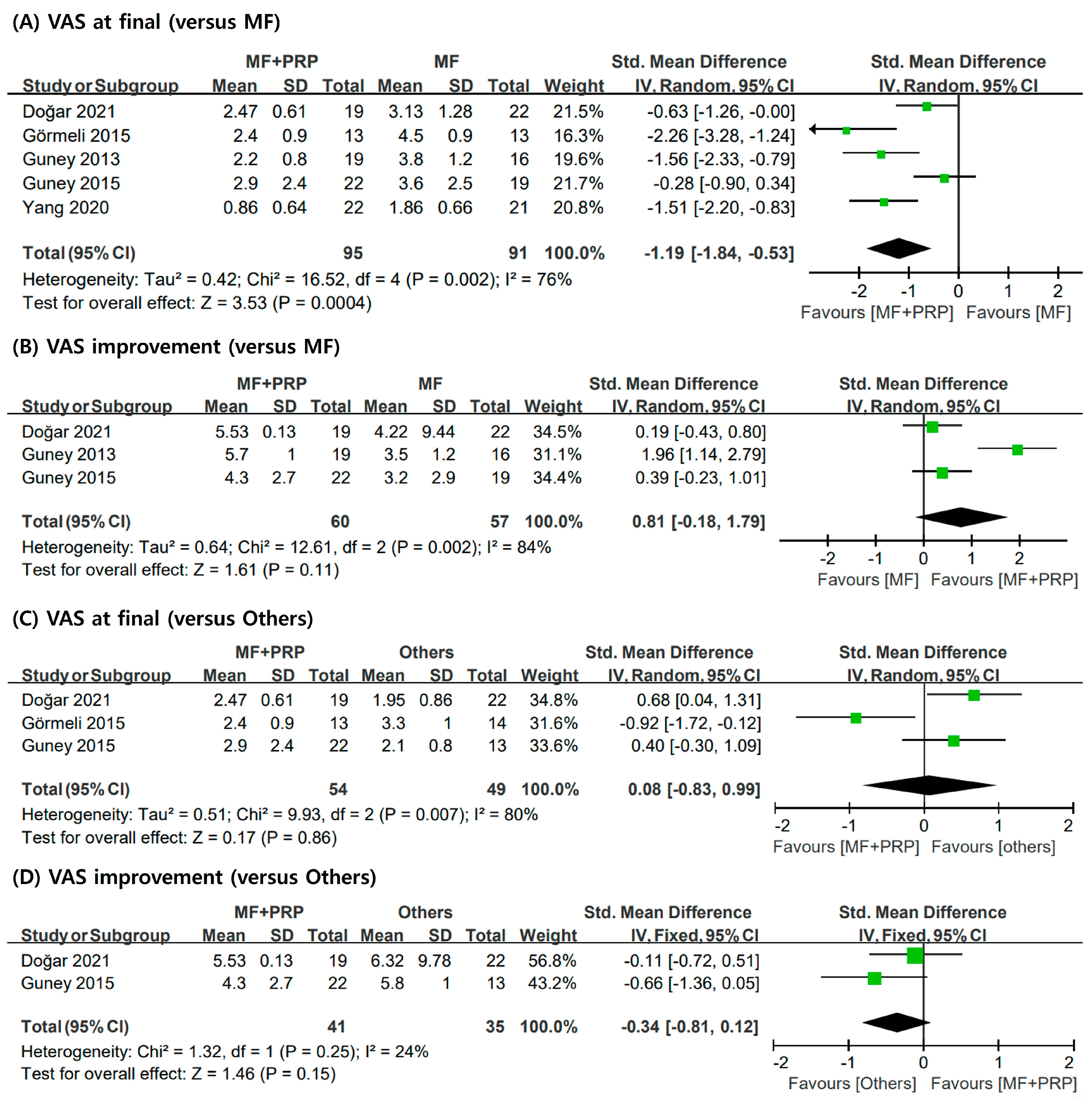
3.4. VAS
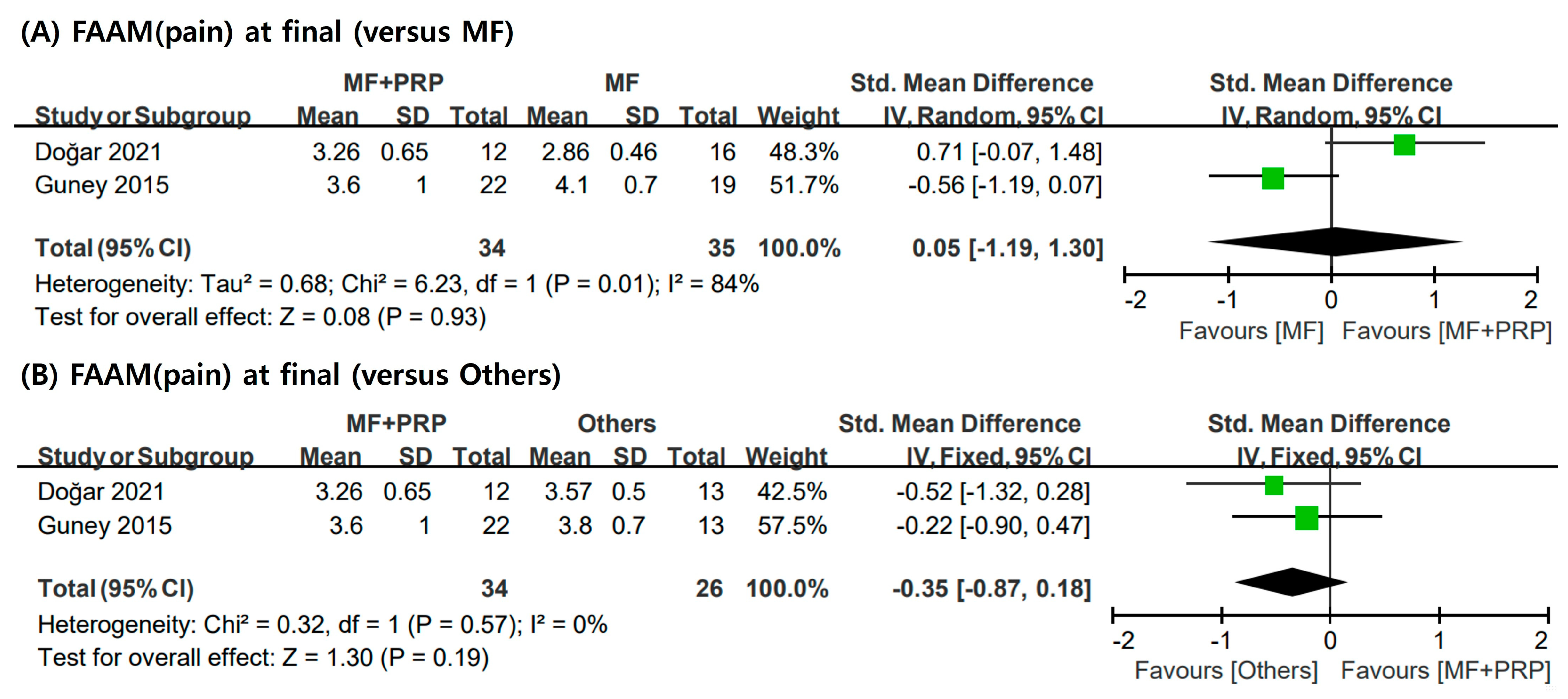
3.5. FAAM
3.6. Complications
3.7. Methodological Quality
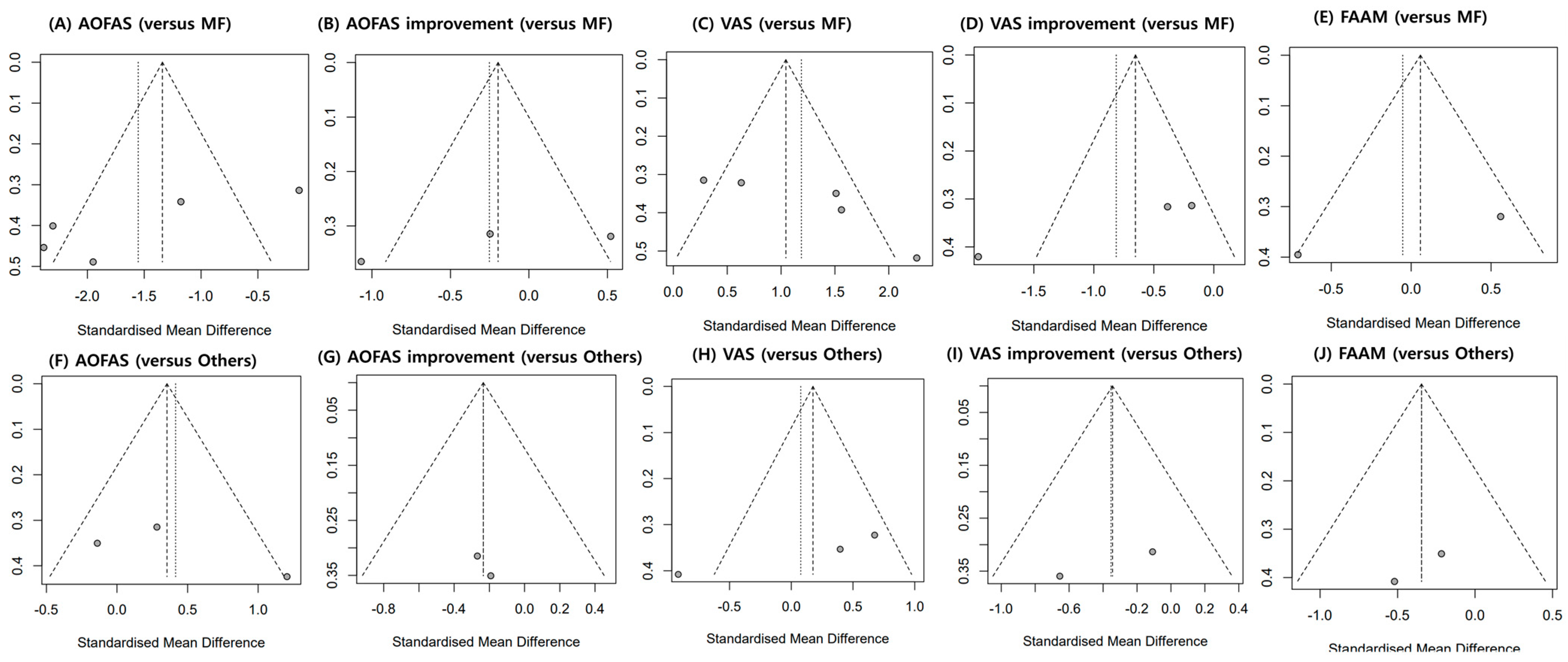
3.8. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berndt, A.L.; Harty, M. Transchondral fractures (osteochondritis dissecans) of the talus. JBJS 1959, 41, 988–1020. [Google Scholar] [CrossRef]
- Easley, M.E.; Latt, D.L.; Santangelo, J.R.; Merian-Genast, M.; Nunley, J.A. Osteochondral lesions of the talus. JAAOS-J. Am. Acad. Orthop. Surg. 2010, 18, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Regier, M.; Petersen, J.P.; Hamurcu, A.; Vettorazzi, E.; Behzadi, C.; Hoffmann, M.; Großterlinden, L.G.; Fensky, F.; Klatte, T.O.; Weiser, L. High incidence of osteochondral lesions after open reduction and internal fixation of displaced ankle fractures: Medium-term follow-up of 100 cases. Injury 2016, 47, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Steman, J.A.; Dahmen, J.; Lambers, K.T.; Kerkhoffs, G.M. Return to sports after surgical treatment of osteochondral defects of the talus: A systematic review of 2347 cases. Orthop. J. Sports Med. 2019, 7, 2325967119876238. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, C.N.; Reilingh, M.L.; Zengerink, M.; Van Bergen, C.J. Osteochondral defects in the ankle: Why painful? Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Guney, A.; Akar, M.; Karaman, I.; Oner, M.; Guney, B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Schachter, A.K.; Chen, A.L.; Reddy, P.D.; Tejwani, N.C. Osteochondral lesions of the talus. JAAOS-J. Am. Acad. Orthop. Surg. 2005, 13, 152–158. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Battaglia, M.; Cavallo, M.; Ruffilli, A.; Ramponi, L.; Pagliazzi, G.; Vannini, F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am. J. Sports Med. 2013, 41, 511–518. [Google Scholar] [CrossRef]
- Perisano, C.; Cannella, A.; Polichetti, C.; Mascio, A.; Comisi, C.; De Santis, V.; Caravelli, S.; Mosca, M.; Spedicato, G.A.; Maccauro, G.; et al. Tibiotalar and Tibiotalocalcaneal Arthrodesis with Paragon28 Silverback(TM) Plating System in Patients with Severe Ankle and Hindfoot Deformity. Medicine 2023, 59, 344. [Google Scholar] [CrossRef]
- Tokgöz, M.A.; Atik, O.Ş.; Esendağlı, G.; Öğüt, B.; Bozkurt, H.H. Is it possible that the pathogenesis of osteoarthritis could start with subchondral trabecular bone loss like osteoporosis? Jt. Dis. Relat. Surg. 2018, 29, 152–158. [Google Scholar] [CrossRef]
- Badekas, T.; Takvorian, M.; Souras, N. Treatment principles for osteochondral lesions in foot and ankle. Int. Orthop. 2013, 37, 1697–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuckpaiwong, B.; Berkson, E.M.; Theodore, G.H. Microfracture for osteochondral lesions of the ankle: Outcome analysis and outcome predictors of 105 cases. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 106–112. [Google Scholar] [CrossRef]
- Choi, S.-W.; Lee, G.-W.; Lee, K.-B. Arthroscopic microfracture for osteochondral lesions of the talus: Functional outcomes at a mean of 6.7 years in 165 consecutive ankles. Am. J. Sports Med. 2020, 48, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Becher, C.; Driessen, A.; Hess, T.; Longo, U.G.; Maffulli, N.; Thermann, H. Microfracture for chondral defects of the talus: Maintenance of early results at midterm follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.E.; Everhart, J.S.; Klitzman, R.G. Hyaluronic acid as an adjunct to microfracture in the treatment of osteochondral lesions of the talus: A systematic review of randomized controlled trials. BMC Musculoskelet. Disord. 2022, 23, 313. [Google Scholar] [CrossRef]
- Smyth, N.A.; Murawski, C.D.; Haleem, A.M.; Hannon, C.P.; Savage-Elliott, I.; Kennedy, J.G. Establishing proof of concept: Platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J. Orthop. 2012, 3, 101. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Bendinelli, P.; Matteucci, E.; Dogliotti, G.; Corsi, M.M.; Banfi, G.; Maroni, P.; Desiderio, M.A. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: Mechanisms of NF-κB inhibition via HGF. J. Cell. Physiol. 2010, 225, 757–766. [Google Scholar] [CrossRef] [Green Version]
- De Vos, R.J.; Weir, A.; van Schie, H.T.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; Weinans, H.; Tol, J.L. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA 2010, 303, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Kajikawa, Y.; Morihara, T.; Sakamoto, H.; Matsuda, K.i.; Oshima, Y.; Yoshida, A.; Nagae, M.; Arai, Y.; Kawata, M.; Kubo, T. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J. Cell. Physiol. 2008, 215, 837–845. [Google Scholar] [CrossRef]
- Kitoh, H.; Kitakoji, T.; Tsuchiya, H.; Katoh, M.; Ishiguro, N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J. Pediatr. Orthop. 2007, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.-P.; Chadjichristos, C.; Legendre, F.; Baugé, C.; Beauchef, G.; Andriamanalijaona, R.; Galéra, P.; Boumediene, K. Interleukin-1 and transforming growth factor-ß 1 as crucial factors in osteoarthritic cartilage metabolism. Connect. Tissue Res. 2008, 49, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.W.; Shetty, A.A.; Kim, S.J.; Kim, Y.J.; Choi, N.Y.; Jun, Y.J.; Park, I.J. The effect of platelet rich plasma combined with microfracture for the treatment of chondral defect in a rabbit knee. Tissue Eng. Regen. Med. 2014, 11, 178–185. [Google Scholar] [CrossRef]
- Manunta, A.F.; Manconi, A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints 2013, 1, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venosa, M.; Calafiore, F.; Mazzoleni, M.; Romanini, E.; Cerciello, S.; Calvisi, V. Platelet-rich plasma and adipose-derived mesenchymal stem cells in association with arthroscopic microfracture of knee articular cartilage defects: A pilot randomized controlled trial. Adv. Orthop. 2022, 2022, 6048477. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.P.; Hondke, S.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J. Orthop. Res. 2012, 30, 845–852. [Google Scholar] [CrossRef]
- Schallmo, M.S.; Marquez-Lara, A.; Luo, T.D.; Rosas, S.; Stubbs, A.J. Arthroscopic Treatment of Hip Chondral Defect With Microfracture and Platelet-Rich Plasma–Infused Micronized Cartilage Allograft Augmentation. Arthrosc. Tech. 2018, 7, e361–e365. [Google Scholar] [CrossRef] [Green Version]
- Doğar, F.; Uzun, E.; Gürbüz, K.; Topak, D.; Akar, M.; Bilal, Ö.; Güney, A. Comparison of arthroscopic treatment methods in talar osteochondral lesions: A multicenter, prospective, randomized clinical trial. J. Am. Podiatr. Med. Assoc. 2021, 111. [Google Scholar] [CrossRef]
- Görmeli, G.; Karakaplan, M.; Görmeli, C.A.; Sarıkaya, B.; Elmalı, N.; Ersoy, Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: A prospective randomized clinical trial. Foot Ankle Int. 2015, 36, 891–900. [Google Scholar] [CrossRef]
- Guney, A.; Yurdakul, E.; Karaman, I.; Bilal, O.; Kafadar, I.H.; Oner, M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1293–1298. [Google Scholar] [CrossRef]
- Yang, J. Micro-fracture therapy combined with intra-articular injection of platelet-rich plasma for small sized osteochondral lesion of the talus. J. Reparative Reconstr. Surg. 2020, 34, 53–56. [Google Scholar]
- Boffa, A.; Previtali, D.; Altamura, S.A.; Zaffagnini, S.; Candrian, C.; Filardo, G. Platelet-Rich Plasma Augmentation to Microfracture Provides a Limited Benefit for the Treatment of Cartilage Lesions: A Meta-analysis. Orthop. J. Sports Med. 2020, 8, 2325967120910504. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Mao, Z.; Zhang, L.; Zhang, L.; Zhao, Y.; Yin, P.; Gao, L.; Tang, P.; Kang, H. Meta-analysis of locking plate versus intramedullary nail for treatment of proximal humeral fractures. J. Orthop. Surg. Res. 2015, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Walther, M.; Gottschalk, O.; Madry, H.; Müller, P.E.; Steinwachs, M.; Niemeyer, P.; Niethammer, T.R.; Tischer, T.; Petersen, J.; Feil, R.; et al. Etiology, Classification, Diagnostics, and Conservative Management of Osteochondral Lesions of the Talus. 2023 Recommendations of the Working Group “Clinical Tissue Regeneration” of the German Society of Orthopedics and Traumatology. Cartilage 2023, 19476035231161806. [Google Scholar] [CrossRef]
- Sandlin, M.I.; Charlton, T.P.; Taghavi, C.E.; Giza, E. Management of Osteochondral Lesions of the Talus. Instr. Course Lect. 2017, 66, 293–299. [Google Scholar]
- Doral, M.N.; Huri, G.; Turhan, E.; Dönmez, G.; Kaya, D. Arthrosurfacing in Talar Osteochondral Lesions. In Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation; Doral, M.N., Karlsson, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–11. [Google Scholar]
- Zengerink, M.; Struijs, P.A.; Tol, J.L.; van Dijk, C.N. Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Buckwalter, J.A.; Mow, V.C.; Ratcliffe, A. Restoration of injured or degenerated articular cartilage. JAAOS-J. Am. Acad. Orthop. Surg. 1994, 2, 192–201. [Google Scholar] [CrossRef]
- Nehrer, S.; Spector, M.; Minas, T. Histologic analysis of tissue after failed cartilage repair procedures. Clin. Orthop. Relat. Res. 1999, 365, 149–162. [Google Scholar] [CrossRef]
- Abrams, G.D.; Frank, R.M.; Fortier, L.A.; Cole, B.J. Platelet-rich plasma for articular cartilage repair. Sports Med. Arthrosc. Rev. 2013, 21, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, P.M.; Lekovic, V.; Weinlaender, M.; Vasilic, N.; Madzarevic, M.; Kenney, E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J. Periodontal Res. 2002, 37, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, F.; Liu, Y.; Ma, Q.; Mao, T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: Experimental study in a rabbit model. J. Oral. Maxillofac. Surg. 2007, 65, 1951–1957. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Wang, H.; Wei, X.; Fu, X.; Zhang, J.; Yu, C. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-κBp65-specific siRNA. Osteoarthr. Cartil. 2008, 16, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Daheshia, M.; Yao, J.Q. The interleukin 1β pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Mei-Dan, O.; Carmont, M.R.; Laver, L.; Mann, G.; Maffulli, N.; Nyska, M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am. J. Sports Med. 2012, 40, 534–541. [Google Scholar] [CrossRef]
- Sánchez, M.; Guadilla, J.; Fiz, N.; Andia, I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology 2012, 51, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Spaková, T.; Rosocha, J.; Lacko, M.; Harvanová, D.; Gharaibeh, A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am. J. Phys. Med. Rehabil. 2012, 91, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, Q.; Xu, Y.; He, H. Platelet-rich plasma treatment for talar cartilage repair: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 366. [Google Scholar] [CrossRef]
- Yausep, O.E.; Madhi, I.; Trigkilidas, D. Platelet rich plasma for treatment of osteochondral lesions of the talus: A systematic review of clinical trials. J. Orthop. 2020, 18, 218–225. [Google Scholar] [CrossRef]
- Fu, S.; Yang, K.; Li, X.; Chen, C.; Mei, G.; Su, Y.; Xue, J.; Zou, J.; Zhang, J.; Shi, Z. Radiographic and clinical outcomes after arthroscopic microfracture for osteochondral lesions of the talus: 5-year results in 355 consecutive ankles. Orthop. J. Sports Med. 2022, 10, 23259671221128772. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Hurtig, M.; Rossomacha, E.; Sun, J.; Chevrier, A.; Shive, M.S.; Buschmann, M.D. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J. Bone Jt. Surg. Am. 2005, 87, 2671–2686. [Google Scholar] [CrossRef]
- Vilá y Rico, J.; Dalmau, A.; Chaqués, F.J.; Asunción, J. Treatment of Osteochondral Lesions of the Talus With Bone Marrow Stimulation and Chitosan–Glycerol Phosphate/Blood Implants (BST-CarGel). Arthrosc. Tech. 2015, 4, e663–e667. [Google Scholar] [CrossRef] [Green Version]
- Frappier, J.; Stanish, W.; Brittberg, M.; Steinwachs, M.; Crowe, L.; Castelo, D.; Restrepo, A. Economic evaluation of BST-CarGel as an adjunct to microfracture vs microfracture alone in knee cartilage surgery. J. Med. Econ. 2014, 17, 266–278. [Google Scholar] [CrossRef]
- Sun, S.F.; Hsu, C.W.; Sun, H.P.; Chou, Y.J.; Li, H.J.; Wang, J.L. The effect of three weekly intra-articular injections of hyaluronate on pain, function, and balance in patients with unilateral ankle arthritis. J. Bone Jt. Surg. Am. 2011, 93, 1720–1726. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsiao, M.Y.; Chen, W.S.; Wang, T.G.; Chien, K.L. Effectiveness of intra-articular hyaluronic acid for ankle osteoarthritis treatment: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 951–960. [Google Scholar] [CrossRef]
- Hangody, L.; Ráthonyi, G.K.; Duska, Z.; Vásárhelyi, G.; Füles, P.; Módis, L. Autologous osteochondral mosaicplasty. Surgical technique. J. Bone Jt. Surg. Am. 2004, 86 (Suppl. S1), 65–72. [Google Scholar] [CrossRef]
- Valderrabano, V.; Leumann, A.; Rasch, H.; Egelhof, T.; Hintermann, B.; Pagenstert, G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am. J. Sports Med. 2009, 37 (Suppl. S1), 105s–111s. [Google Scholar] [CrossRef]
- Marmotti, A.; Rossi, R.; Castoldi, F.; Roveda, E.; Michielon, G.; Peretti, G.M. PRP and articular cartilage: A clinical update. BioMed Res. Int. 2015, 2015, 542502. [Google Scholar] [CrossRef]
- Pinski, J.M.; Boakye, L.A.; Murawski, C.D.; Hannon, C.P.; Ross, K.A.; Kennedy, J.G. Low level of evidence and methodologic quality of clinical outcome studies on cartilage repair of the ankle. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 214–222.e211. [Google Scholar] [CrossRef]
- Paget, L.D.; Reurink, G.; de Vos, R.-J.; Weir, A.; Moen, M.H.; Bierma-Zeinstra, S.M.; Stufkens, S.A.; Kerkhoffs, G.M.; Tol, J.L.; Goedegebuure, S. Effect of platelet-rich plasma injections vs placebo on ankle symptoms and function in patients with ankle osteoarthritis: A randomized clinical trial. JAMA 2021, 326, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
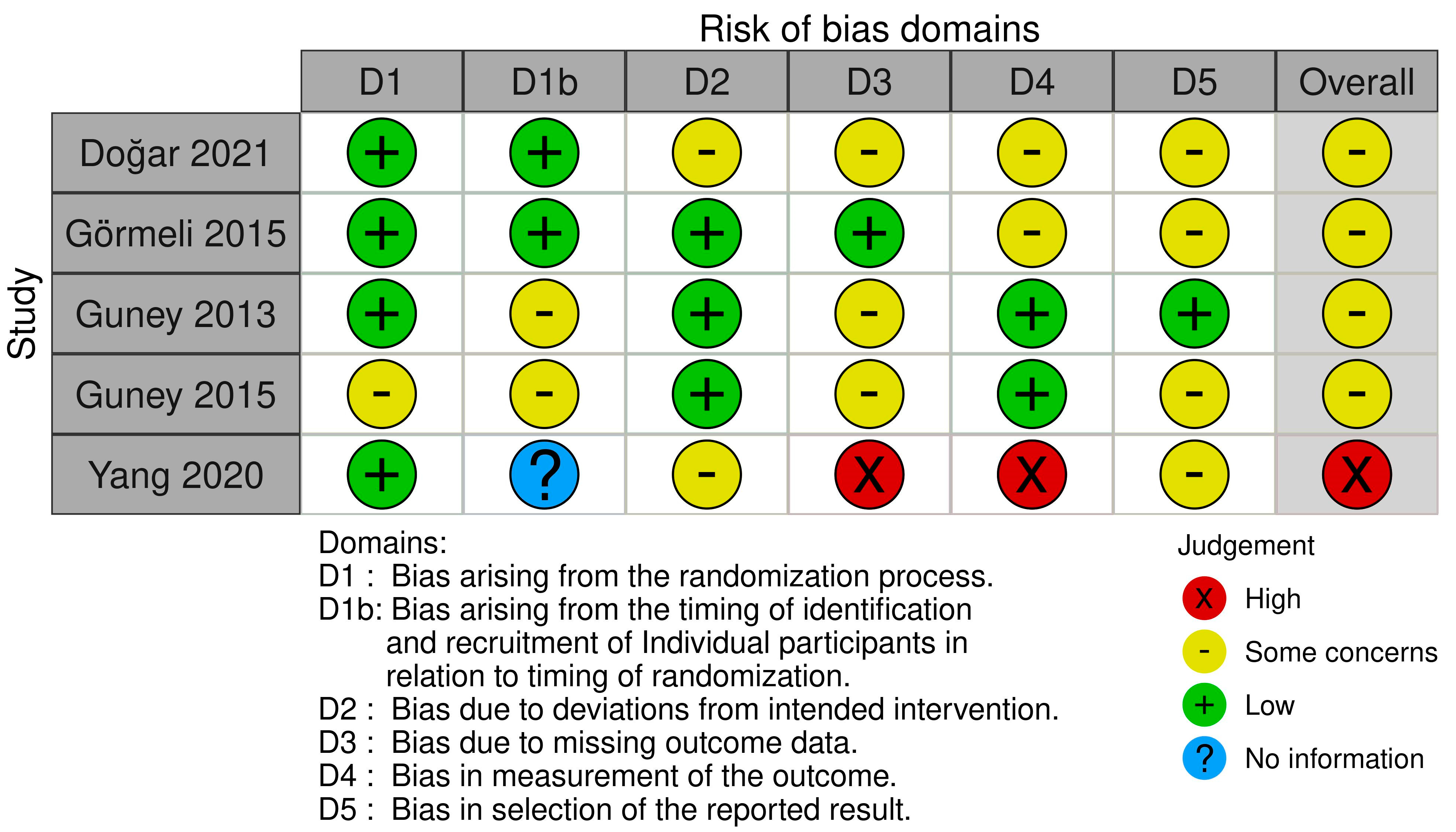
| Authors (Year) | Study Design (LOE) | MQOE | Intervention | Comparator(s) | Mean Age, Years | Follow Up (FU), Months | MF, n | MF + PRP, n | others, n | OLT size | Outcomes Recorded | Administration Modalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Doğar et al. [28] (2021) | RCT (2) | 83 (good) | Arthroscopic MF with PRP | Arthroscopic MF only/arthroscopic MF with BST-CarGel | MF: 33 ± 13.2 MF + PRP: 45.6 ± 11.8 MF + BST: 35.6 ± 12 | Mean FU: MF: 24 ±15 MF + PRP: 30 ± 24 MF + BST: 25 ± 12.2 Range: - | 22 | 19 | 22 | Control: 1.2 cm2 MF: 1.14 cm2 MF + BST-CarGel: 1.5 cm2 | AOFAS, FAAM, VAS, time to postoperative return to sport activity | Single injection of PRP (SmartPReP2 system) 24 h after the operation/BST-CarGel mixed with blood through arthroscopic cannula intraoperatively |
| Görmeli et al. [29] (2015) | RCT (1) | 86 (excellent) | Arthroscopic MF with PRP | Arthroscopic MF with HA/arthroscopic MF with saline | MF + Saline: 40.3 ± 9.4 MF + HA: 39.7 ± 8.7 MF + PRP: 38.6 ± 9.1 | 15.3 Range: 11–25 | 13 | 13 | 14 | PRP: 1.3 cm2 (0.5–1.4) Control: 1.2 cm2 | AOFAS, VAS | Single injection of PRP (SmartPReP2 system) 6 and 24 h after the operation |
| Guney et al. [6] (2013) | RCT (2) | 73 (good) | Arthroscopic MF with PRP | Arthroscopic MF only | MF: 42.8 ± 14.7 MF + PRP: 38.6 = 5 ± 12.7 | Mean FU 16.2 Range 12–84 | 16 | 19 | - | - | AOFAS, FAAM, VAS | Single injection of PRP (SmartPReP2 system [Harvest Autologous Hemobiologics]) 6 and 24 h after the operation |
| Guney et al. [30] (2015) | Quasi-RCT (2) | 75 (good) | Arthroscopic MF with PRP | Arthroscopic MF only/mosaicplasy | MF: 33 ± 13.2 MF + PRP: 45.6 ± 11.8 Mosaicplasty: 35.6 ± 12 | Mean FU: MF: 47.3 ± 16.9 MF + PRP: 40.4 ± 10.4 Mosaicplasty: 30.1 ± 13.1 Range: 12–24 | 19 | 22 | 13 | PRP: <20 mm Control: <20 mm | AOFAS, FAAM, VAS | Single injection of PRP (SmartPReP2 system) 6 and 24 h after the operation |
| Yang et al. [31] (2020) | RCT (2) | 78 (good) | Arthroscopic MF with PRP | Arthroscopic MF only | MF: 36.5 MF + PRP: 35.9 | Mean FU: 15.6 Range: 12–18 | 21 | 22 | - | 0.93 ± 0.41 cm | AOFAS, VAS | Single injection of PRP (3 mL autologous PRP plus 10% CaCl2 0.3 mL), 4 weekly injections after the operation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, I.; Park, J.J.; Seok, H.-G. The Efficacy of Platelet-Rich Plasma Augmentation in Microfracture Surgery Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4998. https://doi.org/10.3390/jcm12154998
Woo I, Park JJ, Seok H-G. The Efficacy of Platelet-Rich Plasma Augmentation in Microfracture Surgery Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(15):4998. https://doi.org/10.3390/jcm12154998
Chicago/Turabian StyleWoo, Inha, Jeong Jin Park, and Hyun-Gyu Seok. 2023. "The Efficacy of Platelet-Rich Plasma Augmentation in Microfracture Surgery Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 15: 4998. https://doi.org/10.3390/jcm12154998
APA StyleWoo, I., Park, J. J., & Seok, H.-G. (2023). The Efficacy of Platelet-Rich Plasma Augmentation in Microfracture Surgery Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(15), 4998. https://doi.org/10.3390/jcm12154998





